Abstract
BACKGROUND:
Very preterm infants (born 24–32 weeks’ gestation) undergo numerous invasive procedures during neonatal care. Repeated skin-breaking procedures in rodents cause neuronal cell death, and in human preterm neonates higher numbers of invasive procedures from birth to term-equivalent age are associated with abnormal brain development, even after controlling for other clinical risk factors. It is unknown whether higher numbers of invasive procedures are associated with long-term alterations in brain microstructure and cognitive outcome at school age in children born very preterm.
METHODS:
Fifty children born very preterm underwent MRI and cognitive testing at median age 7.6 years (interquartile range, 7.5–7.7). T1- and T2-weighted images were assessed for the severity of brain injury. Magnetic resonance diffusion tensor sequences were used to measure fractional anisotropy (FA), an index of white matter (WM) maturation, from 7 anatomically defined WM regions. Child cognition was assessed using the Wechsler Intelligence Scale for Children–IV. Multivariate modeling was used to examine relationships between invasive procedures, brain microstructure, and cognition, adjusting for clinical confounders (eg, infection, ventilation, brain injury).
RESULTS:
Greater numbers of invasive procedures were associated with lower FA values of the WM at age 7 years (P = .01). The interaction between the number of procedures and FA was associated with IQ (P = .02), such that greater numbers of invasive procedures and lower FA of the superior WM were related to lower IQ.
CONCLUSIONS:
Invasive procedures during neonatal care contribute to long-term abnormalities in WM microstructure and lower IQ.
What’s Known on This Subject:
Greater numbers of invasive procedures from birth to term-equivalent age, adjusted for clinical confounders, are associated with altered brain microstructure during neonatal care and poorer cognitive outcome at 18 months’ corrected age in children born very preterm.
What This Study Adds:
Altered myelination at school age is associated with greater numbers of invasive procedures during hospitalization in very preterm children without severe brain injury or neurosensory impairment. Greater numbers of invasive procedures and altered brain microstructure interact to predict lower IQ.
Advances in neonatal care have greatly improved survival of infants born very preterm (≤32 weeks’ gestational age [GA]); however, cognitive impairment may have increased among children with birth weight ≤800 g.1–3 Even in the absence of severe disability (eg, blindness, nonambulatory cerebral palsy, IQ <70), cognitive problems and school difficulties are common among children born very preterm.4–6
Infants born very preterm undergo frequent invasive procedures during neonatal care. Greater numbers of invasive procedures, adjusted for clinical confounders, are associated with abnormal brain development up until term-equivalent age7,8 and altered functional cortical activity at school age.9 Moreover, higher numbers of invasive procedures have been found to be associated with poorer cognitive outcome at 18 months’ corrected age in children born very preterm.10 However, the relationship between the number of invasive procedures and long-term alterations of brain microstructure and cognitive outcomes in children born very preterm remains unknown.
The current study examined whether the number of invasive procedures during neonatal care was associated with white matter (WM) microstructure at age 7 years and whether the number of invasive procedures and measures of brain microstructure interact to predict cognitive outcome at school age in children born very preterm.
Methods
This study was approved by the University of British Columbia/Children’s and Women’s Health Centre of British Columbia Research Ethics Board. Parental written informed consent and child assent were obtained.
Participants
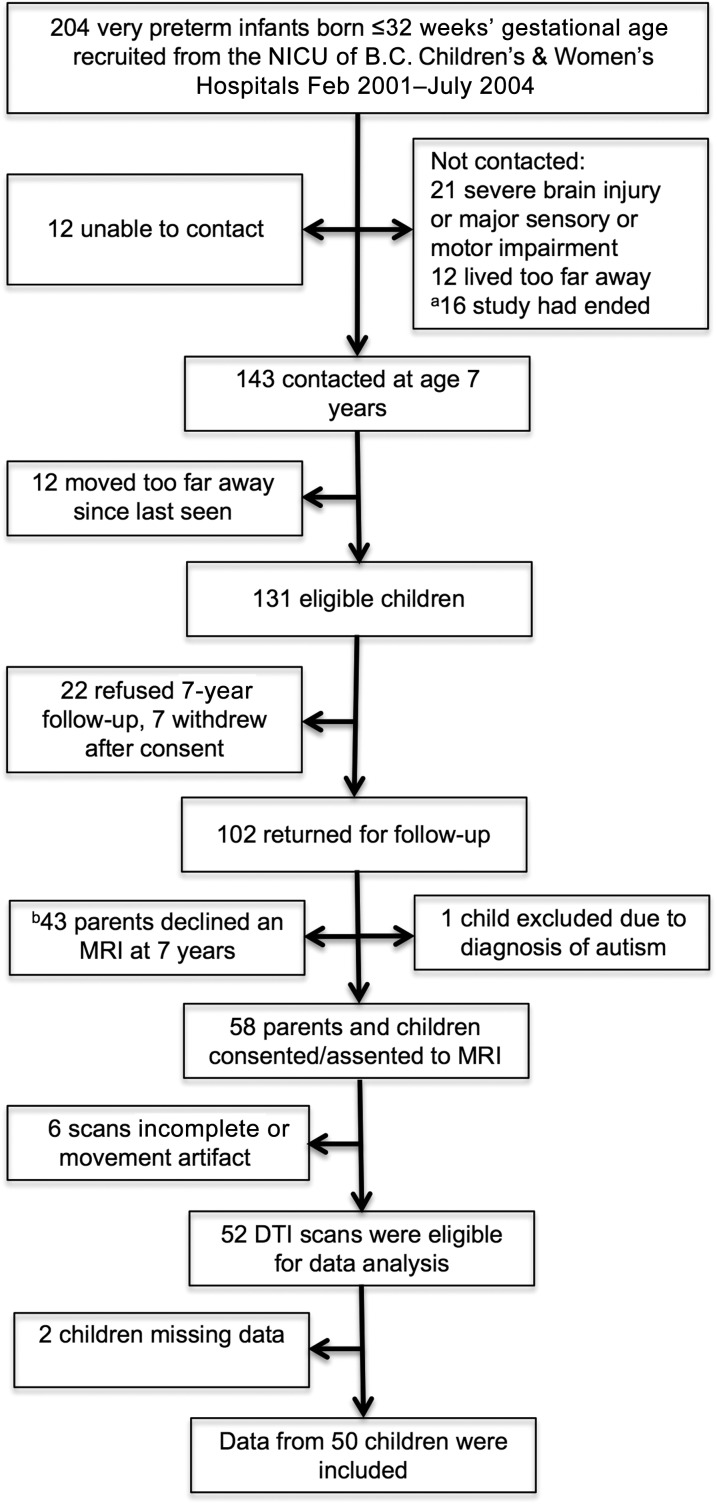
Fifty children born very preterm (≤32 weeks’ GA) recruited from the NICU of the BC Children’s & Women’s Hospitals between February 2001 and July 2004 underwent MRI at median age 7.6 years (interquartile range [IQR], 7.5–7.7) as part of an ongoing study on the effects of neonatal pain on neurodevelopment of children born very preterm.10,11 Children were excluded if they had a major congenital anomaly, major neurosensory impairment (legally blind, nonambulatory cerebral palsy, sensorineural hearing impairment), or severe brain injury evident on neonatal ultrasound (periventricular leukomalacia or grade 3 or 4 intraventricular hemorrhage; Fig 1).
