Abstract
Cocaine-induced stroke is among the most serious medical complications associated with its abuse. However the extent to which acute cocaine may induce silent microischemia predisposing the cerebral tissue to neurotoxicity has not been investigated; in part, because of limitations of current neuroimaging tools, i.e., lack of high spatiotemporal resolution and sensitivity to simultaneously measure cerebral blood flow (CBF) in vessels of different calibers (including capillaries) quantitatively and over a large field of view. Here we combine ultrahigh-resolution optical coherence tomography to enable tracker-free 3D microvascular angiography (μOCA) and a new phase-intensity-mapping algorithm to enhance the sensitivity of 3D optical Doppler tomography (μODT) for simultaneous capillary CBF quantization. We apply the technique to study the responses of cerebral microvascular networks to single and repeated cocaine administration in the mouse somatosensory cortex. We show that within 2–3 minutes after cocaine administration CBF markedly decreased (e.g., ~70%) but the magnitude and recovery differed for the various types of vessels; arterioles had the fastest recovery (~5min), capillaries varied drastically (from 4–20min) and venules showed relatively slower recovery (~12min). More importantly, we showed that cocaine interrupted CBF in some arteriolar branches for over 45min and this effect was exacerbated with repeated cocaine administration. These results provide evidence that cocaine doses within the range administered by drug abusers induces cerebral microischemia and that these effects are exacerbated with repeated use. Thus cocaine-induced microischemia is likely to be a contributor to its neurotoxic effects.
Keywords: 3D optical angiography, 3D optical Doppler tomography, cocaine toxicity, microischemia
INTRODUCTION
Vasoactive effects of cocaine result in marked disruption in cerebral blood flow (CBF) in cocaine abusers1 and are also likely to contribute to the reported occurrences of hemorrhagic and ischemic strokes in cocaine abusers.2–7 However, effective treatment remains elusive in part due to lack of knowledge regarding the nature and the mechanisms that underlie the cerebrovascular changes resulting from cocaine abuse. Studies on the vasoactive effects of cocaine (and other drugs of abuse) in animal models have been hindered by the technical limitations of current neuroimaging techniques. Conventional techniques (e.g., MRI, CT angiography) fail to provide sufficient spatiotemporal resolutions to measure rapid CBF changes in small vessel compartments8; while multiphoton microscopy (MPM)9–11 can detect capillary CBF, its small field of view (FOV) restricts its use for assessing cocaine’s cerebrovascular network effects, and it may not be suitable for repeated imaging of disease progression12 or the dynamics to cocaine responses (e.g., due to complications associated with exogenous fluorescence dye loading and clearance). Recent advances in optical coherence angiography (OCA)12–15 have markedly improved in vivo visualization of the microvascular networks, including 3D microscopy of tumor microenvironments. Yet, methods to enable quantitative capillary CBF imaging remain a technical challenge in Doppler OCT (ODT).
We developed a novel optical imaging technique that allowed us to image 3D capillary cerebrovascular networks quantitatively and at ultrahigh spatial resolution. Specifically, we combined ultrahigh-resolution optical coherence angiography (μOCA)16, 17 to enable visualization of capillary cerebrovascular networks, and a new phase-intensity-mapping (PIM) algorithm to optimize the detection sensitivity of ultrahigh-resolution Doppler flow imaging (μODT). Additionally, this technique allowed separation of arterial and venous branches and thus characterization of their differences in response to stimuli (e.g., cocaine). After validating the technique by imaging the microcirculatory responses to laser disruptions in the mouse brain, we apply it to study the cerebral microvascular network changes induced by acute and repeated cocaine administration using clinically relevant doses. Our findings show for the first time, that cocaine-induced neurovascular ‘like’ microischemia, and that these effects were exacerbated with repeated administration (1 vs. 3 doses). Inasmuch as cocaine abusers repeatedly administer cocaine in binges, this indicates that the vasoactive effects of cocaine will jeopardize oxygen delivery to cerebral tissue making it vulnerable to ischemia and neuronal death.
MATERIALS AND METHODS
Mice
CD1 mice (Charles River, 35~40g/each, female) were used to conduct the CBF imaging studies. All of the mouse experiments were approved by the Institutional Animal Care and Use Committee of Stony Brook University.
Surgery
Mice were anesthetized with inhalational 2% isoflurane (in 100% O2) and mounted on a custom stereotaxic frame to minimize motion artifacts. A ~ϕ5mm cranial window was created above the right somatosensory motor cortex. The exposed cortical surface was immediately covered with 2% agarose gel and affixed with a 100μm-thick glass coverslip using biocompatible cyanocrylic glue. The physiological state of the mice, including electrocardiography (ECG), mean arterial blood pressure (MABP), respiration rate and body temperature, was continuously monitored (SA Instruments, NY).
