Abstract
OBJECTIVE:
This prospective longitudinal study examined the long-term physical and psychosocial outcomes of adolescents with juvenile-onset fibromyalgia (JFM), compared with healthy control subjects, into early adulthood.
METHODS:
Adolescent patients with JFM initially seen at a pediatric rheumatology clinic (n = 94) and age- and gender-matched healthy control subjects (n = 33) completed online measures of demographic characteristics, pain, physical functioning, mood symptoms, and health care utilization at ∼6 years’ follow-up (mean age: 21 years). A standard in-person tender-point examination was conducted.
RESULTS:
Patients with JFM had significantly higher pain (P < .001), poorer physical function (P < .001), greater anxiety (P < .001) and depressive symptoms (P < .001), and more medical visits (P < .001)than control subjects. The majority (>80%) of JFM patients continued to experience fibromyalgia symptoms into early adulthood, and 51.1% of the JFM sample met American College of Rheumatology criteria for adult fibromyalgia at follow-up. Patients with JFM were more likely than control subjects to be married and less likely to obtain a college education.
CONCLUSIONS:
Adolescent patients with JFM have a high likelihood of continued fibromyalgia symptoms into young adulthood. Those who met criteria for fibromyalgia in adulthood exhibited the highest levels of physical and emotional impairment. Emerging differences in educational attainment and marital status were also found in the JFM group. JFM is likely to be a long-term condition for many patients, and this study for the first time describes the wide-ranging impact of JFM on a variety of physical and psychosocial outcomes that seem to diverge from their same-age peers.
Keywords: early adulthood, juvenile fibromyalgia, long-term outcomes, pediatric chronic pain
What’s Known on This Subject:
Juvenile-onset fibromyalgia (JFM) is a poorly understood chronic pain condition, typically identified in adolescence and accompanied by physical and social impairment and mood difficulties. There are no long-term studies on the prognosis of adolescents with JFM into adulthood.
What This Study Adds:
This prospective study demonstrated that pain and other symptoms persisted into adulthood for >80% of JFM patients, with associated impairments in physical functioning and mood. At follow-up, one-half of the sample met full criteria for adult fibromyalgia.
Chronic widespread musculoskeletal pain in childhood is common, affecting 6% to 7% of school-aged children,1–4 and often encountered in pediatric primary care.5–7 For a subset of patients, usually adolescent females, symptoms initially viewed by physicians and parents as temporary “growing pains” may be persistent (beyond 3 months), accompanied by headaches, fatigue, and sleep difficulties, and be medically unexplained. Furthermore, high levels of physical impairment, school absences, and emotional distress are reported,8–11 often prompting families to seek medical attention. Such patients may be referred to numerous pediatric specialists (eg, neurology, gastroenterology, rheumatology, pain medicine) before symptoms of juvenile-onset fibromyalgia (JFM) are finally identified. Until recently, there was little published research on JFM (also known as juvenile primary fibromyalgia syndrome). Despite some continuing debate,12 JFM is now increasingly recognized as a pain syndrome (such as pediatric migraine, functional abdominal pain), and more is known about its proper diagnosis and management.13–16 Although some progress has been made in the recognition and treatment of JFM, very little is known about long-term prognosis. Physicians often reassure their patients (with little scientific evidence) that they will likely “outgrow” symptoms. This outcome may be the case for nontreatment-seeking or less severely affected patients,17,18 but some studies have documented that JFM is indeed a chronic condition for a majority of adolescents who seek medical care.19–21
There are relatively few prospective studies of adolescent patients with JFM; previous studies were limited by small sample sizes (N ≤ 50), and they usually assessed only physical symptoms at 1- to 3-year follow-up.19,21 One published study, based on a subsample of our current study, included a healthy comparison group and assessed both physical and emotional functioning in late adolescence (∼4-year follow-up).20 To the best of our knowledge, no studies have thus far comprehensively examined long-term physical and psychosocial functioning, health care use, and educational/vocational outcomes as adolescents with JFM transition into young adulthood. Pediatric primary care providers and specialists are therefore unable to provide adequate patient education regarding planning for medical care and psychosocial support as these patients begin to make the crucial life decisions typical of early adulthood (eg, establishing independence from their families, planning for college). The objectives of the current long-term study of 94 adolescent JFM patients (and a smaller cohort of healthy control subjects [matched for age and gender]) were to: (1) determine the prognosis of JFM by assessing the proportion of patients who met criteria for adult fibromyalgia (FM) in their early adult years; (2) examine pain characteristics, physical functioning, mood symptoms, health care utilization, and living circumstances (education, vocation, marital status, sources of financial support, and living situation) of the JFM group compared with control subjects at follow-up; and (3) compare those with FM, those who have subclinical FM (sc-FM) symptoms, and healthy control subjects on pain, physical, and psychological functioning. On the basis of our previous research,20 we hypothesized that the majority of the JFM group (>70%) would continue to have fibromyalgia symptoms at follow-up. In addition, we anticipated that the JFM group would have significantly higher levels of physical and psychological impairment than healthy control subjects at follow-up, and that the most severely affected (active FM group) would have the highest levels of impairment.