FIGURE 1.
Participant flow chart. aRecruitment stopped after the goal of 100 very preterm children seen at 7 years follow-up was reached. bResearch scans were available only on weekdays after 4 pm, and booking limitations affected study consents for MRI.
Neonatal Medical Chart Review
Neonatal data were acquired from medical chart review performed from birth to term-equivalent age or discharge (whichever came first) by a neonatal research nurse. We defined the number of invasive procedures as every attempt at a procedure as listed in Table 1, from birth to term-equivalent age, adjusted for clinical confounders (eg, illness severity on day 1 [Score for Neonatal Acute Physiology (SNAP-II)])12; days of mechanical ventilation, confirmed infection, morphine exposure).
TABLE 1.
Invasive Procedures in the NICU
| Injectiona | Umbilical artery catheter insertion |
| Chest tube insertiona | Umbilical venous catheter insertion |
| Pleural tapa | Lumbar puncture reservoir tapa |
| Peripheral artery line insertiona | Brainz needle insertiona |
| Peripherally inserted central line insertion or removala | Heel poke (including glucometer pokes)a |
| Penrose insertion or removala | Suprapubic bladder tapa |
| Abscess draineda | Catheter insertion for urine collection |
| Peripheral intravenous catheter sited or resiteda | Venous blood collectiona |
| Endotracheal tube prong change or retaping | Glycerin suppository |
| Nasogastric tube insertion | Orogastric tube insertion |
| Healon or Wydase for intravenous burns | Insuflon device site changea |
| Pericentesisa | Endotracheal or nasopharyngeal intubationa |
| Eye examination |
Each attempt was counted.
Skin-breaking procedures: The Pearson correlation between all procedures listed in this table and the skin-breaking procedures denoted by an asterisk was r = 0.99 for the 50 children born very preterm. The results of this study were the same whether the number of invasive procedures or the number of skin-breaking procedures was entered into the model.
MRI
Children were scanned at a median age of 7.6 years (IQR, 7.5–7.7). A Siemens 1.5 Tesla Avanto magnet, standard 12-channel head coil, and VB 16 software were used to obtain the following sequences: 3-dimensional T1-weighted spoiled gradient recalled acquisition (repetition time [ms] 18/echo time [ms] 9.2/field of view [mm] 256/slice thickness [mm] 1/gap [mm] 0/matrix 256 × 256) and T2-weighted images axial fast spin echo (4030/90/220/3/1/512 × 354) and axial fluid attenuation inversion recovery (8900/87/220/5/1/256 × 154). Neuroradiologist K.J.P., blinded to the child’s medical history, assessed these images for brain injury (ie, evidence of cerebellar hemorrhage, ventriculomegaly, or moderate to severe WM injury, as described previously).13
Diffusion Tensor Imaging
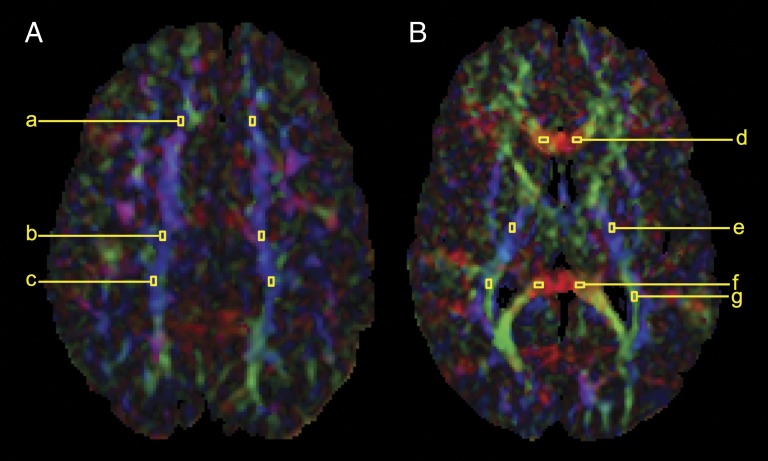
Diffusion tensor imaging (DTI) is a MRI technique that can be used to characterize the spatial distribution of water diffusion in each voxel (3-dimensional pixel) of the image as an ellipsoid, providing a measure of regional brain microstructural development.14 The size, shape, and orientation of the ellipsoid are given by eigenvalues (λ1, λ2, and λ3). λ1 corresponds to axial diffusion and is considered to reflect axonal integrity.15 λ2 and λ3 correspond to radial diffusion and reflect myelin integrity.15 Fractional anisotropy (FA), a measure of overall directionality, reflects the variance of λ1, λ2, and λ3. DTI was acquired with a multirepetition, single-shot echo planar sequence with 12 gradient directions (7800/82/256/2/0/128 × 128), 3 averages of diffusion weighting 700 (b value). DTI parameters of FA, λ1, λ2, and λ3 were obtained from 7 bilateral regions of interest in the WM (Fig 2), consistent with our neonatal studies.8,16 Intrarater reliability, based on the repeated analysis of a random 20% of regions of interest, was comparable with previously published findings (FA mean difference of −0.002 [Bland–Altman limits of agreement, −0.011 to 0.007]).8,16
FIGURE 2.
