Abstract
Controlled proliferation of cardiac myocytes remains a major limitation in cell biology and one of the main underlying hurdles for true modern regenerative medicine. Here we provide a technique to robustly expand early fetal-derived mouse ventricular cardiomyocytes on a platform usable for high-throughput molecular screening, tissue engineering or potentially useful for in vivo translational experiments. This method provides a small molecule-based approach to control proliferation or differentiation of early beating cardiac myocytes through modulation of the Wnt/β-catenin signaling pathway. Moreover isolation and expansion of fetal cardiomyocytes takes less than 3 weeks, yields a relatively pure (~70%) functional myogenic population and is highly reproducible.
Keywords: Cardiomyocyte proliferation, differentiation, isolation, expansion, GSK-3 inhibitor, Wnt/β-catenin signaling
INTRODUCTION
During cardiogenesis, cells from the endocardium, myocardium and epicardium give rise to the appearance of the ventricular wall (Moorman & Christoffels, 2003). The myogenic commitment of multipotent cardiovascular progenitors sources the myocardial compartment herein. And while the right ventricular myocardium originates from an Isl1/Nkx2.5 positive cell population, up to date, it remains unknown what transcription factor marks the origin of left ventricular myocardium (Buckingham et al, 2005; Cai et al, 2003; Laugwitz et al, 2005). Early Isl1+ and Nkx2.5+ right ventricular marked cardiomyocytes isolated from a double transgenic renewable cell source can be used for functional tissue engineering and show limited intrinsic capacity to proliferate in vitro (Domian et al, 2009). Previous work showed that Wnt/β-catenin plays a pivotal role in self-renewal and myogenic differentiation of early embryonic multipotent progenitors (Kwon et al, 2007; Lin et al, 2007; Qyang et al, 2007). In addition, recently, we demonstrated that Wnt/β-catenin signaling pathway also controls spatiotemporal proliferation and differentiation of early ventricular myocytes derived from pluripotent cell sources as well as mouse fetal ventricular myocytes. Furthermore, constitutively activated β-catenin in fetal ventricular myocardium promotes proliferation of cardiac myocytes in the left and right ventricle up to the early neonatal stage, while abrogation of β-catenin signaling attenuates proliferation of early ventricular myocytes.
Therefore, we explored the effect of a defined set of small molecules, known to modulate the Wnt/β-catenin signaling pathway, on proliferation and differentiation of early fetal-isolated ventricular myocytes. We found that a group of small molecules (Table 1), directly inhibiting cytoplasmic glycogen synthase kinase 3 (GSK-3) and thereby activating Wnt/β-catenin signaling, robustly enhanced the ex vivo proliferation capacity of early cardiomyocytes. Conversely, treatment with molecules abrogating Wnt/β-catenin signaling resulted in reduced intrinsic proliferation and enhanced differentiation as found with quantitative reverse transcription polymerase chain reaction (qRT-PCR) for structural cardiac genes.
Table 1.
Selected small molecule inhibitors and activators of the Wnt/β-catenin signaling pathway
| Small molecule | Molecular target | Wnt/β-catenin signaling | Effect | Reference |
|---|---|---|---|---|
| BIO* | GSK-3 inhibition | Activation | Proliferation | (Meijer et al, 2003) |
| CHIR99021 | GSK-3 inhibition | Activation | Proliferation | (Ying et al, 2008) |
| 1-Azakenpaullone | GSK-3 inhibition | Activation | Proliferation | (Kunick et al, 2004) |
| IWR-1* | Axin stabilization | Inhibition | Differentiation | (Lu et al, 2009) |
| IWP-3 | Porcupine inhibition | Inhibition | Differentiation | (Chen et al, 2009) |
| PNU7747 | Nuclear β-catenin binding | Inhibition | Differentiation | (Trosset et al, 2006) |
GSK-3, glycogen synthase kinase 3.
Small-molecules used in this protocol.
Herein, we describe a reliable and reproducible method to isolate relatively high numbers of ventricular cardiomyocytes and provide the techniques to expand or differentiation these cells. Although, several strategies for isolation of rat and murine cardiac myocytes have been reported before, yet no easy and effective culture method exists to efficiently expand these. Mouse fetal (E11.5-14.5) cardiomyocytes have a limited capacity to proliferate ex vivo. This intrinsic capacity can be robustly enhanced with treatment of a GSK-3 inhibitor. Moreover, isolation and expansion or differentiation of fetal cardiomyocytes takes less than 3-weeks and yields high numbers of functional myocytes with relatively high purity (~70%).