Optical instrumentation
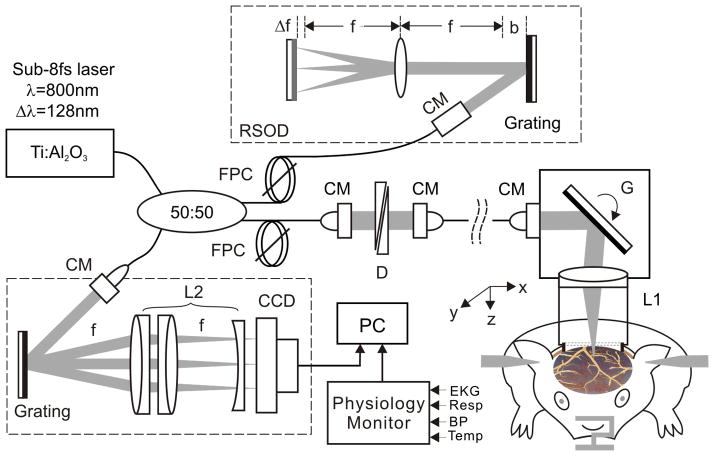
As illustrated in Fig. 1, we used a custom ultrahigh-resolution optical coherence tomography (μOCT) system17 which acquired 3D cross-sectional images of cortical brain structures characterized by their backscattering properties at near real time and over a large FOV (e.g., 2×2×1mm3) through the cranial window, and applied post-image processing14, 15, 18 to render μOCA and quantitative μODT images of the cerebral microvascular networks in vivo. The axial resolution of μOCA/μODT is 1.8μm, as determined by the coherence length (Lc =2(ln2)1/2/π·λ2/Δλcp) of a 8fs Ti: Sapphire laser system used (λ=800nm; Δλcp≈154nm, cross-spectrum); its transverse resolution is 3μm, as determined by the focal spot size of the microscopic objective employed (f16mm/NA0.25). The technical details of μOCA/μODT are provided in Supplementary Information.
Fig. 1.
A schematic of μOCT for simultaneous 3D μOCA/μODT imaging of mouse cortical cerebrovascular networks in vivo. CM: fiberoptic collimator, FPC: fiber polarization controller; D: paired wedge prisms; RSOD: grating-lens-based rapid optical delay (f: focal length, Δf: spectral modification to maximize Δλcp, b: dispersion compensation along with D); G: servo mirror; Obj: achromatic lens (f16mm/NA0.25); L1, L2: achromatic lens group for field correction.
Laser disruption
A pigtailed green laser (60mW, 532nm) was coupled into the sample arm of the μOCT system to disrupt an individual vessel on focus (spot size: ~ϕ3μm) whose coordinates (x, y, z) were accurately determined by prior 3D μOCA image. Laser exposure (2min) was used to disrupt a capillary vessel and repeated exposures were used to disrupt a branch vessel.
Intravenous cocaine induction
A mouse tail vein was cannulated with a 30-gauge hypodermic needle connected to PE10 tubing, through which a bolus of cocaine (2.5mg/kg, body weight) was administered (<15sec).
Statistical Analysis
Data are presented as mean ± s.e.m. P-values to determine significant difference between groups were analyzed by performing a paired t-test (two tail) or a 1-way ANOVA test (Systat software).
RESULTS
Microangiography and quantitative capillary CBF imaging
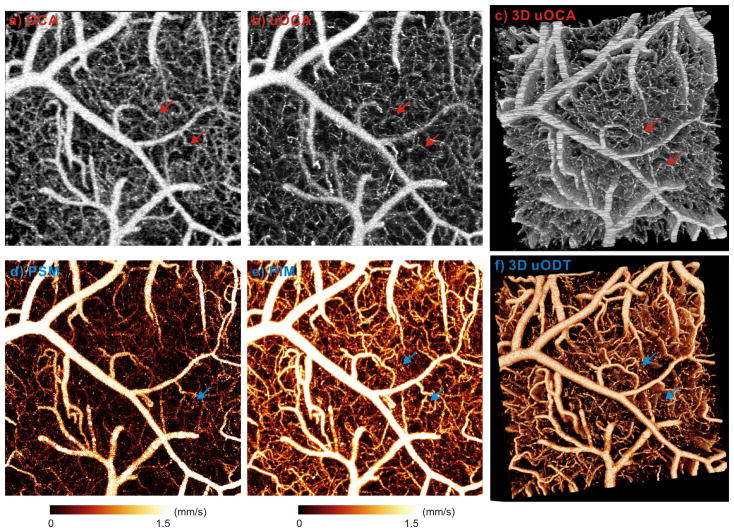
Arterial and capillary vasculatures play a crucial role in maintaining the energy requirements of the functioning brain and can accommodate to increasing tissue demands by modifying the diameters and speeds of flow in the vessel. Although the contrast of both μOCA and μODT originates from the Doppler shifts induced by moving scatterers including red and white blood cells flowing in a blood vessel, they are detected under two distinct regimes (Supplementary Information). μOCA uses dynamic laser speckle contrast to offset moving parts (causing speckle variance) against the surrounding ‘stationary’ brain tissue, whereas μODT uses intrinsic Doppler phase shift to render CBF quantification. The upper panels in Fig. 2 illustrate how the enhanced spatial resolution of μOCA (b) enabled us to accurately resolve the vessel-size diversity of the capillary beds on mouse cortical brain, which would have been otherwise overestimated by conventional OCA (a). For instance, the red arrows show that the two capillaries measured by OCA (ϕ15μm, ϕ13μm) were accurately determined by μOCA (ϕ5.4μm, ϕ3.5μm). Fig. 2c illustrates the advantage of 3D μOCA to image capillary cerebrovascular networks at high resolution and across a large FOV (1×1×1mm3). The lower panels show the efficacy of our new phase detection technique (PIM vs. PSM) for eliminating the phase noise induced by tissue motion, so that capillary CBF with both small vessel sizes (<ϕ5μm) and slow flow speeds (≤10μm/s) could be readily detected and quantified. A comparison between Fig. 2c and Fig. 2f illustrates the capability for quantitative μODT of cerebral microvascular networks with spatial resolution (for measuring capillary vessels) fairly comparable to that of μOCA. More importantly, as both images were acquired simultaneously, it allowed us to study vasculatural (μOCA) and physiological (μODT) changes in response to functional and pharmacological interventions.
