Abstract
Background
Telemedicine is defined as the use of electronic information and communication technologies to provide health care between distant people. Many activities in transfusion medicine could benefit from the application of telemedicine. To map the spread of the use of telemedicine in transfusion medicine in Italy, the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) performed a nationwide survey: the results are presented in this paper.
Materials and methods
A survey, dealing with different aspects of the use of telemedicine, was performed by sending a questionnaire to 280 Italian Blood Centres. The survey was designed to evaluate the diffusion of telemedicine and the features of the systems, with special attention to the systems’ safety and legal adequacy. One section of the questionnaire was designed to identify the features of the systems considered essential by the respondents.
Results
Out of 280 Blood Services contacted, 196 (70%) filled in at least one of the questions of the online questionnaire. Globally the use of some form of telemedicine was reported by 70% of the respondents. Telemedicine is used for remote validation of laboratory tests by 32% of the Centres that responded, for remote biological validation of blood units by 34% and for assignment of blood components by 29%. Less frequently, telemedicine is used to control electronic refrigerators, for electronic blood requests and for bed-side identification of patients.
Discussion
The use of telemedicine is widespread in Italian Blood Services. There appears to be some heterogeneity between structures with regards to the evaluation of the systems’ safety and their legal adequacy. No telemedicine system should be introduced into practice until it has proven to have the same standards of safety as the corresponding “on site” activity.
Keywords: telemedicine, blood transfusion, survey, questionnaire
Introduction
Many definitions of telemedicine are reported in the literature but, ultimately, it could simply be described as the use of electronic information and communication technologies to provide health care between distant people. The broader description adopted by the World Health Organisation defines telemedicine as “the delivery of health care services, where distance is a critical factor, by all health care professionals using information and communication technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and for the continuing education of health care providers, all in the interests of advancing the health of individuals and their communities”1. In recent years, telemedicine has excited growing interest, particularly in the context of needing to control healthcare costs: its use could, in fact, expand health care in a large number of settings with no need for a contemporaneous increase in human resources, thus maximising the system’s efficiency. Although its cost-benefit ratio has been questioned2–3, telemedicine is currently used in many medical specialties4–11. The ongoing tendency to centralise the control of processes related to blood collection, processing, validation and distribution makes telemedicine attractive to Blood Banks12–15. Supervision and interpretation of immunohaematological and serological analyses performed at distant sites, as well as the biological validation of blood components stored elsewhere, are both activities which could benefit from the use of telemedicine. Telemedicine could also be of help in some emerging aspects of transfusion medicine, such as the management of remote electronic blood refrigerators or the issue of blood to patients in hospitals lacking a Blood Centre. Information and communication technologies could provide a solution for the control of the entire blood transfusion process, starting with the electronic “order-entry” and ending with the registration of each transfusion episode in the Blood Bank information system16.
Despite its great potential, the use of telemedicine remains highly fragmented: besides technical problems, some others issues need to be resolved, especially those of legal and ethical nature. The lack of accepted standard procedures represents a further obstacle to the widespread diffusion of telemedicine17,18.
In 2011, a survey aimed to determine the degree of spread of telemedicine techniques among Italian Blood Services was carried out by the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI): the results were presented, in part, at the second National Conference of Blood Centres, held in Pisa in May 2011.
Materials and methods
Every Italian Blood Transfusion Service was asked to fill in an online questionnaire at the SIMTI web site (www.simti.it). A letter of invitation to fill in the questionnaire was sent by the President of SIMTI to all Directors of Transfusion Services, and the request was subsequently repeated every week by e-mail in periodic SIMTI newsletters. The importance of participating in the survey was stressed both in the letter and in subsequent messages sent by SIMTI, in order to provide the community of Italian transfusion specialists with up-to-date data about telemedicine. The expected time to fill in the questionnaire was about 1 hour although no information is available about how long it really took the participants. The questionnaire was structured in seven sections (Table I) and is described in detail in Tables II to VIII. The first six sections were aimed to get a snapshot of telemedicine use across the country, while in section 7 participants were asked to provide opinions about the issues to be resolved prior to the adoption of telemedicine techniques. The results were analysed using simple mathematic functions present in Microsoft Excel version 2007; no specific statistical software was necessary.