BASIC PROTOCOL 1. Isolation of fetal ventricular cardiomyocytes
Introductory paragraph
This protocol is used to isolate ventricular cardiac myocytes from murine embryonic hearts (E11.5-14.5) using micro dissection and enzymatic digestion techniques. The steps describe how to dissect fetal tissues and how to process the cardiac tissue to yield dissociated cardiomyocytes. This protocol will yield between 50.000–200.000 ventricular myocytes per embryonic heart depending on the embryonic stage.
Materials
Sterile Phosphate Buffered Saline (PBS) 1x
Collagenase Solution (see Reagents & Solutions)
Trypsin/EDTA (Invitrogen, 5200-056)
Culture Media (see Reagents & Solutions)
15-ml centrifuge tubes (BD, 352097)
Scissor
Forceps
Scalpel
Microscope
Inverted light microscope
70% Ethanol
Laminar flow hood
37°C water bath
37°C/5% CO2 tissue incubator
Protocol steps
Euthanize mouse (preferable through cervical dislocation) and open up the abdominal skin and peritoneum with scissor and forceps.
Dissect out the uterus by cutting the following structures; Fallopian tube on one side, the cervix and the Fallopian tube on the other side. Transfer the uterus containing the embryos to a 10cm cell culture dish with ice-cold PBS 1x (Figure 1A).
Open up the uterus wall with a scissor and collect the embryos by opening up the yolk sacs. Cut-off the head with one incision in the neck and the lower structures with one incision above the liver. The heart now becomes visible and can be dissected-out. Remove the pericardial sac, atrial and vascular tissue (Figure 1B).
Incise ventricles multiple (3–4) times with a scalpel and put in 15mL collection tube(s) while in PBS 1x on ice (Figure 1C)
Wash ventricles with PBS 1x, spin down at 850 RPM for 3 minutes and discard wash buffer. Repeat this step up to 3 times.
Add 2–4mL of Collagenase Digestion Solution to 15mL tube and incubate ventricular tissue for 1–1.5 hours at 37°C water bath.
Gently pipet ventricular tissue up and down every 15 minutes to enhance enzymatic digestion.
After 1–1.5 hours, add 1 volume of 0.25% trypsin and incubate for 3 minutes at 37°C.
Gently pipet up and down to create a single cells suspension and neutralize the trypsin with 1 volume of FBS.
Fill up 15mL tube(s) with PBS1x and spin cells down for 5 minutes at 850 RPM.
Discard supernatant and re-suspend cells in 1mL of Culture Media (see Reagents & Solutions).
Pipet up 10μL and count cells using standard counting method.
Figure 1. Isolation and plating of ventricular myocytes.
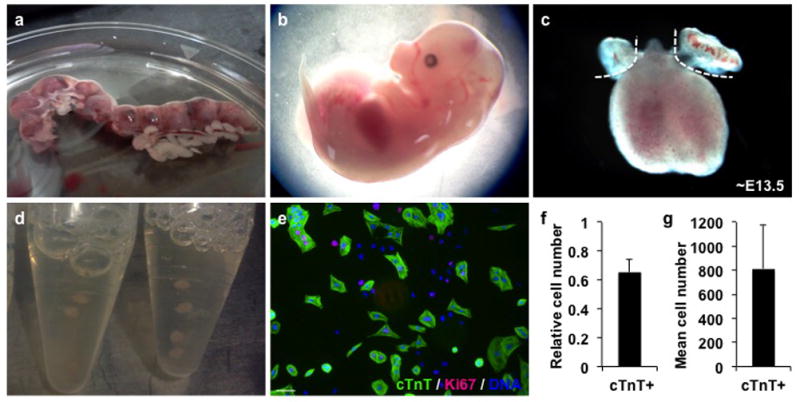
(a) Image of dissected mouse uterus containing multiple embryos. (b) mouse embryo at ~E12.5. Dashed lines indicate where incisions should be made to yield the heart. (c) fetal mouse heart. Dashed lines indicate excision of atrial tissue. (d) pooling of ventricular tissue in 15mL tubes. 2–3 tubes can be used for biological replicates. (e) representative image of ventricular cells stained for cardiac troponin T (cTnT) (green), Ki67 (red) and DAPI (blue). (f) percentage of cTnT+ cells 1 day after isolation (E12.5+1). Scale bar represents 50μm. (g) quantification of cTnT+ cell number per well of a 384-well plate. Error bars indicate standard deviation. (n=3, each in 6 technical replicates).