Fig. 2.
Cerebral vasculature (upper panel) and CBF (lower panel) of mouse somatosensory cortex imaged by 3D μOCA/μODT. a)–b) Maximum intensity projection (MIP) images of vasculature by OCA (~12μm) and by μOCA (~3μm). Capillaries that appear largely identical (~ϕ15μm) in OCA are fully restored to their real sizes (e.g., ϕ3.5μm) by μOCA. c) 3D μOCA image. d)–e) The corresponding MIP images of quantitative CBF by existing PSM vs. new PIM algorithms. PIM effectively enhanced flow detection sensitivity to uncover capillary CBF embedded in the noise background. f) 3D μODT image. Image size (FOV): 1×1×1mm3. Arrows: 2 capillaries (ϕ5.4μm, ϕ3.5μm) for comparison.
Laser-induced microvascular disruption
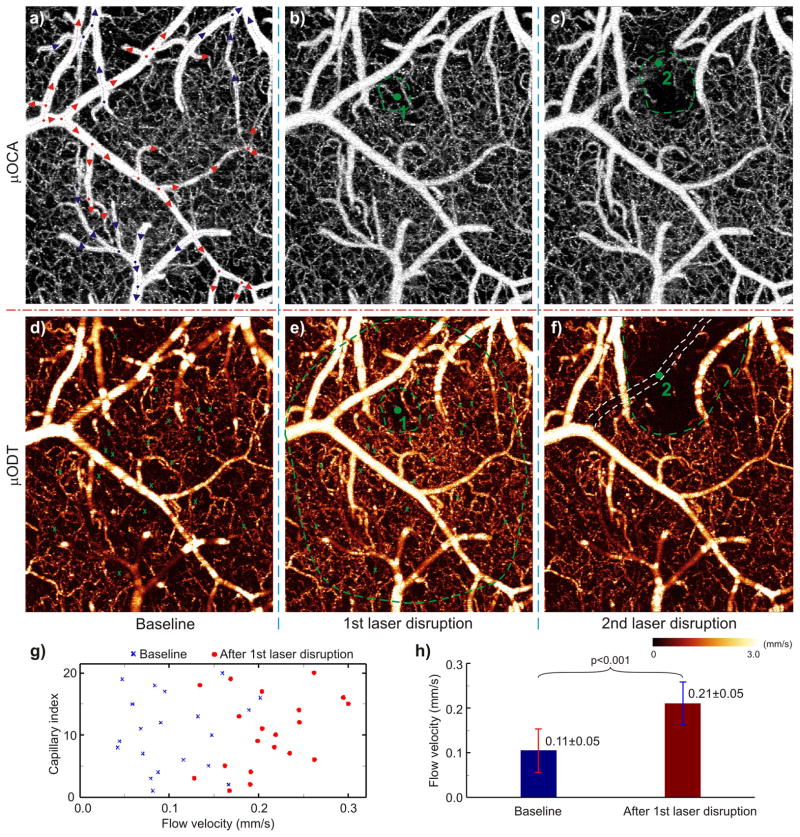
To validate the utility of this technique for assessing microvascular networks, we used laser disruption as a reference intervention since it allowed us to assess the downstream and compensatory responses of cerebral microvascular networks to an insult that is restricted to a single microvessel. Fig. 3 shows the results of laser disruption of a small ϕ35.8±1.2μm arteriole (see Supplementary Fig. S4 for vein and artery separation) and of a ϕ9μm capillary. The laser’s disruption of the capillary (1) elicited only a small localized change in the μOCA image (Fig. 3b); in contrast, the μODT image showed not only the disruption of flow in this capillary but also the CBF decrease in the surrounding microenvironment (~ϕ0.13mm, inner circle) as well as a massive vasodilatation across almost the entire FOV (~ϕ1mm, outer circle). Quantitative analyses in Fig. 3g–h showed a significant CBF increase (p<0.001) within the outer circle from 0.105±0.049mm/s (baseline) to 0.211±0.048mm/s (30min after laser disruption), which corresponds to the average CBF change from 20 capillaries. This is interesting, for it documents that interruption of flow in a single capillary will have a significant effect on CBF of the surrounding cerebral microvascular networks. Similarly, for disruption of the branch arteriole (2), both μOCA and μODT detected laser-induced occlusion of the vessel, but μODT was able to track the quenching effect of local microvascular networks, i.e., the capillaries with an active circulation, over a much large area (~ϕ0.5mm, dashed half circle) than that detected by μOCA (~ϕ0.21mm, dashed circle). The results show the value of quantitative μODT for monitoring not only local but also downstream CBF responses to a circumscribed insult to a small vessel in cerebrovascular networks, which is necessary for understanding the buffering capacity of microvascular networks to cerebrovascular pathology.