Methods
Participants
For this study, we re-contacted 144 participants (100 patients previously diagnosed with JFM by a pediatric rheumatologist and 44 healthy control subjects) who were enrolled in our earlier studies investigating psychological treatments for and psychosocial factors related to JFM from 2002 to 2010.15,22,23 Inclusion criteria for JFM patients at initial enrollment included: (1) age 11 to 18 years; (2) diagnosis of primary JFM according to the criteria of Yunus and Masi24; and (3) no underlying rheumatologic conditions (eg, systemic lupus erythematosus, juvenile idiopathic arthritis). A smaller cohort of healthy control subjects was originally recruited as part of a substudy of peer relationships in JFM and were selected from classroom rosters of JFM patients; they were matched based on closest birth date, age, gender, and having no chronic illness.22 We have continued to follow up this group of matched contemporaneous control subjects because they serve as a useful normative reference point for comparing developmental milestones, such as going to college and getting married. Criteria for inclusion in this follow-up study were that individuals were enrolled in 1 of our previous studies based on the aforementioned inclusion criteria and they were at least 19 years old.
Procedure
Participants were contacted by telephone to obtain verbal consent, and if they agreed, signed informed consent was obtained by mail. Participants received a unique login code and password to access a secure Web site to complete study questionnaires. Thereafter, an in-person visit was scheduled for a trained examiner (postdoctoral fellow) to administer the tender-point examination. Retention in this long-term study was maximized by requesting contact information for 2 family members/close friends to reach the participant in case they moved and permission to contact these individuals if needed; an annual newsletter to enhance engagement; minimally burdensome assessments (Web-based surveys and home visits); and modest incentives (gift cards) for participation. This study was approved by the Cincinnati Children’s Hospital Medical Center institutional review board and conducted in accordance with current ethical standards for human subject research.
Measures
Background and Demographic Characteristics
Demographic information, including information about marital status, educational, and vocational background, was obtained, as well as current living situation and sources of financial support.
Pain Intensity, Pain Locations, and Symptom Severity
An 11-point numeric rating scale based on the Brief Pain Inventory25,26 was used to obtain ratings of average pain intensity (in the past week) (0 = “no pain” to 10 = “pain as bad as you can imagine”). Participants also reported whether they had experienced pain over the past 3 months. The Widespread Pain Index (WPI) and symptom severity (SS) scale27 adapted for self-report were completed by patients to gather comprehensive information about fibromyalgia symptoms at follow-up. On the WPI, participants indicated up to 19 body areas in which they experienced pain during the past week. Higher scores represent a greater number of pain locations (range: 0–19). The SS scale assesses cardinal (eg, fatigue, waking unrefreshed, cognitive symptoms) and other somatic (eg, dizziness, numbness, irritable bowel, nausea) symptoms associated with FM. The severity of each cardinal FM symptom was rated by participants on a 4-point Likert scale. Participants then endorsed (on a checklist) whether they experienced 40 somatic items within the previous week. Based on the number of symptoms endorsed, the following ratings were assigned: 0 = no symptoms, 1 = few symptoms, 2 = moderate symptoms, or 3 = great deal of symptoms. The SS score comprises the sum of the 3 cardinal symptoms with the rating of somatic symptom severity (final range: 0–12).