Regions of interest obtained within the WM. A, Superior WM: (a) anterior, (b) middle, and (c) posterior subcortical WM. B, WM tracts: (d) genu of the corpus callosum, (e) posterior limb of the internal capsule, (f) splenium of the corpus callosum, and (g) optic radiations.
Cognitive Testing
At age 7 years, IQ was measured by using the standardized Wechsler Intelligence Scale for Children–4th Edition (WISC-IV),17 which includes 4 index scores that make up the Full Scale IQ (FSIQ): Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed.
Statistical Analyses
Statistical analyses were performed by using Stata 9.2 (Stata Corp, College Station, TX). Normality plots were examined, and skewed variables (number of invasive procedures, days on mechanical ventilation, morphine exposure, FA values, and age at scan) were log transformed. IQ, GA, birth weight, and illness severity on day 1 of the included and excluded preterm infants were compared by using t tests. Demographic characteristics of the preterm infants exposed to lower and higher numbers of invasive procedures were compared by using t tests or χ2, when appropriate. Multivariate analyses were adjusted for confounders: GA, size at birth (small for gestational age versus appropriate for gestational age), illness severity on day 1, days of mechanical ventilation, morphine exposure, infection, gender, age at scan, and concurrent brain injury. A generalized estimating equation was used to examine whether the number of invasive procedures was associated with FA at age 7 years in an initial pain model. This model was repeated for the axial and radial axes. The pain model was extended to include variables for surgery and fentanyl exposure (surgery model), and corticosteroids and midazolam (steroid model). The regression coefficients for these models are reported as effect sizes. FA values were then grouped a priori into superior WM (anterior, middle, and posterior subcortical WM) and WM tracts (genu and splenium of the corpus callosum, posterior limb of the internal capsule, optic radiations), and group means were used for analysis. Generalized linear modeling was used to examine whether the number of invasive procedures interacted with FA values from either the superior WM or WM tracts to predict FSIQ.
Results
Participant Characteristics
Of the 131 eligible children contacted for the 7-year follow-up (Fig 1), 22 refused to participate and 7 withdrew, so that 102/131 (78%) were seen at school age. One child diagnosed with autism was excluded, leaving 101 children in this study. Of the 101 who returned for follow-up (psychometric assessment) at median age 7.6 years (IQR, 7.5–7.8), 58 (57%) parents and children consented/assented to an MRI. Research scans were available only on weekdays after 4 pm, and booking limitations affected study consents for MRI. Scans were not completed for 3 of the participants, and 3 were of poor quality because of motion artifact. Moreover, 2 children were missing either neonatal or follow-up data. Therefore, data from 50 children born very preterm were included in the current study. Importantly, the FSIQ of the children included (n = 50) did not differ from that of the other 51 children who returned for 7-year follow-up (95% confidence interval [CI], −7.18 to 3.86, P = .55). Moreover, children included in the current study did not differ in GA (95% CI, −1.40 to 0.44, P = .30), birth weight (95% CI, −187.33 to 147.52, P = .81), or early illness severity (95% CI, −2.81 to 5.73, P = .50) from the children who returned for follow-up or from the 81 infants in the original sample (95% CI, −1.10 to 0.63, P = .59; CI, −134.99 to 194.17, P = .72; and 95% CI, −2.50 to 5.73, P = .44; respectively).
Among the 50 children with imaging data at age 7 years, exposure to higher numbers of invasive procedures (median 122; IQR, 81–210) was associated with lower GA, higher illness severity on day 1, more days on mechanical ventilation, and a greater exposure to surgery, infection, dexamethasone, and morphine compared with children exposed to lower numbers of procedures (median 46; IQR, 30–55) (Table 2). Among the 101 children born very preterm who returned for follow-up at 7 years, exposure to higher numbers of invasive procedures (median 127; IQR, 87–200) were also associated with increased exposure to midazolam and fentanyl and a significantly lower FSIQ compared with children exposed to lower numbers of procedures (median 43; IQR, 32–52) (Table 3).
TABLE 2.