Fig. 3.
Laser disruptions of a cerebral capillary (1) and a branch vessel (2) on mouse cortex. Upper panel: MIP images of microvasculature (μOCA) at baseline (a), after laser disruption of a ϕ9μm capillary (b), and of a ϕ35.8μm arteriole (c). Red/blue arrows: flow directions of arterioles/venules, dots: vessel junctures. Lower panel: MIP images of CBF (μODT) at baseline (d) and after laser disruptions (e, f). Angiography detects no difference except reduced vasculature in the immediate areas (dashed green circles in b, c) around laser disruption (green dots); whereas quantitative CBF reveals vastly expanded vasodilatation almost over the entire field after laser disruption of a capillary (dashed outer green circle in e) and the quenching of local CBF networks over a much larger area (dashed green circle in e). Quantitative comparisons among 20 capillaries indicate that CBF increased significantly from 0.105±0.049mm/s at baseline to 0.211±0.048mm/s at 30min after laser disruption (g and h; p<0.001). Image size: 1×1.2×1mm3. Laser radiation: 532nm/60mW, ~ϕ3μm focal spot; 2min and 6min exposures for capillary (1) and arteriole (2), respectively.
Repeated cocaine evokes cerebral microischemia
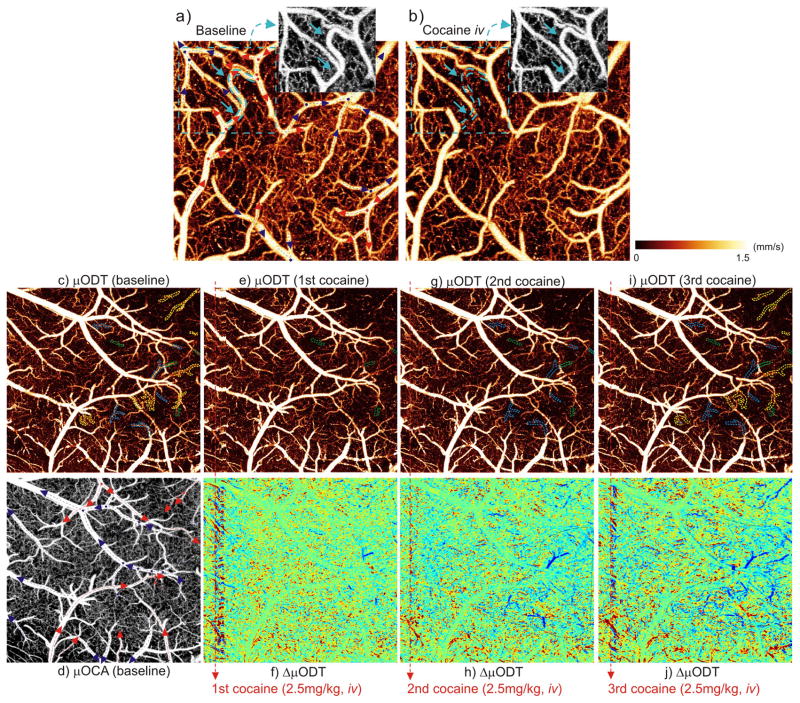
Neuroimaging studies on the hemodynamic effects of cocaine are crucial to elucidating the mechanisms underlying its neurotoxicity including microcirculatory pathology (micro-hemorrhagic stroke) and hemodynamic dysfunction (microischemic stroke). The ultrahigh resolution/sensitivity and large FOV of μOCA/μODT (Figs.2–3) show its relevance for these studies. Fig. 4 shows the results of mouse cortex before and after acute cocaine challenge (2.5mg/kg, i.v.) and identifies the occurrence of what appears to be a cocaine-induced microischemia along with the CBF response patterns of the adjacent cerebrovascular networks. The upper panel (a, b) shows the shunt of an arcade (~ϕ23μm) interconnecting two side branch arterioles. Cocaine abolished the flow in this vessel which appeared as indiscernible by μODT even though it was fully detectable by μOCA (due to Brownian motion of blood in a deactivated vessel, see Supplementary Tab. S1). This suggests that the cocaine-induced ischemic dysfunction probably reflected vasoconstriction of an isolated vessel rather than vessel rupture (Supplementary Fig. S3: vessel rupture resulting in hemorrhage would be evidenced by local blurring due to pronounced blood backscattering) or upstream vasoconstriction. Moreover, the fact that there was no CBF drop in the surrounding microvascular networks suggests that the interruption of flow in this arcade was compensated by the microcirculatory networks, even when the shunt was long lasting (remained for >40min). More importantly, this result suggests quantitative CBF imaging (μODT) is more sensitive for detecting ischemic events than angiography (μOCA).