Table I.
Structure of the questionnaire.
| Section | Heading |
|---|---|
| 1 | Supervision of analysis |
| 2 | Biological validation of blood components |
| 3 | Remote assignment of blood components |
| 4 | Remote management of electronic blood refrigerators |
| 5 | Blood transfusion order-entry |
| 6 | Bed-side identification of patients |
| 7 | Process control, legal issues and risk management |
Table II.
Interpretation and supervision of analysis.
| Question | Yes (n∘-%) |
|---|---|
| Do you use remote validation of analyses in your centre? | 63–32% |
| For immunohaematology | 20–10% |
| For serology | 27–14% |
| For immunohaematology and serology | 16–8% |
|
| |
| Do you use specific or remote control software? | |
| - Specific | 46–73% |
| - Remote control | 10–16% |
| - No answer | 7–11% |
|
| |
| Do you use this modality: | |
| - Always | 37–59% |
| - In the absence of personnel | 12–19% |
| - When considered opportune | 14–22% |
|
| |
| Are analytical instruments interfaced with your information system? | 57–90% |
|
| |
| Do you have written procedures to be followed in the case the system crashes? | 51–81% |
|
| |
| Does the system have digital signature software? | 19–30% |
|
| |
| Do you consider that the system complies with legislation on the protection of privacy? | 53–84% |
Table VIII.
Process control, legal issues and risk management.
| Question | % |
|---|---|
| Which of the following do you consider essential in a telemedicine system for Blood Banks? | |
| - Interfaced instruments | 91% |
| - Complete traceability | 78% |
| - Procedures for system failures | 73% |
| - Possibility of seeing the transfusion request | 66% |
| - Possibility of consulting the patient’s transfusion history | 69% |
| - Possibility of seeing images of the tests | 81% |
| - Accurate analyses comparing telemedicine and standard processes | 78% |
| - Systems compliant with legislation on the protection of privacy | 94% |
| - Digital signature | 87% |
Results
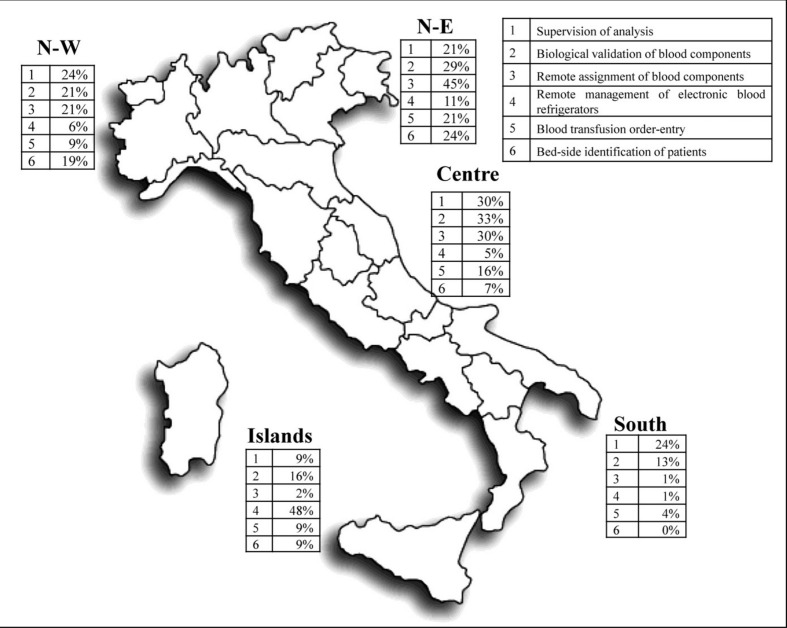
Out of the 280 Blood Services contacted, 196 (70%) filled in at least one of the questions of the online questionnaire, with geographic variations between the different areas of the country, as defined by Eurostat19: north-east (71%), north-west (84%), centre (87%), south (57%) and the islands (52%). Overall, 132 Services (67% of respondents) reported using some form of telemedicine, with this use being more frequent in the north-west and centre (68% and 67%, respectively) than in the north-east (44%), south (28%) and the islands (34%): the geographic distribution of telemedicine use is shown in Figure 1.