Characteristics of Children With MRI at Age 7 Years
| Neonatal Characteristics | n = 50 | Lower Number of Invasive Procedures, Median 46, IQR 30–55 | Higher Number of Invasive Procedures, Median 122, IQR 81–210 | P Value |
|---|---|---|---|---|
| n = 25 | n = 25 | |||
| Gestational age (wk), median (IQR) | 29.8 (28.1–31.9) | 31.4 (29.7–32.3) | 28.4 (26.9–30.4) | <0.001 |
| Small for gestational age, n (%) | 6 (12) | 2 (8) | 4 (16) | 0.13 |
| Illness severity on day 1 (SNAP-II), median (IQR) | 8 (0–18) | 0 (0–9) | 14 (5–23) | 0.01 |
| Invasive procedures (n), median (IQR) | 74 (46–124) | — | — | |
| Surgery, n (%) | 8 (16) | 0 (0) | 8 (32) | <0.001 |
| Infection, n (%) | 11 (22) | 1 (4) | 10 (40) | <0.001 |
| Mechanical ventilation (days), median (IQR) | 2 (0–8) | 0 (0–1) | 7 (3–20) | <0.001 |
| Dexamethasone or hydrocortisone, number exposed (%) | 5 (10) | 0 (0) | 5 (20) | <0.001 |
| Total morphine exposure (g/kg), median (IQR), number exposed (%) | 0.0 (0.0–0.6) | 0.0 (0.0–0.0) | 0.5 (0.1–1.8) | 0.03 |
| 24 (48) | 4 (16) | 20 (80) | ||
| Total midazolam exposure (g/kg), median (IQR), number exposed (%) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.12 |
| 3 (6) | 0 (0) | 3 (12) | ||
| Total fentanyl exposure (µg/kg), median (IQR), number exposed (%) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–3.0) | 0.12 |
| 6 (12) | 0 (0) | 6 (24) | ||
| Child Characteristics | n = 50 | n = 25 | n = 25 | P Value |
| Gender (male), n (%) | 21 (42) | 6 (24) | 15 (60) | <0.001 |
| Age at scan (y), median (IQR) | 7.6 (7.5–7.7) | 7.6 (7.5–7.6) | 7.6 (7.5–7.7) | 0.82 |
| Moderate to severe brain injury, n (%) | 6 (12) | 4 (16) | 2 (8) | 0.13 |
| WISC-IV FSIQ, median (IQR) | 102 (91–110) | 103 (92–110) | 95 (85–112) | 0.30 |
| WISC-IV VCI, median (IQR) | 98 (93–109) | 99 (94–109) | 98 (89–109) | 0.56 |
| WISC-IV PRI, median (IQR) | 104 (94–113) | 104 (98–112) | 100 (91–119) | 0.68 |
| WISC-IV WMI, median (IQR) | 98 (91–110) | 97 (91–109) | 99 (88–115) | 0.80 |
| WISC-IV PSI, median (IQR) | 94 (86–108)a | 100 (88–115) | 91 (83–105) | 0.09 |
2 children did not complete the PSI.
TABLE 3.
Characteristics of Children Who Returned for Follow-Up at Age 7 Years
| Neonatal Characteristics | n = 101 | Lower Number of Invasive Procedures, Median 43, IQR 32–52 | Higher Number of Invasive Procedures, Median 127, 87–200 | P Value |
|---|---|---|---|---|
| n = 49 | n = 51 | |||
| Gestational age (wk), median (IQR) | 29.9 (27.5–31.7) | 31.6 (29.9–32.4) | 27.7 (26.3–29.3) | <0.001 |
| Small for gestational age, n (%) | 10 (10)a | 4 (8) | 6 (12) | 0.48 |
| Illness severity on day 1 (SNAP-II), median (IQR) | 9 (0–19)a | 5 (0–9) | 16 (8–25) | <0.001 |
| Invasive procedures (n), median (IQR) | 73 (43–129)a | — | — | |
| Surgery, n (%) | 17 (17)a | 1 (2) | 16 (31) | <0.001 |
| Infection, n (%) | 24 (24)a | 1 (2) | 23 (45) | <0.001 |
| Mechanical ventilation (days), median (IQR) | 2 (0–10)a | 0 (0–0) | 9 (3–31) | <0.001 |
| Dexamethasone or hydrocortisone, number exposed (%) | 8 (8) | 0 (0) | 8 (16) | <0.001 |
| Total morphine exposure (g/kg), median (IQR), number exposed (%) | 0.0 (0.0–0.6)a | 0.0 (0.0–0.0) | 0.6 (0.1–1.8) | 0.001 |
| 49 (49) | 6 (12) | 43 (84) | ||
| Total midazolam exposure (g/kg), median (IQR), number exposed (%) | 0.0 (0.0–0.0)a | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.02 |
| 8 (8) | 0 (0) | 8 (16) | ||
| Total fentanyl exposure (µg/kg), median (IQR), number exposed (%) | 0.0 (0.0–0.0)a | 0.0 (0.0–0.0) | 0.0 (0.0–3.0) | 0.04 |
| 12 (12) | 0 (0) | 12 (24) | ||
| Child Characteristics | n = 101 | n = 49 | n = 51 | P Value |
| Gender (male), n (%) | 50 (50) | 19 (39) | 31 (61) | 0.003 |
| Age at follow-up (y), median (IQR) | 7.6 (7.5–7.8) | 7.6 (7.5–7.7) | 7.6 (7.5–7.8) | 0.80 |
| WISC-IV FSIQ, median (IQR) | 100 (91–110)b | 104 (94–114) | 95 (87–108) | 0.009 |
| WISC-IV VCI, median (IQR) | 98 (93–108)b | 99 (95–114) | 98 (89–104) | 0.09 |
| WISC-IV PRI, median (IQR) | 100 (92–116)b | 104 (98–117) | 100 (88–110) | 0.07 |
| WISC-IV WMI, median (IQR) | 97 (88–110)b | 99 (91–110) | 94 (88–108) | 0.10 |
| WISC-IV PSI, median (IQR) | 94 (85–106)b | 100 (90–113) | 88 (83–99) | 0.003 |
1 child was missing neonatal data, and 2 children did not have neonatal infection data.
4 children did not complete the FSIQ, 2 children did not complete the VCI, 1 child did not complete the PRI, 3 children did not complete the WMI, and 6 children did not complete the PRI.
Number of Invasive Procedures in Relation to WM Microstructure at Age 7 Years
Children born very preterm exposed to a greater numbers of invasive procedures in the NICU had lower FA values at age 7 years (effect size = −0.02, P = .01; CI, −0.04 to −0.005) after adjusting for confounders (GA, birth weight, illness severity on day 1, days of mechanical ventilation, morphine exposure, infection, gender, age at scan, and concurrent brain injury) (pain model, Table 4). Infants who received the lowest number of invasive procedures (ie, 10 invasive procedures) had 7% higher FA values than infants who underwent the highest number of invasive procedures (ie, 267 invasive procedures). The relationship between the number of invasive procedures and FA of the WM was driven by the radial diffusion axes (λ2 and λ3: effect size = 0.05; CI, 0.01 to 0.09; P = .01), such that greater numbers of invasive procedures from birth to term-equivalent age were associated with higher radial diffusion values. In contrast, the number of invasive procedures was not associated with the axial diffusion axis (λ1: effect size = −0.05; CI, −0.15 to 0.06; P = .38). Neither adjustment for surgery and fentanyl nor corticosteroids and midazolam significantly changed the results of the pain model (surgery and steroid models, Table 4).