Fig. 4.
Cocaine-evoked microvascular disruptions on the mouse somatosensory cortex. Upper panel: quantitative CBF (μODT) to show disruption of a ~ϕ23μm arteriole (arcade); low panels: progression of cocaine-evoked deactivations of branch vessels and spreading of vasoconstriction (dark dashed areas) with repeated cocaine challenges. a) baseline, b) 30min after cocaine injection (2.5mg/kg, i.v.); insets: vasculature (μOCA) of the dashed area. Image size: 1×1×1mm3. c–d) μODT/μOCA at baseline, e–j) μODT and ΔμODT after 1–3 cocaine doses (green/blue/yellow dashed circles: occluded vessels after 1–3 cocaine doses; dark circles: vasoconstrictive clouds). Image size: 2×2×1mm3. Red/blue arrows: flow directions of arteriolar/venial vessels; dots: vessel junctures.
In contrast, the lower panels (c–j) show the progression of vasoconstrictions and local ischemia (mostly shunts of terminal vessels) after repeated acute cocaine injections (3 repeated doses of 2.5mg/kg/each, i.v.), although no vasculatural impairment such as vessel rupture was observed. The dashed green, blue and yellow circles outline the deactivated branch vessels elicited by 1–3 cocaine doses, respectively (c–i). A comparison between panels (c, d) shows that terminal arterioles (~77%) were more vulnerable to ischemic shunts than terminal venules (~23%). Noticeable drops of active circulation in the immediate capillary circuits and the spreading of vasoconstrictive clouds (dashed dark circles) with repeated cocaine revealed that the microcirculation was bypassed (local cerebral microvascular network was unable to compensate). Such microischemic events were focal and probably undetectable by current imaging methods, including OCA (e.g., no obvious disruption was detected by μOCA in panel (d) even after the 3 repeated cocaine injections). Note that the differences between the ΔμODT responses of different vessels (f, h, j) are likely to reflect the heterogeneity in neurovascular responses to cocaine, e.g., some areas in the bottom show increased (red) flow. This type of approach will enable to systematically evaluate the effects of acute and repeated cocaine administration on vascular architecture and CBF and to help understand the mechanisms underlying cocaine-induced ischemic and hemorrhagic strokes and provide with a tool to monitor potential therapeutic interventions.
Inhomogenity of spatiotemporal responses of CBF to cocaine
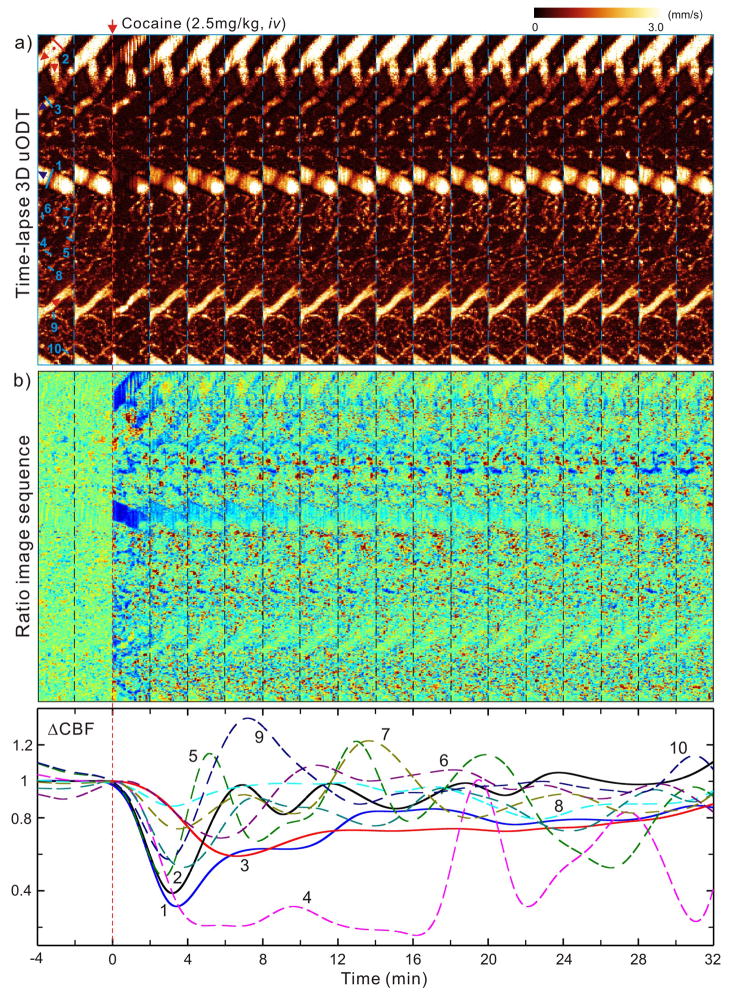
As a prolonged time is needed for quantitative detection of capillary CBF, a full-size 3D μODT image (e.g., Figs.2–4) might require over 8min of scanning, which may not be adequate to observe the fast dynamic responses of the cerebrovascular networks during functional or pharmacological activation such as those that occur after an intravenous cocaine challenge. As a compromise, we reduced the image size in the y-axis (anterior-posterior) so that the spatial and the temporal dynamics of cocaine-evoked CBF responses could be visualized. Fig. 5 illustrates the time-lapse 3D μODT images (1×0.12×1mm3) following a bolus injection of cocaine (2.5mg/kg, i.v.). The upper panels show the quantitative CBF images with extended flow dynamic range (Supplementary Fig. S2). The middle panels plot the time-lapse ratio images defined as ΔCBF(t)≡[CBF(t)−CBF(tb)]/CBF(tb) to illustrate the spatiotemporal evolutions of arterioles, venules, and capillaries. The lower panel shows cocaine-evoked dynamic responses of CBF in vein (1), arteriole (2), venule (3) and capillaries (4–6). The transient ΔCBF(t) of branch vessels (1–3) involved a rapid CBF drop (2~4min) followed by a slow recovery lasting 10–30min, with the arteriole (2) showing more pulsate features. In contrast, the capillary flows exhibited vigorous pulsive changes in response to cocaine challenge and more diverse patterns including transient overshooting.