Tender-Point Examination
At the in-person visit, a trained psychology pain research fellow administered a standard tender-point examination by using manual thumb palpation of 18 predefined body sites to determine the number of painful sites (pain in 11 of 18 sites is required for a classification of FM).1,28,29 Fellows were trained in conducting standardized tender-point assessments by a senior fibromyalgia researcher (Dr Arnold) on the team, and their accuracy was confirmed by reliability checks with the study rheumatologist (Dr Ting).
Determination of FM Status
The most stringent criteria were used, including both American College of Rheumatology 1990 and 2010 suggested criteria,27,29 to determine whether participants met criteria for adult FM at follow-up. Patients were considered to have FM if they had: (1) WPI score ≥7 and SS score ≥5, or WPI score of 3 to 6 and SS score ≥9; (2) symptom duration of at least 3 months; (3) no underlying medical condition that would otherwise explain the pain; and (4) reported pain in 11 of 18 tender points. They were classified as having sc-FM symptoms if they continued to experience pain and ≥1 of the cardinal symptoms (fatigue, sleep difficulty, and cognitive symptoms) but did not meet the full criteria for FM described earlier. Patients were considered “pain free” if they reported no pain and were not using any FM medication.
Physical Function and Perceived Health Status
The 36-item Short-Form Health Survey, version 2,30 was used to evaluate impairment in physical function (physical function subscale) and all other domains of perceived health status (including general health, social, emotional functioning, and role functioning). This survey is a self-report instrument designed for individuals ≥14 years of age that is frequently used to assess pain-related disability in adult patients with FM.31 Scores were transformed according to norm-based scoring (mean ± SD t score: 50 ± 10), with lower scores reflecting poorer functioning.
Anxiety and Depressive Symptoms
The Beck Anxiety Inventory and the Beck Depression Inventory (second revision) are well-validated, brief, self-report instruments used to assess mood in older adolescents and adults.32,33 Participants were asked to rate the severity of each mood symptom on a 4-point Likert scale. Higher scores on each instrument reflect greater symptom severity (range: 0–63).
Health Care Utilization
Participants reported their use of health care services in the past 3 months on a questionnaire modified from our previous research.34 Items included the number of outpatient visits (primary care, specialty care, and counselor/psychologist visits) and emergency department visits within the past year.
Analytic Plan
All data were entered and analyzed by using SPSS version 20 (IBM SPSS Statistics, IBM Corporation, Armonk, NY). First, descriptive data on demographic characteristics, physical and psychological symptoms, medication usage, and health care utilization were computed separately for the JFM group (n = 94) and healthy control subjects (n = 33). These groups were then compared by using independent-samples t tests for all continuous outcome variables. Effect sizes (Cohen’s d) and confidence intervals were computed for group differences.
Using the criteria listed for FM diagnosis described earlier, the JFM group was then further divided into 2 groups: (1) those who met active criteria for adult FM (FM group); and (2) those who had subclinical symptoms (sc-FM group) or were pain free. The subclinical and the pain-free group were combined because there were very few pain-free individuals (n = 14), and the majority of these reported continued sleep difficulty and/or cognitive symptoms. The FM group and the sc-FM group were then compared with healthy control subjects by using analysis of variance models.