TABLE 4.
Higher Number of Invasive Procedures Was Associated With Lower FA at Age 7 Years
| Fractional Anisotropy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Pain Model, n = 50 | Surgery Model, n = 50 | Steroid Model, n = 50 | ||||||
| Effect Size | CI | P | Effect Size | CI | P | Effect Size | CI | P | |
| Number of invasive procedures | −0.02 | −0.04 to −0.005 | .01 | −0.02 | −0.04 to −0.006 | .007 | −0.02 | −0.04 to -0.006 | .01 |
| GA | −0.001 | −0.004 to 0.003 | .71 | −0.001 | −0.004 to 0.003 | .73 | −0.001 | −0.005 to 0.004 | .76 |
| Small for GA | −0.003 | −0.02 to 0.01 | .68 | −0.003 | −0.02 to 0.01 | .67 | −0.005 | −0.02 to 0.01 | .52 |
| Illness severity | <0.001 | −0.0004 to 0.001 | .57 | <0.001 | −0.0004 to 0.001 | .65 | <0.001 | −0.001 to 0.001 | .84 |
| Mechanical ventilation | −0.003 | −0.02 to 0.01 | .70 | −0.002 | −0.02 to 0.01 | .78 | <0.001 | −0.01 to 0.02 | 1.00 |
| Postnatal infection | 0.009 | −0.004 to 0.02 | .16 | 0.008 | −0.005 to 0.02 | .21 | 0.008 | −0.006 to 0.02 | .26 |
| Gender | −0.002 | −0.01 to 0.007 | .71 | −0.002 | −0.01 to 0.008 | .72 | −0.004 | −0.01 to 0.007 | .49 |
| Age at scan | −0.19 | −0.47 to 0.08 | .17 | −0.20 | −0.48 to 0.09 | .17 | −0.18 | −0.46 to 0.10 | .21 |
| Brain injury | −0.009 | −0.02 to 0.002 | .10 | −0.009 | −0.02 to 0.002 | .10 | −0.007 | −0.02 to 0.003 | .16 |
| Surgery | — | — | — | 0.002 | −0.02 to 0.02 | .84 | — | — | — |
| Morphine exposure | 0.008 | −0.007 to 0.02 | .28 | 0.008 | −0.009 to 0.03 | .37 | 0.003 | −0.02 to 0.02 | .77 |
| Fentanyl exposure | — | — | — | <−0.001 | −0.0001 to 0.00002 | .24 | — | — | — |
| Corticosteroids | — | — | — | — | — | — | −0.007 | −0.02 to 0.01 | .43 |
| Midazolam | — | — | — | — | — | — | 0.001 | −0.0003 to 0.001 | .22 |
Number of Invasive Procedures Interacts With the Superior WM to Predict FSIQ at Age 7 Years
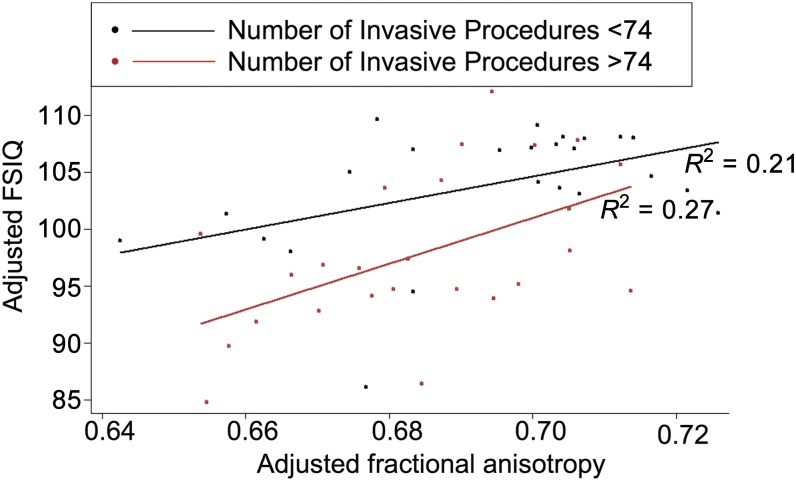
The interaction between number of invasive procedures and FA values of the superior WM was significantly associated with FSIQ (B = 412.18; P = .02; CI, 55.59 to 768.77; adjusted R2 = 0.22; Table 5), such that greater numbers of invasive procedures (adjusted for confounders) and lower FA of the superior WM were associated with lower FSIQ at age 7.5 years in children born very preterm (Fig 3). To assist with the interpretation of this interaction, post hoc analyses were conducted. We used a cutoff of FSIQ <100 versus FSIQ >100, because children with major impairments had been excluded. Among children exposed to either higher or lower numbers of invasive procedures (median split), we examined whether a change in FA from the 75th percentile to the 25th percentile corresponded with a decrease in FSIQ >2.60 (ie, beyond the SE of measurement). Specifically, among the children with lower FSIQ (<100), exposed to higher numbers of invasive procedures (>74 invasive procedures), a change in FA in the posterior subcortical WM from the 75th percentile (0.67) to the 25th percentile (0.58) corresponded to a 13.1 point decrease in FSIQ. In contrast, a change in FA from the 75th percentile to the 25th percentile for children exposed to lower numbers of invasive procedures (<74 invasive procedures) corresponded to a nonsignificant 0.86 point change in FSIQ, less than the SE of measurement for FSIQ.
TABLE 5.