Fig. 5.
Spatiotemporal responses of CBF to an acute cocaine challenge (2.5mg/kg, i.v.). a) Time-lapse CBF images (1×0.12×1mm3/panel); b) Normalized ratio images (ΔCBF) with time, showing heterogeneous responses to cocaine; c) time-lapse ΔCBF curves of 3 branch vessels (solid curves) and 7 capillary vessels (dashed curves) whose positions are marked by light green lines in a). CBF in larger branch vessels (1: vein, 2: arteriole) show a dramatic transient drop (~60–70%) within 2–3min followed by a slow recovery, similar responses are seen in venule (3) except the dip is smaller (~40%) and lagged to 6–7min. Noteworthily, arteriole (2) recovers faster (at ~5min) and exhibits more pulsive patterns than the 2 venules (e.g., at ~12min for 1). In contrast, capillaries show dramatically different patterns and more vibrant or pulsive changes with cocaine. Red/blue arrows: flow directions of arterioles/venules; dots: vessel junctures.
DISCUSSION
Here we show that cocaine interrupted CBF in some arteriolar branches for over 45min and this effect was exacerbated with repeated cocaine administration. In addition we show that cocaine produced marked decreases in CBF (e.g., ~70%) shortly after acute cocaine administration (2–3min) and that the magnitude and recovery differed between vessels, showing faster recovery in arterioles (~5min) than in venules (~12min) and revealing marked variability and pulsatility in capillaries (recovery varied from 4–20min). These findings provide evidence that acute cocaine elicits cerebral microischemic dysfunction that seems to get exacerbated with repeated cocaine administration. It also uncovers significant heterogeneity in the cerebrovascular responses to cocaine, highlighting the importance of separately assessing vessels of different calibers. Our findings were possible due to the enhanced capabilities of μOCA/μODT, which demonstrates its value as a novel and more sensitive tool for investigating neurovascular toxicity by drugs or other insults.
Our cocaine findings are relevant since stroke is one of the most serious clinical complications associated with cocaine abuse. Indeed cocaine is a main risk factor for stroke among young abusers.19 Though it was hypothesized that cocaine-induced cerebral microischemia was involved in some of the clinical complications seen in cocaine abusers, there was no data to support this. Here we show evidence of long-lasting CBF interruptions in cerebral microvessels (>45min) that are exacerbated with repeated cocaine administrations. Specifically, 3 sequential cocaine doses induced greater changes than those induced after a single dose, which is clinically relevant since cocaine when abused is repeatedly administered in binges and rarely used as a single administration.20 Thus a sensitized response of cerebral microvessels to repeated cocaine administration could contribute to cocaine’s neurotoxic effects. More specifically, the long-lasting interruption in flow observed in some of the vessels, if it is exacerbated with repeated cocaine use could result in microischemic dysfunction and if prolonged could lead to neuronal death and loss of function. We had previously used Doppler OCT to show decreases in CBF after acute cocaine,18, 21, 22 but the limited resolution and sensitivity did not allow us to measure the effects of cocaine on capillary beds. In the current study the enhanced capabilities of μOCA/μODT allowed us to document cocaine-induced microischemic events in capillaries and to show marked differences in the responses to cocaine between arterioles, venules and capillaries in the cerebrovascular networks (Figs.4–5). Of these the capillaries showed the greatest variability and pulsatility upon intravenous cocaine administration, and the terminal arterioles (~77%) seemed more vulnerable to cocaine-elicited microischemia than terminal venules (~23%).
The mechanisms underlying cocaine vasoactive effects are likely to reflect in part its dopaminergic effects. Indeed there is evidence of dopamine terminals in close contact with arterioles and capillaries in cortical tissue that when stimulated results in vasoconstriction.23 Studies on isolated cerebral arterioles have shown that application of cocaine or its metabolites induced vasoconstriction corroborating a direct effect of cocaine on blood vessels as opposed to indirect effects secondary to neuronal actions24. Moreover, vasoconstriction from cocaine was prevented by haloperidol, which suggests the involvement of dopamine (D2) receptors in cocaine induced vasoconstriction24. There is also evidence of dopamine transporter expression (target of cocaine’s effects) in cerebral blood vessels in the brain25. However, it is also possible that the local anesthetic effects of cocaine may contribute to its vasoactive actions26.