Step annotations
-
1
Note: Experiments involving live animals must be according to the institutional regulations and require approval of the Institutional Animal Care and Use Committee (IACUC) or equivalent.
-
2
Note: The mouse uterus is located in the peritoneal cavity. The uterus tube runs from the cervix up to the Fallopian tubes on both sides.
-
4
Optional: The left and right ventricle can be collected separate if the study design requires this.
-
6
Note: Alternatively the 15mL can be placed horizontally on rotational platform in a 37°C incubator to enhance enzymatic digestion with continuous mechanical force.
-
9
TROUBLESHOOTING: Before pipetting the tissue up and down in the digestion buffer, wet pipet tips to avoid adhesion of cardiac tissue on the inside wall.
-
10
IMPORTANT: Pipet gently to avoid shear stress.
BASIC PROTOCOL 2 (optional). 2-Dimensional culture of fetal ventricular cardiomyocytes
This protocol describes the procedures for the in vitro culture of fetal-derived ventricular cardiomyocytes. Depending on the purpose of the experiment, cells can be plated (2-dimensional) or cultured in aggregates (3-dimensional) (alternate protocol 2) (Table 2). 2-dimensional cultures allow better quantification options for proliferation or differentiation assays, while 3-dimensional cultures preserve a better physiological cellular context useful for tissue engineering experiments. GSK-3 inhibitor treatment in a 2-dimensional culture conditions results in up to a 20-fold increase of cardiac myocyte within one week (Figure 2a–d).
Table 2.
Overview of 2 and 3-dimensional culture methods.
| Environment (Plate) | Media (volume) | Cells (number) |
|---|---|---|
| 2-Dimensional culture | ||
| 24-well culture plate | 1000μl | 15.000–45.000 |
| 96-well culture plate | 200μl | 2.500–7.500 |
| 384-well culture plate | 75μl | 500–1500 |
| 3-Dimensional culture | ||
| 10 or 15 cm petri-dish | 10–20μl/drop | 250–500 |
Figure 2. 2-dimensional expansion of differentiation of ventricular myocytes.
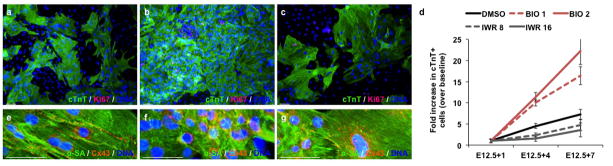
Representative images of ventricular cells cultured in (a) DMSO, (b) BIO or (c) IWR stained for cardiac troponin T (cTnT) (green), Ki67 (red) and DAPI (blue). Scale bar represents 50μm. (d) Quantification of cTnT+ cells at day 1 (baseline) (E12.5+1), 3 (E12.5+4) and 6 (E12.5+7) additional days of culture in the presence or absence of BIO or IWR. Error bars indicate standard deviation. (n=3, each in 6 technical replicates for each time point).
Materials
0.1% Gelatin solution (see Reagents & Solutions)
Collagen solution 1:20 (see Reagents & Solutions)
Culture Media (see Reagents & Solutions)
6-Bromoindirubin-3′-oxime (BIO) (Sigma, B1686) (see Reagents & Solutions)
Inhibitor of Wnt Response-1 (IWR) (Sigma, I0161) (see Reagents & Solutions)
Sterile pipet basin
Multichannel pipet
24, 96 or 384-well cell culture plate(s)
Inverted light microscope
Laminar flow hood
37°C water bath
37°C/5% CO2 tissue incubator
4% Paraformaldehyde (PFA) solution
TRIzol Reagent (Invitrogen, 15596-026)
Protocol steps
Coat plates with 0.1% gelatin solution for 20 minutes at RT.
Re-suspend cells in the desired amount of culture media to end-up with cell densities per volume as listed in table 2. Next, transfer the cell suspension to a sterile pipet basin.
Use a single or multichannel pipet to plate cells on 0.1% gelatin pre-coated plates.