Higher Number of Invasive Procedures and Lower FA of the Superior WM Predicts Lower IQ
| Predictors | Full Scale IQ, n = 50 | ||
|---|---|---|---|
| B | CI | P | |
| Number of invasive procedures × FA | 412.18 | 55.59 to 768.77 | .02 |
| FA | −735.63 | −1411.08 to −60.18 | .03 |
| Number of invasive procedures | 83.35 | 25.16 to 141.53 | .005 |
| Gestational age | 0.88 | −2.16 to 3.93 | .57 |
| Small for GA | −1.64 | −14.36 to 11.07 | .80 |
| Illness severity | −0.50 | −0.95 to −0.05 | .03 |
| Mechanical ventilation | −5.22 | −21.72 to 11.29 | .54 |
| Morphine exposure | 1.75 | −15.55 to 19.04 | .84 |
| Postnatal infection | 6.17 | −4.09 to 16.43 | .24 |
| Gender | 0.37 | −7.54 to 8.27 | .93 |
| Age at scan | −168.10 | −382.31 to 46.12 | .12 |
| Brain injury | −2.41 | −13.41 to 8.59 | .67 |
FIGURE 3.
Number of invasive procedures and brain microstructure predicts FSIQ. Higher numbers of invasive procedures (above median: red) and lower FA values of the superior WM were associated with lower FSIQ after adjustment for neonatal and clinical confounders, age at scan, and concurrent brain injury.
Interaction Between Number of Invasive Procedures and WM Tracts in Relation to FSIQ
The interaction between the number of invasive procedures and FA values of the WM tracts was not associated with FSIQ (B = −304.22; P = .46; CI, −1106.38 to 497.94).
Discussion
This is the first study, to our knowledge, to show that greater numbers of invasive procedures during neonatal care are associated with altered WM microstructure at school age in children born very preterm, after accounting for degree of prematurity, systemic illness, drug exposures, and concurrent brain injury. Specifically, in 7-year-olds without severe brain injuries or major neurosensory impairment, a higher number of invasive procedures during NICU care was associated with an increase in radial diffusion values at age 7 years, suggestive of abnormal myelination. Morphine did not appear to ameliorate or exacerbate the effects of the number of invasive procedures on the microstructural integrity of the WM. Greater numbers of invasive procedures and reduced myelination of the superior WM were associated with lower IQ in children born very preterm at school age. The relationship between the number of procedures, brain microstructure, and IQ was driven by 2 frontoparietal functions, verbal comprehension and working memory, which share common neural substrates (see Supplemental Information).18
Both the peripheral and central nociceptive systems are functionally active in infants born very preterm,19,20 although both the ascending and descending nociceptive pathways are still immature. Subsequently, infants born very preterm have lower tactile thresholds and become sensitized to repeated tactile and skin-breaking stimulation.21,22 Repeated stimulation of physiologically immature neurons can lead to excitotoxic damage and increased neuronal cell death.23,24 Previously, it has been demonstrated that a higher number of invasive and stressful procedures was associated with delayed WM maturation in infants born very preterm both during NICU care and at term-equivalent age.7,8,25 Higher numbers of invasive procedures are associated with lower stress hormone cortisol responses at 32 weeks’ postmenstrual age and higher levels at 8 and 18 months’ corrected age.26,27 Brain regions rich in glucocorticoid receptors (eg, prefrontal cortex) are particularly vulnerable to the effects of ongoing stress.28,29 This may explain why the number of invasive procedures and the subcortical WM, rather than the WM tracts, predicted IQ at 7 years.
Preoligodendrocytes actively develop between 24 and 40 weeks’ gestation to form mature, myelinating oligodendrocytes.30 However, during this peak developmental period, preoligodendrocytes are sensitive to reactive oxygen, nitrogen species, and cytokines secreted by microglia.31–35 Neonatal hypoxia–ischemia and neuroinflammation lead to the activation of astrocytes and microglia, which induces myelin deficiency, associated with long-term cognitive problems.30,36 Similarly, pain also induces both oxidative stress and inflammatory reactions.37,38 Therefore, greater exposure to invasive procedures in the NICU may arrest the development of premyelinating cells.
In this study, we could not discern why the strongest association with invasive procedures was observed with the subcortical WM, rather than with WM tracts. Myelination first appears in the central WM tracts, therefore the subcortical WM may have been more enriched in progenitor stages of the oligodendrocyte lineage in contrast with the WM tracts. Alternatively, the subcortical and central WM tracts may have been similarly affected, but the latter had greater potential for recovery.
Another important consideration involves the distribution of opioid receptors. Electrical stimulation of the periaqueductal gray and rostroventral medulla, regions responsible for the release of opioids within the spinal cord, does not result in the inhibition of the pain signal in rat pups until postnatal day 21.39,40 Repeated invasive procedures during this maturational period of the descending inhibitory system may lead to hyperinnervation of the periaqueductal gray and rostroventral medulla, thereby altering their functional integrity.41 Inflammatory pain in neonatal rats has been found to increase the adult endogenous opioid tone.42 Therefore, repeated exposure to invasive procedures in the NICU may lead to chronically elevated opiate peptides, affecting the integrity of the subcortical WM, which connects to the periaquaductal gray, a region rich in opioid receptors.
Invasive procedures may also have indirectly affected the neonatal brain. Greater numbers of invasive procedures were associated with slower growth,43 and slower growth during neonatal care was associated with delayed cerebral cortical maturation in infants born very preterm.44 FA values reach the noise floor in the cortical gray matter by approximately 36 weeks’ postmenstrual age,44,45 due to neuronal maturation, synaptogenesis, and the disappearance of radial glial cells.46–50 Therefore, we could not examine the long-term effects of the number of invasive procedures on the DTI measures of cortical gray matter. Future studies using alternative methods for quantifying neuronal integrity (eg, cortical thickness, volumetrics) are needed.
In contrast with findings in other cohorts, midazolam and corticosteroids did not have an effect on brain development in this cohort of infants without severe brain injury.51–53 The quantity, timing, or duration of the exposure may not have been sufficient to negatively affect myelination. We previously reported that postnatal infections were significantly associated with 8% lower overall FA in infants born very preterm.54 After accounting for clinical confounders, the magnitude of change observed in this study relative to the number of invasive procedures was comparable (ie, 7% lower FA in infants exposed to higher numbers of invasive procedures).