Our findings also demonstrate the enhanced capabilities of our μOCA/μODT tool for simultaneously rendering angiographic (μOCA) and quantitative CBF (μODT) images of 3D cerebrovascular networks with capillary details comparable to those by MPM. Specifically, we incorporate ultrahigh-resolution OCT for improving spatial resolution (~3μm) and PIM (based on FFT analysis in lateral direction14, 15, 18) for optimizing phase detection sensitivity (≤10μm/s, Supplemental Tab. S1), and show that the new μOCA/μODT platform offers several unique capabilities that are highly relevant to brain functional studies, yet lacking in current imaging modalities (e.g., MPM, OCA). This technique, based on intrinsic Doppler effect (i.e., tracker free), enables time-lapse imaging of the dynamic responses to brain functional activation (Fig. 5) and disease progression12. It extends the image depth of MPM (~300μm) to 700μm~1mm and the vastly increased FOV (e.g., 2×2×1mm3) is crucial for mapping cerebral microvascular network effects. Noteworthily, μODT is uniquely capable of CBF quantization in both capillaries and branch vessels (Figs.3–5), which provides more sensitive physiological changes (e.g., microischemia) in the local microvascular networks than μOCA (Figs.3–4). Additionally, it allowed us to separate venous and arterial vasculatures and thus to study their respective physiological responses to various functional and pharmacological interventions (Figs.3–5).
A limitation in our study was that the mice had to be anesthetized (as is the case for most rodent imaging studies), which raises concerns of potential interactions between cocaine and the anesthetic agent. However, we specifically chose isoflurane since in a prior study addressing the influence of anesthetic drugs on cocaine’s effects we showed that the findings from the isoflurane-anesthetized rodents agreed with those reported in human subjects27 and more recently with those reported in awake macaques.28 Moreover, isoflurane did not uncouple cocaine’s effects on CBF from those in oxygen metabolism, which suggests that at the doses used to anesthetize the mice, isoflurane did not disrupt the autoregulation of CBF. Also to control for potential confounds secondary to cocaine-induced peripheral vascular effects28 we monitored the mean arterial blood pressure (MABP) throughout the experiments. Although MABP decreased in response to cocaine (Supplementary Fig. S10), this effect was modest (MABP>70mmHg) and short lasting (<5min), suggesting that neither the immediate (Fig. 5) nor the long-lasting (Fig. 4f, h, i) decreases in CBF following cocaine administration were due to cocaine’s peripheral effects. In addition, the measured apparent CBF comprises artifacts (e.g., underestimation) due to Doppler angle effect, especially when the angle θ→90°. The error can be accurately corrected; however, angle correction of the entire CBF network is challenging because of high correction errors for flows with θ→90° and limited sensitivity for capillary beds (see Supplementary Fig. S8 and Tab. S2 for details). It should also be noted that due to limited temporal resolution of 3D μODT (e.g., 1min), the imaged CBF change in response to cocaine (e.g., Fig. 5) was confounded with the inherent CBF fluctuation over time (e.g., basal ΔCBF(t: t<0) variations in Fig. 5). Although this change in larger vessel (e.g., >ϕ50μm) was negligible (~8%) compared to the changes induced by cocaine (e.g., 50%), the influence was more obvious in smaller vessels and capillaries (see Supplementary Fig. S7).
In summary, we provide evidence that cocaine induced cerebral microischemic changes that in some vessels were long lasting (>45min) and were exacerbated with repeated administration. This could underlie some of the neurologic deficits reported in cocaine abusers ranging from mild and transient facial paralysis, to severe and irreversible tetraplegia.29 We also show evidence of the enhanced capabilities of μOCA/μODT for studying the dynamic responses of cerebral microvessels to drugs and other insults.