Missing data (7% missing American College of Rheumatology thumb palpation) were handled via multiple imputation with auxiliary correlates.35,36 Due to the multiple comparisons in this study, a number of adjustments were made to control for spurious findings. For 1-way analysis of variance models, the least significant difference post hoc mean comparison technique was used to best control for per-comparison type I errors. If the assumption of homogeneity of independent variable group variances was not met, the Games-Howell post hoc mean comparison technique was used.37–39 To address the additional concern of possible family-wise type I error inflation based on several statistical tests performed on data from the same dataset, the false discovery rate was used; this rate has also been shown to best control type I errors without negatively affecting statistical power.40
Results
Sample Characteristics
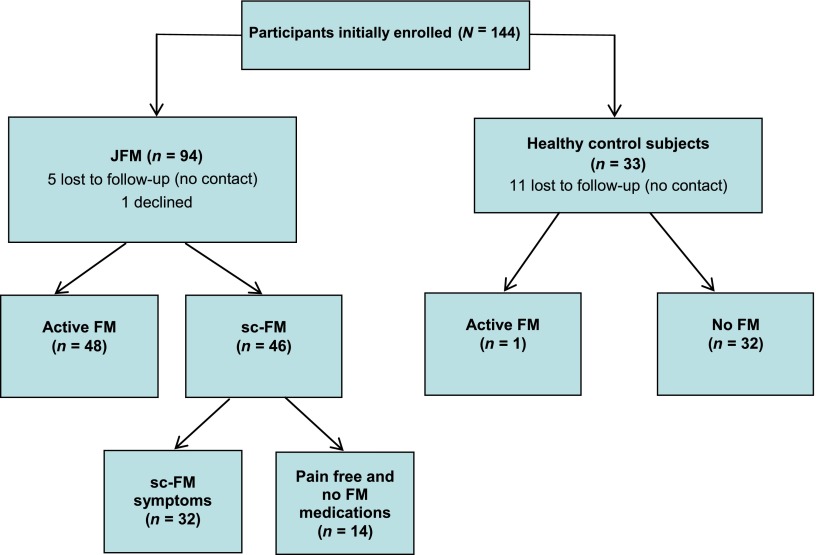
Of the 144 participants in the original cohort, 127 completed the follow-up study (88.2% retention; 94 JFM patients and 33 healthy control subjects). Retention in the JFM group was higher (94%) than in the healthy control group (75%). There were no significant differences between participants and dropouts based on age or baseline socioeconomic status, pain, or depressive symptoms. The mean duration of time elapsed from initial assessment to follow-up was 5.9 years. The mean age of the cohort at initial assessment was 15.1 ± 1.7 years and at follow-up was 21.6 ± 1.9 years. The majority of participants were female (95.6%) and white (86.7%), with no significant age and gender differences between JFM and healthy control subjects. The JFM patients reported that their symptoms began at a mean age of 12.7 ± 2.6 years. Of the 94 patients with JFM, 48 (51.1%) met the criteria for FM at follow-up. The remaining patients (n = 32 [34.0%]) reported subclinical symptoms, and only 14 participants (14.9%) were pain free but continued to experience sleep or cognitive difficulties (Fig 1). Of note, 1 healthy control subject was diagnosed with FM at follow-up; this participant was retained in the control group for analyses.
FIGURE 1.
Study flow diagram.
Comparisons Between JFM Sample and Healthy Control Subjects
Pain, Physical Impairment, and Emotional Functioning
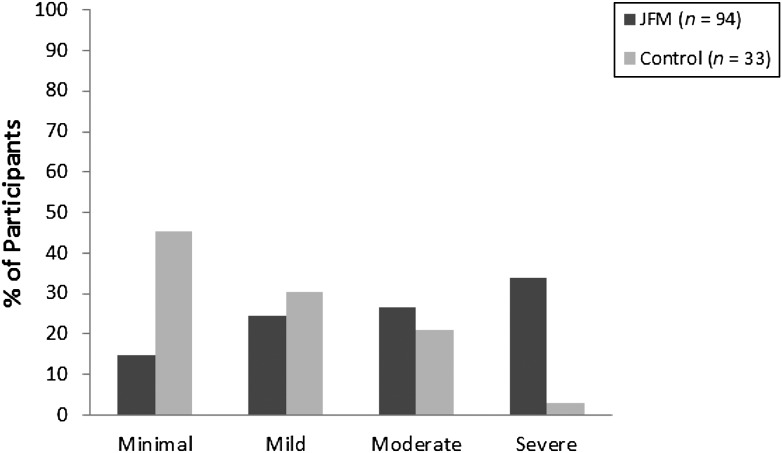
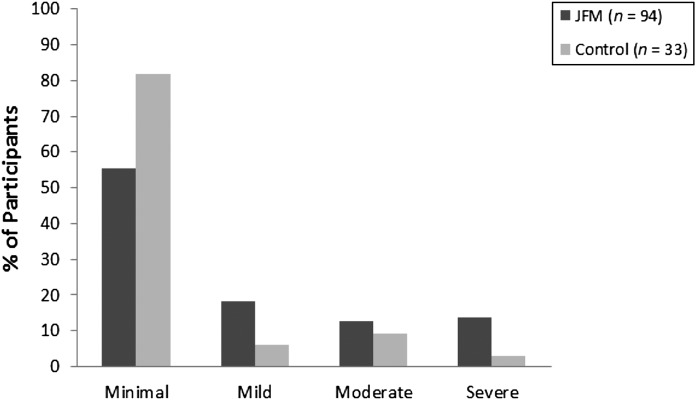