The study sample was limited; therefore, the results of this article are a first step in understanding the relationship between the number of invasive procedures in the NICU, brain microstructure, and neurodevelopmental outcomes at school age. Similarly, the post hoc analyses should be interpreted with caution. The sample size also limited the number of variables we could include, such that residual confounding for clinical condition associated with invasive procedures may remain. Future research with larger samples is needed to address whether recurrent life-threatening complications such as necrotizing enterocolitis may also have predisposed infants to ischemia and related myelination disturbances. Preterm infant responses to invasive procedures vary depending on GA, sleep–wake state, illness severity, and previous exposures to pain,55–62 therefore it is difficult to determine the extent of pain perceived for a specific procedure. Furthermore, although we adjusted for cumulative morphine exposure in our models, we could not account for pain management that may have been provided during a given procedure. Therefore, in the present study we related the number of procedures that are normally considered painful with brain development but without being able to quantify the amount of pain each infant experienced.
Associations between brain volume, microstructure, and cognitive function have been demonstrated in children and adults born preterm.63–68 In this cohort of preterm children without severe brain injury or major neurosensory/motor/cognitive impairment, we demonstrated that after accounting for degree of prematurity, systemic illness, medication exposures, and concurrent brain injury, greater numbers of invasive procedures interacted with alterations in WM to predict lower IQ.
Conclusions
This article provides the first evidence for the association between the number of invasive procedures in the NICU and altered WM and IQ at school age, in very preterm children who escaped major brain injury and neurosensory impairment, after adjusting for other risk factors associated with prematurity. Given the apparently limited efficacy of morphine in preventing adverse effects of repeated invasive procedures, it is important to continue to examine methods for pain management. Reducing pain during neonatal care may help optimize brain development and improve cognitive outcomes in this vulnerable population.
Supplementary Material
Acknowledgments
We thank the children and their parents who participated in this study and Dr Deborah E. Giaschi for providing the magnetic resonance simulation prior to scanning. R.E.G. is supported by a Senior Scientist award from the Child and Family Research Institute. S.P.M., currently Bloorview Children’s Hospital Chair in Paediatric Neuroscience, was supported by a Tier 2 Canadian Research Chair in Neonatal Neuroscience and a Michael Smith Foundation for Health Research Scholar Award. J.V. holds a Canadian Institutes of Health Research Frederick Banting and Charles Best Doctoral Award and is a member of the Pain in Child Health Canadian Institutes of Health Research Strategic Training Initiative in Health Research.
Glossary
- CI
confidence interval
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FSIQ
full scale intelligence quotient
- GA
gestational age
- IQR
interquartile range
- PRI
perceptual reasoning index
- PSI
processing speed index
- SNAP-II
Score for Neonatal Acute Physiology
- VCI
verbal comprehension index
- WISC-IV
Wechsler Intelligence Scale IV
- WM
white matter
- WMI
working memory index
Footnotes
Ms Vinall assisted with the acquisition of data and statistical analyses, contributed to the interpretation of the data, drafted the manuscript for review, and provided critical review of the manuscript; Drs Miller and Synnes contributed to the interpretation of the data and provided critical review of the manuscript; Drs Bjornson and Poskitt assisted with the acquisition of data, contributed to the interpretation of the data, and provided critical review of the manuscript; Mr Fitzpatrick assisted with the acquisition of data and provided critical review of the manuscript; Dr Brant conducted statistical analyses and provided critical review of the manuscript; Dr Cepeda provided critical review of the manuscript; Dr Grunau conceptualized and designed the study, contributed to the interpretation of the data, and provided critical review of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R01 HD39783 to R.E.G.) funded this study. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Synnes AR, Anson S, Arkesteijn A, et al. School entry age outcomes for infants with birth weight </= 800 grams. J Pediatr. 2010;157(6):989–994.e1 [DOI] [PubMed] [Google Scholar]
- 2.Doyle LW, Roberts G, Anderson PJ, Victorian Infant Collaborative Study Group . Changing long-term outcomes for infants 500–999 g birth weight in Victoria, 1979–2005. Arch Dis Child Fetal Neonatal Ed. 2011;96(6):F443–F447 [DOI] [PubMed] [Google Scholar]
- 3.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson S, Hennessy E, Smith R, Trikic R, Wolke D, Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F283–F289 [DOI] [PubMed] [Google Scholar]
- 5.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269 [DOI] [PubMed] [Google Scholar]
- 6.Larroque B, Ancel PY, Marret S, et al. EPIPAGE Study group . Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371(9615):813–820 [DOI] [PubMed] [Google Scholar]
- 7.Zwicker JG, Grunau RE, Adams E, et al. Score for Neonatal Acute Physiology–II and neonatal pain predict corticospinal tract development in premature newborns. Pediatr Neurol. 2013;48(2):123–129.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71(3):385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doesburg SM, Chau CM, Cheung TP, et al. Neonatal pain-related stress, functional cortical activity and visual-perceptual abilities in school-age children born at extremely low gestational age. Pain. 2013;154(10):1946–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunau RE, Whitfield MF, Petrie-Thomas J, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143(1–2):138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150(2):151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100 [DOI] [PubMed] [Google Scholar]
- 13.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–616 [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23(9):1445–1456 [PMC free article] [PubMed] [Google Scholar]
- 15.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436 [DOI] [PubMed] [Google Scholar]
- 16.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66(2):155–164 [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. WISC-IV: Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 2003 [Google Scholar]