Allow the cells to settle overnight in a tissue incubator at 37°C (12–24 hours)
The next day, dissolve compounds in DMSO to create a 10mM stock concentration.
Prepare 10x of final concentrations in Culture Media (for BIO make 15–25μM and IWR 80–160μM solutions (which is 10x if the appropriate end concentration, i.e 1.5–2.5μM for BIO and 8–16μM for IWR)
Add the small-molecules and DMSO carrier control in 10x concentration of final to the cell culture (i.e. add 8.3μl of 10x compound to 75μl of media in a 384-well, to make ~83μl)
Change the Culture Media every 2–3 days, or when color changes to orange/yellow, and add compounds again in 10x of the final concentration on top.
Fix cells with 4% PFA at desired time-point(s) and perform standard immunocytochemistry. Alternatively lyse unfixed cells in TRIzol Reagent and process for RT-PCR analysis.
Step annotations
NOTE: To mimic a more organic environment plates can also be coated with collagen type I solution for 20 minutes at RT.
ALTERNATE PROTOCOL 2 (optional). 3-Dimensional culture of fetal ventricular cardiomyocytes
3-Dimensional aggregate culture of cardiomyocytes has the advantage that physiological cell-cell communication is maintained or re-established to some extend. Because of the absence of attachment to the culture plate, cardiomyocytes form aggregates which allow interaction and mechanical forces in 3-dimensions. Previous work suggested that neonatal rat ventricular cells have an innate potential to re-form the complex 3-dimensional organization of cardiac tissue in vitro. The electrophysiological properties of cardiomyocytes cultured in 3-dimensional environment were superior to those of the same cells cultured as monolayers (Akins et al, 1999; Bursac et al, 2003). Using alternative protocol 2, ventricular cells can be cultured, expanded or differentiated using a hanging drop aggregate method.
Materials
Culture Media
TRIzol® reagent (Invitrogen, 15596-026)
Chloroform (Fisher Scientific, 67-66-3)
Pipet basin
Multichannel pipet
Inverted light microscope
Laminar flow hood
37°C water bath
37°C/5% CO2 tissue incubator
Protocol steps
Re-suspend cells in a density of 25–50 cells/μl and add final concentrations of small molecules (BIO 1.5–2.5μM and IWR 8.0–16μM)
Use a multichannel pipet to make 10μl drops (250–500 cells/drop) on a petri dish.
Flip the petri dish upside down and place hanging drops in tissue culture incubator at 37°C.
Within 12 hours the isolated cardiac myocytes will cluster and form cardiac microspheres. Within 24–36 hours spontaneously contracting cardiac microspheres can be observed.
After 2–4 days, pool cardiac microspheres into petri dish or low-attachment with new culture media for additional culture or process tissue for optical, immunohistochemistry or standard real-time reverse-transcription PCR analysis (RT-PCR).
IMPORTANT NOTE: For optimal cell adhesion it is recommended not to move the plate within the first 12 hours of cardiac microsphere formation.
Step annotations
SUPPORT PROTOCOL 2 (). Analysis of expanded or differentiated fetal ventricular myocytes
This section summarizes and discusses a selection of analytical readout options available to study proliferation and/or differentiation of fetal-derived ventricular progenitors.
Materials
PBS 1x
Saponin (Sigma, 47036)
4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI) 1:10.000 (invitrogen, D1306)
Primary antibodies
Cardiac Troponin T (cTnT) 1:250 (mouse monoclonal, NeoMarkers, Ms-295)
Ki67 1:300 (rabbit monoclonal, Abcam 16667)
α-Sarcomeric Actinin (α-SA) 1:250 (mouse monoclonal, Sigma, A7811)
Connexin-43 (Cx43) 1:150 (rabbit polyclonal, Sigma, C6219)
Secondary antibodies
Alexa 488nm donkey anti-mouse 1:400 (Invitrogen, A-21202)
Alexa 594nm donkey anti-rabbit 1:400 (Invitrogen, A-21206)
Immunofluorescence microscope
Qiagen RNeasy Mini Kit (Qiagen, 74104)
iScript cDNA Synthesis Kit (BIO-RAD, 170-8891)
Immunofluorescence
Standard immunofluorescence can be used to visualize cell number, proliferation and differentiation or commitment. Commonly used antibodies for a basic analysis of cardiac myocytes (and/or differentiation or commitment) are cTnT and α-SA. Assays to analyze cell number and proliferation include staining for nuclear DNA with DAPI or for proliferation marker Ki67. In this protocol, cTnT staining is used to discriminate between the cardiomyocyte and non-cardiomyocyte populations. Furthermore, scoring of total cTnT+ cell number, as well all Ki67+/cTnT+ cells are useful methods to evaluate proliferation.