Supplementary Material
Acknowledgments
The work was supported in part by National Institutes of Health grants K25-DA021200 (CD), 2R01-DK059265 (YP), 1RC1DA028534 (CD and YP), 1R21-DA032228 (CD and YP), and NIAAA Intramural Research Program (NDV).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at Molecular Psychiatry’s website
References
- 1.Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. The British Journal of Psychiatry. 1988;152(5):641. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- 2.Howington JU, Kutz SC, Wilding GE, Awasthi D. Cocaine use as a predictor of outcome in aneurysmal subarachnoid hemorrhage. Journal of neurosurgery. 2003;99 (2):271–275. doi: 10.3171/jns.2003.99.2.0271. [DOI] [PubMed] [Google Scholar]
- 3.Pozzi M, Roccatagliata D, Sterzi R. Drug abuse and intracranial hemorrhage. Neurological Sciences. 2008;29:269–270. doi: 10.1007/s10072-008-0960-z. [DOI] [PubMed] [Google Scholar]
- 4.Bartzokis G, Beckson M, Hance DB, Lu PH, Foster JA, Mintz J, et al. Magnetic resonance imaging evidence of “silent” cerebrovascular toxicity in cocaine dependence. Biological psychiatry. 1999;45(9):1203–1211. doi: 10.1016/s0006-3223(98)00228-5. [DOI] [PubMed] [Google Scholar]
- 5.Toossi S, Hess CP, Hills NK, Josephson SA. Neurovascular Complications of Cocaine Use at a Tertiary Stroke Center. Journal of Stroke and Cerebrovascular Diseases. 2010;19(4):273–278. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Nanda A, Vannemreddy P, Willis B, Kelley R. Stroke in the young: relationship of active cocaine use with stroke mechanism and outcome. Brain Edema XIII. 2006:91–96. doi: 10.1007/3-211-30714-1_22. [DOI] [PubMed] [Google Scholar]
- 7.Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Archives of general psychiatry. 2007;64(4):495–502. doi: 10.1001/archpsyc.64.4.495. [DOI] [PubMed] [Google Scholar]
- 8.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drew PJ, Shih AY, Driscoll JD, Knutsen PM, Blinder P, Davalos D, et al. Chronic optical access through a polished and reinforced thinned skull. Nature Methods. 2010;7(12):981–984. doi: 10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, et al. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nature medicine. 2001;7(7):864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- 11.Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):13081. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nature medicine. 2009;15(10):1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan VJ, Jiang JY, Yaseen MA, Radhakrishnan H, Wu W, Barry S, et al. Rapid volumetric angiography of cortical microvasculature with optical coherence tomography. Optics letters. 2010;35(1):43–45. doi: 10.1364/OL.35.000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Optics Express. 2007;15(7):4083–4097. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
- 15.An L, Qin J, Wang RK. Ultrahigh sensitive optical microangiography for in vivo imaging of microcirculations within human skin tissue beds. Optics Express. 2010;18 (8):8220–8228. doi: 10.1364/OE.18.008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Z, Chen B, Ren H, Pan Y. On the possibility of time-lapse ultrahigh-resolution optical coherence tomography for bladder cancer grading. Journal of Biomedical Optics. 2009;14:050502. doi: 10.1117/1.3223246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nature biotechnology. 2003;21(11):1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Z, Luo Z, Volkow N, Pan Y, Du C. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta SV, Gluncic V, Iqbal SM, Frank J, Ansari SA. Role of Perfusion Imaging in Differentiating Multifocal Vasospasm-related Ischemia versus Thromboembolic Stroke in a Setting of Cocaine Abuse. Journal of Stroke and Cerebrovascular Diseases. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Hatsukami DK, Thompson TN, Pentel PR, Flygare BK, Carroll ME. Self-administration of smoked cocaine. Experimental and Clinical Psychopharmacology. 1994;2(2):115. [Google Scholar]
- 21.Luo Z, Wang Z, Yuan Z, Du C, Pan Y. Optical coherence Doppler tomography quantifies laser speckle contrast imaging for blood flow imaging in the rat cerebral cortex. Opt Lett. 2008;33(10):1156–1158. doi: 10.1364/ol.33.001156. [DOI] [PubMed] [Google Scholar]
- 22.Luo Z, Yuan Z, Tully M, Pan Y, Du C. Quantification of cocaine-induced cortical blood flow changes using laser speckle contrast imaging and Doppler optical coherence tomography. Applied optics. 2009;48(10):D247–D255. doi: 10.1364/ao.48.00d247. [DOI] [PubMed] [Google Scholar]
- 23.Krimer LS, Muly EC, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nature Neuroscience. 1998;1(4):286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- 24.He GQ, Zhang A, Altura BT, Altura BM. Cocaine-induced cerebrovasospasm and its possible mechanism of action. Journal of Pharmacology and Experimental Therapeutics. 1994;268(3):1532. [PubMed] [Google Scholar]
- 25.Ohtsuki S, Yamaguchi H, Kang YS, Hori S, Terasaki T. Reduction of L-Type Amino Acid Transporter 1 mRNA Expression in Brain Capillaries in a Mouse Model of Parkinson’s Disease. Biological & pharmaceutical bulletin. 2010;33(7):1250–1252. doi: 10.1248/bpb.33.1250. [DOI] [PubMed] [Google Scholar]
- 26.Albuquerque MLC, Dean Kurth C. Cocaine constricts immature cerebral arterioles by a local anesthetic mechanism. European journal of pharmacology. 1993;249(2):215–220. doi: 10.1016/0014-2999(93)90435-k. [DOI] [PubMed] [Google Scholar]
- 27.Du C, Tully M, Volkow ND, Schiffer WK, Yu M, Luo Z, et al. Differential effects of anesthetics on cocaine’s pharmacokinetic and pharmacodynamic effects in brain. European Journal of Neuroscience. 2009;30(8):1565–1575. doi: 10.1111/j.1460-9568.2009.06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandeville JB, Choi JK, Jarraya B, Rosen BR, Jenkins BG, Vanduffel W. fMRI of Cocaine Self-Administration in Macaques Reveals Functional Inhibition of Basal Ganglia. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollander JE. Cocaine intoxication and hypertension. Annals of emergency medicine. 2008;51(3):18. doi: 10.1016/j.annemergmed.2007.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.