- 18.Gläscher J, Tranel D, Paul LK, et al. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61(5):681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage. 2010;52(2):583–589 [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6(7):507–520 [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39(1):31–36 [DOI] [PubMed] [Google Scholar]
- 22.Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56(1):95–101 [DOI] [PubMed] [Google Scholar]
- 23.Dührsen L, Simons SH, Dzietko M, et al. Effects of repetitive exposure to pain and morphine treatment on the neonatal rat brain. Neonatology. 2013;103(1):35–43 [DOI] [PubMed] [Google Scholar]
- 24.Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62(3):283–290 [DOI] [PubMed] [Google Scholar]
- 25.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70(4):541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1). Available at: www.pediatrics.org/cgi/content/full/114/1/e77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunau RE, Holsti L, Haley DW, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113(3):293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7 [DOI] [PubMed] [Google Scholar]
- 29.Meaney MJ, Diorio J, Francis D, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18(1–2):49–72 [DOI] [PubMed] [Google Scholar]
- 30.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress–induced death caused by glutathione depletion. J Neurosci. 1998;18(16):6241–6253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Back SA, Luo NL, Mallinson RA, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58(1):108–120 [DOI] [PubMed] [Google Scholar]
- 33.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62(5):441–450 [DOI] [PubMed] [Google Scholar]
- 34.Buntinx M, Moreels M, Vandenabeele F, et al. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res. 2004;76(6):834–845 [DOI] [PubMed] [Google Scholar]
- 35.Pang Y, Cai Z, Rhodes PG. Effect of tumor necrosis factor–alpha on developing optic nerve oligodendrocytes in culture. J Neurosci Res. 2005;80(2):226–234 [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Liu J, Cheung PY, Chen C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic–ischemic brain injury. Brain Res. 2009;1301:100–109 [DOI] [PubMed] [Google Scholar]
- 37.Slater L, Asmerom Y, Boskovic DS, et al. Procedural pain and oxidative stress in premature neonates. J Pain. 2012;13(6):590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol (Oxf). 2006;187(1–2):321–327 [DOI] [PubMed] [Google Scholar]
- 39.van Praag H, Frenk H. The development of stimulation-produced analgesia (SPA) in the rat. Brain Res Dev Brain Res. 1991;64(1–2):71–76 [DOI] [PubMed] [Google Scholar]
- 40.Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem–spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587(pt 12):2927–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaPrairie JL, Murphy AZ. Long-term impact of neonatal injury in male and female rats: sex differences, mechanisms and clinical implications. Front Neuroendocrinol. 2010;31(2):193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laprairie JL, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci. 2009;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinall J, Miller SP, Chau V, Brummelte S, Synnes AR, Grunau RE. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain. 2012;153(7):1374–1381 [DOI] [PubMed] [Google Scholar]
- 44.Vinall J, Grunau RE, Brant R, et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med. 2013;5(168):168ra8. [DOI] [PubMed] [Google Scholar]
- 45.McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12(12):1237–1243 [DOI] [PubMed] [Google Scholar]
- 46.Marín-Padilla M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J Comp Neurol. 1992;321(2):223–240 [DOI] [PubMed] [Google Scholar]
- 47.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–470 [DOI] [PubMed] [Google Scholar]
- 48.Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271(3):355–386 [DOI] [PubMed] [Google Scholar]
- 49.Kostović I, Jovanov-Milosević N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11(6):415–422 [DOI] [PubMed] [Google Scholar]
- 50.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62(1):1–35 [DOI] [PubMed] [Google Scholar]
- 51.Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2012;6:CD002052. [DOI] [PubMed] [Google Scholar]
- 52.Parikh NA, Lasky RE, Kennedy KA, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119(2):265–272 [DOI] [PubMed] [Google Scholar]
- 53.Tam EW, Chau V, Ferriero DM, et al. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci Transl Med. 2011;3(105):105ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. 2012;71(3):274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks’ postconceptional age. Pediatrics. 2001;107(1):105–112 [DOI] [PubMed] [Google Scholar]
- 56.Holsti L, Grunau RE, Whifield MF, Oberlander TF, Lindh V. Behavioral responses to pain are heightened after clustered care in preterm infants born between 30 and 32 weeks gestational age. Clin J Pain. 2006;22(9):757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holsti L, Grunau RE, Oberlander TF, Whitfield MF. Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Hum Dev. 2005;81(3):293–302 [DOI] [PubMed] [Google Scholar]
- 58.Holsti L, Grunau RE, Oberlander TF, Osiovich H. Is it painful or not? Discriminant validity of the Behavioral Indicators of Infant Pain (BIIP) scale. Clin J Pain. 2008;24(1):83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anand KJ. Pain assessment in preterm neonates. Pediatrics. 2007;119(3):605–607 [DOI] [PubMed] [Google Scholar]
- 60.Ranger M, Johnston CC, Anand KJ. Current controversies regarding pain assessment in neonates. Semin Perinatol. 2007;31(5):283–288 [DOI] [PubMed] [Google Scholar]
- 61.Gibbins S, Stevens B, McGrath PJ, et al. Comparison of pain responses in infants of different gestational ages. Neonatology. 2008;93(1):10–18 [DOI] [PubMed] [Google Scholar]
- 62.Slater R, Cantarella A, Yoxen J, et al. Latency to facial expression change following noxious stimulation in infants is dependent on postmenstrual age. Pain. 2009;146(1–2):177–182 [DOI] [PubMed] [Google Scholar]
- 63.Allin MP, Kontis D, Walshe M, et al. White matter and cognition in adults who were born preterm. PLoS ONE. 2011;6(10):e24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947 [DOI] [PubMed] [Google Scholar]
- 65.Van Kooij BJ, Benders MJ, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54(3):260–266 [DOI] [PubMed] [Google Scholar]
- 66.Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131(Pt 12):3201–3208 [DOI] [PubMed] [Google Scholar]
- 67.Thompson DK, Inder TE, Faggian N, et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 2012;59(4):3571–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldman HM, Lee ES, Loe IM, Yeom KW, Grill-Spector K, Luna B. White matter microstructure on diffusion tensor imaging is associated with conventional magnetic resonance imaging findings and cognitive function in adolescents born preterm. Dev Med Child Neurol. 2012;54(9):809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.