Quantitative PCR
RT-PCR analysis for structural cardiac genes provides insights in what effect of small-molecules exert on gene expression. In this protocol it is illustrated for cardiac Troponin T (TnnT2), ventricular specific myosin light chain (Myl2), and cardiac specific α-myosin heavy chain (Myh6) in ventricular myocytes treated with BIO or IWR. Furthermore, activation or inhibition of a molecular pathway can be monitored by RT-PCR analysis for direct target genes.
REAGENTS AND SOLUTIONS
Collagenase solution
Phosphate Buffered Saline (PBS) 1x, Collagenase A (Roche, Cat. No. 11 088 785 103) 1mg/mL, Collagenase B (Roche, Cat. No. 11 088 823 103) 1mg/mL and 20% Fetal Bovine Serum (FBS) (Gemini Bioproducts)
Trypsin
0.25% Trypsin-EDTA 1x (Invitrogen, 25200-056)
0.1% Gelatin solution
Phosphate Buffered Saline (PBS) 1x, 0.1% gelatin (Sigma, G1890)
Collagen 1:20 solution
Phosphate Buffered Saline (PBS) 1x, collagen type I 3.37mg/mL 1:20 (BD, 354236)
Ascorbic Acid
Sterile water, Ascorbic acid 10mg/mL (100x) (Sigma, A4544)
Culture media
Iscove’s Modified Dulbeccos Medium (IMDM) (Thermo Scientific, SH30228.01), 10% Fetal Bovine Serum (FBS) (Gemini Bioproducts, 100–500), Non Essential Amino Acids solution (NEAA) 1x (Invitrogen, 11140-050), Ascorbic acid 1x, Pencillin 50U/mL, Streptomycin 50μg/mL, Mercaptoethanol 1:150.000 (Sigma, M6250)
BIO (GSK-3 inhibitor)
6-Bromoindirubin-3′-oxime (BIO) (Sigma, B1686), prepare 10mM solution in DMSO
IWR (Axin inhibitor)
Inhibitor of Wnt Response-1 (IWR) (Sigma, I0161), prepare 10mM solution in DMSO
Antibodies
Primary antibodies: Cardiac Troponin T (cTnT) 1:250 (mouse monoclonal, NeoMarkers), Ki67 1:300 (rabbit monoclonal, Abcam 16667), α-Sarcomeric Actinin (α-SA) 1:250 (mouse monoclonal, Sigma, A7811), Connexin-43 (Cx43) 1:150 (rabbit polyclonal, Sigma, C6219). Secondary antibodies: Donkey anti-mouse Alexa 488nm 1:400 (Invitrogen, A-21202) and donkey anti-rabbit 594nm 1:400 (Invitrogen, A-21206).
COMMENTARY
Background Information
Annual ventricular myocyte turnover is estimated around 2% in the adult mammalian heart and occurs mostly through refreshment of preexisting myocytes (Senyo et al, 2013). Unlike the mammalian heart, certain fish and amphibians maintain the capacity to repair cardiac damage throughout life (Poss et al, 2002). And while adult mammalian myocardium is almost completely lacking the capacity to regenerate the myocytes lost after injury, it was shown that the early neonatal myocardium has an intrinsic capacity to reconstitute for myocyte loss. This capacity of neonatal cardiomyocytes to proliferate is lost early after birth. The neonatal intrinsic cardiomyocyte response to reconstitute the cell loss, however, is similar to the regenerative capacity of zebrafish hearts (Jopling et al, 2010; Kikuchi et al, 2010).
Pharmacological or cell based therapy, aiming at replacing or augmenting the number of functional myocardial cells represents an attractive therapeutic approach to regenerate the injured mammalian heart. These pharmacological compounds or cells will have to be applied or assembled into the 3-dimensional structure of the myocardial wall. Boosted cardiomyocyte renewal or direct engrafted cardiac cells will then have to be functionally coupled with native myocardium to improve cardiac function. Furthermore, electrophysiological coupling of de novo cardiomyocytes has to occur without resulting in arrhythmias or rejection. For such a pharmacological or cell-based approach to regenerate the adult heart, a more detailed understanding of physiological cardiac myocyte growth and turnover is required.