Figure 1.
Geographic distribution of telemedicine use in Italy.
Seventy-four centres used telemedicine for more than one activity. Supervision of analysis was most frequently observed in concurrence with biological validation of blood components (in 12 cases), with remote blood assignment (in 7 cases), or with both (in 10 cases).
The results are presented in detail in Tables II to VIII.
Remote supervision and interpretation of analysis
The first section of the questionnaire regarded the remote validation of immunohaematological and serological analyses, that is, the authorisation for clinical use of laboratory results performed by someone far from the site where the tests were performed.
Out of the 196 respondents, 63 (32%) reported using remote validation, 20 (10%) for immunohaematological tests, 27 (14%) for serological analyses and 16 (8%) for both: dedicated software was generally used, but 10 centres reported exploiting non-specific programmes for remote control, that is, software allowing a person, through a internet connection, to work on a distant computer as if it were his or her own local machine. Among the Centres performing remote validation, 59% declared that they used the system in every case, 19% only in the absence of personnel and 22% when they considered it more opportune. Nearly always (90%) the analytical instruments were interfaced with the Blood Bank information system.
Fifty centres (81%) had written procedures to be followed in the case that the telemedicine system crashed. In 19 Services the system was supplied with digital signature software considered compliant with national regulations; the great majority of the users (84%) considered the system adequate with regards to legal requirements for protection of privacy.
Biological validation of blood components
The purpose of this section of the questionnaire was to verify the utilisation of telemedicine in the authorisation of therapeutic use of blood components given by someone far from the site of storage. This modality was used in 62 (34%) of the 180 structures that responded to the question (34%) and more frequently involved specific software (71%) than software for remote control (16%): in the great majority of cases (82%) this modality was the only one employed for validation of blood components. In more than half of the cases (61%) there were written procedures to overcome failure of the systems, whereas equipment for digital signatures was present in only 19% of the Centres.
Remote assignment of blood components
This section examined the utilisation of telemedicine in authorising the transfusion of specific blood units to a patient, when the person responsible for the authorisation is located remotely from the site where the compatibility tests are performed. This assignment modality was used in 52 out of 182 respondents to this section (29%), and most frequently involved specific software (75%), during out-of-routine hours when the authorising person was providing an on-call service. The professional figure responsible for blood assignment was almost always (98%) a Blood Bank graduate and more frequently a physician (65%), in half the cases operating from home with, however, the possibility of evaluating the request’s appropriateness, of knowing the patient’s transfusion history, and of seeing the images of the pre-transfusion tests. The manual skill for tests was provided by Blood Bank technicians, called from home in two-thirds of cases, sometimes (17%) sharing turns with non-Blood Bank technicians. In most cases, there were written procedures for dealing with failures of the system, while the use of digital signature software was less frequent (44%).
More than 90% of the respondents considered the system adequate with regards to legal requirements for the protection of privacy.
Remote electronic blood refrigerators
Remote electronic blood refrigerators were used rarely (in 6% of structures responding to this section) and were generally limited to public hospitals, with Blood Bank personnel called only when compatible units for patients (assigned units or valid type and screen) were not available. The blood refrigerators were interfaced with the Blood Bank information system and there were written procedures to solve system failures.
Electronic order-entry
One hundred and seventy-nine centres answered questions in this section: 31 of them reported using an electronic order entry, almost always (97% of cases) interfaced with the Blood Bank information system. An electronic flow-chart was often included (84%), but was designed to block inappropriate requests in only 58% of cases.
Systems for bed-side identification of patients
Among the 175 centres that responded to questions in this section, 30 (17%) declared that they used a system for bed-side identification of patients. In most cases the system was based on bar-code technology (80%); six structures reported using more than one type of technology. In 21 out of 30 (70%) centres, the system used was interfaced with the Blood Bank information system in order to track the transfusion event. There were almost always procedures to deal with a system failure.
Process control, legal issues and risk management
The features most frequently considered essential for telemedicine systems in transfusion medicine are the systems’ adequacy with respect to the legal requirements for protection of privacy, the presence of interfaced instruments and the availability of a digital signature device. More than 80% of respondents also considered it necessary to be able to see the images of pre-transfusion tests.
Discussion
This is the first national survey concerning the use of telemedicine in immunohaematology and transfusion medicine. This field of medical activity raises many questions, the foremost concerning its legal conformity, particularly considering the complexity of national legislation (“Digital Administration Code”)20, and the requirements for a safe digital signature when applied to medical prescriptions.
Most of the respondents to this survey declared that the systems they use are adequate with respect to legislation on the protection of privacy, but only a few considered them conformant with the requirements for the digital signature. This observation supports the conclusion that, at present, the importance of a safe, valid and authentic digital signature as a minimum requirement for telemedicine systems is undervalued by those who actually use them, although most of the respondents consider such a signature as mandatory before introducing telemedicine into routine use.
The safety of telemedicine is dependent not only on digital signatures and legal aspects, but also on most of the other parts of the activities: for example, whether procedures are in place to ensure the continuity of the service if the telemedicine system fails. Even this aspect seems to be underestimated (only 73% considered it essential), although it is critically important for the safety of patients requiring transfusion in urgency or emergency conditions. This situation probably reflects the fact that, in most cases in Italy, telemedicine has been introduced to ensure transfusion supplies after restructuring health services to cover a broader region, or because of shortage of personnel: this seems to have led to a more problematic organisation which, in some instances, does not allow system failures to be covered effectively. This aspect must to be considered with great attention because of the potential risk for patients.
Conclusions
Telemedicine systems are intended to reduce the risk for patients and the specific risk of every medical activity in which telemedicine is used should, therefore, be evaluated with appropriate techniques of risk assessment and risk management before the telemedicine system is introduced into routine practice. This evaluation should demonstrate that the risk score is at least equal, or even lower, than that for the same activities performed in the traditional way. In summary, there seems to be some form of incoherence between what respondents consider as essential in tele-transfusion medicine, and what they really use when applying it. Besides each single aspect of the procedure, no telemedicine system can be allowed until it has been proven to have the same standards of safety as the corresponding “on site” activity.
Table III.
Biological validation of blood components.
| Question | Yes (n∘-%) |
|---|---|
| Do you use remote validation of blood components in your centre? | 62–34% |
|
| |
| Do you use specific or remote control software? | |
| - Specific | 44–71% |
| - Remote control | 10–16% |
| - No answer | 8–13% |
|
| |
| Do you use this modality: | |
| - Always | 51–82% |
| - In the absence of personnel | 4–6% |
| - When considered opportune | 5–8% |
| - No answer | 2–3% |
|
| |
| Do you have written procedures to be followed in the case the system crashes? | 38–61% |
|
| |
| Does the system have digital signature software? | 12–19% |
Table IV.