Up to date, no stable cardiac myocyte cell-line has been described. And although neonatal rat cardiomyocytes have a limited capacity to proliferate ex vivo, neonatal mouse-derived myocytes almost completely lack the intrinsic capacity to further proliferate. Recent work, however, showed that a number of microRNAs efficiently promote the proliferation of murine cardiomyocytes (Eulalio et al, 2012). In this regard, having a small-molecular strategy to direct early cardiomyocytes to expand or further differentiate forms therefore the next step to cardiomyocyte culture. Furthermore, the set up of this protocol allows it to study molecular Wnt signals driving the proliferation and differentiation. In addition, this approach is adaptable into a platform to identify novel small-molecules regulating early cardiomyocyte fate.
Critical Parameters and Troubleshooting
Survival and viability
Low yield is often a result of too much shear stress through vigorously pipetting or too long exposure to enzymatic digestion. Since the cardiac cells in the native myocardium are highly organized and tightly connected to each other by gap junctions and adherens junctions (desmosomes) it requires slow enzymatic dissociation over 1–2 hours. In addition, gentle pipetting enhances the dissociation process and shortens the digestion time. Therefore, the survival and viability of the isolated cells is a balance between the least shear stress and the shortest possible digestion process. To optimize cell dissociation, a 3-minute Trypsin digestion step can be added after 1–2 hours of collagenase treatment. Optionally collagenase digestion can be performed on a rotational shaker.
Adherence
Protein coating of the cell culture plates is necessary to facilitate sufficient attachment of plated cells. As described, we routinely use gelatin and collagen protein-solutions for coating of our culture plates. In addition, fibronectin and laminin are other proteins often used for coating. If adherence of cell is an issue, protein concentrations in the coating solution can be increased up to a 10 fold to promote cell adhesion.
Plating density
For successful expansion of ventricular myocytes it is important to start off with the seeding densities as described in table 2. For RT-PCR analysis it is recommended to use higher densities, while for cell count analysis lower cell numbers per well are time saving.
Small-molecule treatment
It is important to add compounds within 12–24 hours after cell seeding to maintain ventricular myocytes in a proliferative state, while it is not recommended to seed cells together with the final concentration of the compounds, since compounds can have different effects on cell survival, viability and attachment. Furthermore, avoid multiple freezing and thawing cycles of the small molecule stocks and instead prepare small aliquots. 10x diluted compounds are usually last for up to 1 week. Cover tubes with small-molecules in aluminum foil to avoid light exposure.
Contamination
Under aseptic conditions ventricular myocytes cultures can be grown without antibiotics. However, we prefer to perform the animal dissection and isolation of cardiac cells under non-sterile conditions and supplement the Culture Media with low concentrations of antibiotics (see Reagents & Solutions: Culture Media). Potentially, antibiotics can cause bacterial resistance and can interfere with the expansion or differentiation of cardiac cells.
Anticipated Results
This protocol describes in detail the isolation and subsequently expansion or differentiation of fetal ventricular cardiomyocytes. As described in Basic Protocol 1, between 500.000 and 2 million ventricular myocytes can be isolated from one litter, depending on the embryonic stage. Usually, between 60–80% of the fetal ventricular myocytes stain positive for cardiac troponin T (cTnT) at day 1 (E12.5+1) (Figure 1e–g)
As shown in Basic Protocol 2, isolated cells can subsequently be plated and up to a 20-fold expanded in 1 week with GSK-3 inhibitor treatment. Conversely, inhibition of Wnt signaling with IWR, results in the reduced proliferation and ventricular myocyte cell number (Figure 2a–d). Furthermore, expanded and differentiated ventricular myocytes show strong sarcomere expression and gap junction formation as illustrated in figure 2e–g.
Alternate Protocol 2, describes the culture of ventricular myocytes in aggregates. When BIO or IWR are added to the aggregates, it results in increased or decreased diameter of the cardiac tissue aggregates compared to the DMSO control (Figure 3a–d). Furthermore, IWR appears to increase the beating rate while BIO has the opposite effect, when compared to aggregates cultured in DMSO (Video 1–3). Quantitative RT-PCR reveals that BIO decreases and IWR increases mRNA expression of structural cardiac/ventricular genes as Tnnt2, Myl2 and Myh6 as compared to the DMSO controls (Figure 3e).