Remote assignment of blood components.
| Question | Yes (n∘-%) |
|---|---|
| Do you use remote assignment of blood components in your centre? | 52–29% |
|
| |
| Do you use specific or remote control software? | |
| - Specific | 39–71% |
| - Remote control | 12–23% |
| - No answer | 1–2% |
|
| |
| Do you use this modality: | |
| - Always | 8–15% |
| - Only for urgent requests during out-of-routine hours | 12–23% |
| - For urgent requests during out-of-routine hours and when considered opportune | 30–58% |
| - No answer | 2–4% |
|
| |
| Which professional figure is responsible for remote blood assignment? | |
| - A Blood Bank physician | 34–65% |
| - A Blood Bank graduate, who is not necessarily a physician (e.g. a biologist) | 17–33% |
| - A physician or graduate from wards other than the Blood Bank | 1–2% |
|
| |
| During remote blood assignment, is the person responsible: | |
| - In the hospital, away from the site of the transfusion request | 26–50% |
| - At home | 12–23% |
| - In a different hospital or at home, depending on the hours | 13–25% |
| - No answer | 1–2% |
|
| |
| During remote blood assignment, is the person responsible able to: | |
| - Evaluate the request’s appropriateness | 47–90% |
| - Evaluate the patient’s transfusion history | 46–88% |
| - During remote blood assignment, is the person responsible able to see the images of tests? | 48–92% |
|
| |
| During remote blood assignment, who provides the manual skills for pre-transfusion tests: | |
| - A Blood Bank technician | 42–81% |
| - A technician who is not from the Blood Bank | 1–2% |
| - Either a Blood Bank or a non-Blood Bank technician | 9–17% |
|
| |
| During remote blood assignment out of routine hours, is the technician: | |
| - On-call, inside the hospital | 17–33% |
| - On-call, at home | 34–65% |
| - No answer | 1–2% |
|
| |
| Do you have written procedures to be followed in the case the system crashes? | 48–92% |
|
| |
| Does the system have digital signature software? | 23–44% |
|
| |
| Do you consider that the system complies with legislation on the protection of privacy? | 48–92% |
Table V.
Remote electronic blood refrigerators.
| Question | Yes (n∘-%) |
|---|---|
| Do you use electronic refrigerators in your centre? | 11–6% |
|
| |
| Where are the electronic refrigerators located? | |
| - In public hospitals | 9–82% |
| - In private hospitals | 1–9% |
| - In either public or private hospitals | 1–9% |
|
| |
| Are the electronic refrigerators interfaced with the Blood Bank information system? | 9–82% |
|
| |
| To authorise blood unit delivery, the Blood Bank personnel is called: | |
| - In every case | 1–9% |
| - In every case except emergencies | 2–18% |
| - Only if there is not already a valid type & screen for the patient | 4–36% |
| - Only if assigned units to the patient are not already available | 4–36% |
|
| |
| Do you have written procedures to be followed in the case the system fails? | 9–82% |
Table VI.
Electronic order-entry.
| Question | Yes (n∘-%) |
|---|---|
| Do you use an electronic order-entry in your centre? | 31–17% |
| Are the electronic refrigerators interfaced with the Blood Bank information system? | 30–97% |
| Does the system include an electronic flow-chart to guide transfusion appropriateness? | 26–84% |
| Does the system include a block of inappropriate requests? | 18–58% |
Table VII.
Systems for bed-side identification of patients.
| Question | Yes (n∘-%) |
|---|---|
| Do you use systems for bed-side identification of patients in your centre? | 30–17% |
|
| |
| Which technology is used? | |
| - Bar-code | 24–80% |
| - Radio-frequency | 6–20% |
| - Biometric | 6–20% |
|
| |
| Is the system interfaced with the Blood Bank information system? | 21–70% |
|
| |
| Are there written procedures to be followed if the system fails? | 27–90% |
Footnotes
The Authors declare no conflicts of interest.