Figure 3. 3-dimensional culture of ventricular myocytes.
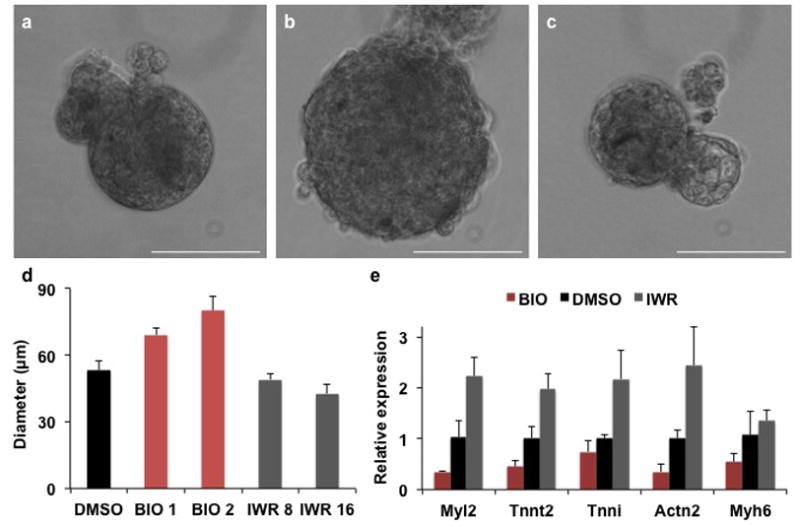
Representative bright field images of ventricular cells cultured in aggregates treated with (a) DMSO, (b) BIO or (c) IWR. Scale bar represents 50μm. (d) Quantification of the diameter of ventricular tissue constructs in μm. (n=3, each in 3 technical replicates). (e) qPCR analysis for structural cardiac genes on cells treated with BIO, DMSO or IWR. (n=3). Error bars indicate standard deviation.
Time Considerations
Animal breeding
The animal breeding for this protocol exists of setting up C57BL/6 or CD1 females with males. Mice mate during night, so plug checks should preferably be performed in the in the morning. Usually, C57BL/6 or CD1 females plug within 3 days in the presence of a male. Fetal cardiomyocytes can be yielded between E11.5 and 14.5. Total time for this part of the protocol is 2–2.5 weeks.
Isolation
Sacrificing the pregnant female and dissecting the fetal ventricular tissue takes approximately 1–1.5 hours. Digestion of tissue 1–2 hours and plating varies on the size of the experiment. Total time is approximately 2–4 hours.
Expansion
Expansion or differentiation assays can be performed varying from 3 days up to 1–2 weeks. Total time of this part depends on experimental design.
Footnotes
KEY REFERENCE (optional)
Buikema et al. ☺
INTERNET RESOURCES (optional)
PCR primer sequences are online available on PrimerBank: http://pga.mgh.harvard.edu/primerbank/
LITERATURE CITED
- Akins RE, Boyce RA, Madonna ML, Schroedl NA, Gonda SR, McLaughlin TA, Hartzell CR. Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng. 1999;5:103–118. doi: 10.1089/ten.1999.5.103. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9:1243–1253. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunick C, Lauenroth K, Leost M, Meijer L, Lemcke T. 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3 beta. Bioorg Med Chem Lett. 2004;14:413–416. doi: 10.1016/j.bmcl.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Ma Z, Hsieh JC, Fan CW, Chen B, Longgood JC, Williams NS, Amatruda JF, Lum L, Chen C. Structure-activity relationship studies of small-molecule inhibitors of Wnt response. Bioorg Med Chem Lett. 2009;19:3825–3827. doi: 10.1016/j.bmcl.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosset JY, Dalvit C, Knapp S, Fasolini M, Veronesi M, Mantegani S, Gianellini LM, Catana C, Sundstrom M, Stouten PF, Moll JK. Inhibition of protein-protein interactions: the discovery of druglike beta-catenin inhibitors by combining virtual and biophysical screening. Proteins. 2006;64:60–67. doi: 10.1002/prot.20955. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]