References
- 1.WHO. A health telematics policy in support of WHO’s Health-For-All strategy for global health development: report of the WHO group consultation on health telematics; 11–16 December; Geneva. Geneva: World Health Organization; 1997. 1998. [Google Scholar]
- 2.Sapirstein A, Lone N, Latif A, et al. Tele ICU: paradox or panacea? Best Pract Res Clin Anaesthesiol. 2009;23:115–26. doi: 10.1016/j.bpa.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Ekeland AG, Bowes A, Flottorp S. Effectiveness of telemedicine: a systematic review of reviews. Int J Med Inform. 2010;79:736–71. doi: 10.1016/j.ijmedinf.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet. 2011;378:731–9. doi: 10.1016/S0140-6736(11)61229-4. [DOI] [PubMed] [Google Scholar]
- 5.McLean S, Nurmatov U, Liu JL, et al. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;7:CD007718. doi: 10.1002/14651858.CD007718.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magann EF, McKelvey SS, Hitt WC, et al. The use of telemedicine in obstetrics: a review of the literature. Obstet Gynecol Surv. 2011;66:170–8. doi: 10.1097/OGX.0b013e3182219902. [DOI] [PubMed] [Google Scholar]
- 7.Young LB, Chan PS, Lu X, et al. Impact of telemedicine intensive care unit coverage on patient outcomes: a systematic review and meta-analysis. Arch Intern Med. 2011;171:498–506. doi: 10.1001/archinternmed.2011.61. [DOI] [PubMed] [Google Scholar]
- 8.Warshaw EM, Hillman YJ, Greer NL, et al. Teledermatology for diagnosis and management of skin conditions: a systematic review. J Am Acad Dermatol. 2011;64:759–72. doi: 10.1016/j.jaad.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Barneveld Binkhuysen FH, Ranschaert ER. Teleradiology: evolution and concepts. Eur J Radiol. 2011;78:205–9. doi: 10.1016/j.ejrad.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Challacombe B, Wheatstone S. Telementoring and telerobotics in urological surgery. Curr Urol Rep. 2010;11:22–8. doi: 10.1007/s11934-009-0086-8. [DOI] [PubMed] [Google Scholar]
- 11.García-Lizana F, Muñoz-Mayorga I. Telemedicine for depression: a systematic review. Perspect Psychiatr Care. 2010;46:119–26. doi: 10.1111/j.1744-6163.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- 12.Meza M, Breskvar M, Kosir A, et al. Telemedicine in the blood transfusion laboratory: remote interpretation of pre-transfusion tests. J Telemed Telecare. 2007;13:357–62. doi: 10.1258/135763307782215370. [DOI] [PubMed] [Google Scholar]
- 13.Wong KF, Kwan AM. Virtual Blood Banking: a 7-year experience. Am J Clin Pathol. 2005;124:124–8. doi: 10.1309/13CUJ61YRB50B1CT. [DOI] [PubMed] [Google Scholar]
- 14.Wong KF. Virtual blood bank. J Pathol Inform. 2011;2:6. doi: 10.4103/2153-3539.76155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagliaro P, Turdo R. Transfusion management using a remote-controlled, automated blood storage. Blood Transfus. 2008;6:101–6. doi: 10.2450/2008.0029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staves J, Davies A, Kay J, et al. Electronic remote blood issue: a combination of remote blood issue with a system for end-to-end electronic control of transfusion to provide a “total solution” for a safe and timely hospital blood transfusion service. Transfusion. 2008;48:415–24. doi: 10.1111/j.1537-2995.2007.01545.x. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros de Bustos E, Moulin T, Audebert HJ. Barriers, legal issues, limitations and ongoing questions in telemedicine applied to stroke. Cerebrovasc Dis. 2009;27(Suppl 4):36–9. doi: 10.1159/000213057. [DOI] [PubMed] [Google Scholar]
- 18.Kluge EH. Ethical and legal challenges for health telematics in a global world: telehealth and the technological imperative. Int J Med Inform. 2011;80:e1–5. doi: 10.1016/j.ijmedinf.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Eurostat. Nomenclature of territorial units for statistics (NUTS) Available at: http://epp.eurostat.ec.europa.eu/portal/page/portal/nuts_nomenclature/introduction. Downloaded on 13/12/2011.
- 20.Codice dell’Amministrazione Digitale (Digital Administration Code). Official Gazette of the Italian Republic n. 112, 16 May 2005.