Mean pain levels were significantly higher in the JFM group compared with the healthy control subjects (Table 1). Similarly, patients with JFM reported significantly more pain locations, with the most common being neck/jaw/shoulder (n = 81 [86.2%]), back (n = 80 [85.1%]), leg (n = 55 [58.5%]), and hip (n = 43 [45.7%]). Participants with JFM had significantly poorer physical functioning, and lower perceived health status and role functioning across physical, social, and emotional domains, and higher levels of anxiety and depressive symptoms than control subjects. In the JFM group, 60% of participants reported moderate to severe levels of anxiety and 26.6% had moderate to severe depressive symptoms compared with 24.2% and 12.1%, respectively, in the healthy control group (Figs 2 and 3).
TABLE 1.
Comparison of Control Subjects Versus JFM Patients on Pain, Perceived Health Status, Mood, and Health Care Utilization
| Variable | Control Group (n = 33) | JFM Group (n = 94) | P | 95% CIa |
|---|---|---|---|---|
| Pain | ||||
| Pain intensity, NRS (0–10) | 0.88 ± 1.71 | 3.79 ± 2.25 | <.001 | 0.94 to 1.80 |
| No. of pain locations | 1.21 ± 1.34 | 3.80 ± 1.59 | <.001 | 1.87 to 5.24 |
| Perceived health status (SF-36; t scoreb) | ||||
| Physical functioning | 52.44 ± 8.86 | 45.44 ± 9.24 | <.001 | 0.36 to 1.17 |
| Physical role | 51.36 ± 8.44 | 42.94 ± 10.24 | <.001 | 0.45 to 1.27 |
| Bodily pain | 52.67 ± 9.12 | 40.05 ± 8.85 | <.001 | 0.98 to 1.85 |
| General health | 51.63 ± 8.82 | 35.09 ± 9.28 | <.001 | 1.35 to 2.26 |
| Vitality | 50.41 ± 9.45 | 39.31 ± 9.97 | <.001 | 0.71 to 1.55 |
| Social functioning | 48.92 ± 10.57 | 40.60 ± 11.75 | <.001 | 0.32 to 1.13 |
| Emotional role | 48.34 ± 11.46 | 40.91 ± 12.90 | <.01 | 0.19 to 0.99 |
| Mental health | 50.01 ± 10.20 | 41.29 ± 11.43 | <.001 | 0.37 to 1.19 |
| Mood | ||||
| Anxiety, BAI (0–60) | 9.58 ± 7.13 | 20.86 ± 12.42 | <.001 | 0.58 to 1.41 |
| Depression, BDI (0–60) | 7.19 ± 8.26 | 15.05 ± 11.46 | <.001 | 0.44 to 1.26 |
| Health care utilization (within the past year) | ||||
| All outpatient visits (physician + mental health) | 6.09 ± 10.40 | 11.54 ± 17.02 | <.01 | 1.24 to 2.90 |
| Emergency department | 0.24 ± 0.44 | 0.80 ± 1.21 | <.01 | 1.44 to 7.55 |
| Medications | ||||
| No. of current medications | 0.33 ± 0.78 | 1.40 ± 1.60 | <.001 | 2.03 to 8.76 |
| n (%) | n (%) | |||
| Antidepressants | 1 (3.03) | 38 (40.40) | ||
| Pain medications | 6 (18.18) | 38 (40.40) | ||
| Anticonvulsants | 0 (0) | 15 (16.00) | ||
| Opioid analgesics | 2 (6.06) | 5 (5.30) | ||
Data are presented as mean ± SD or n (%). BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; SF-36, 36-item Short-Form Health Survey, version 2.
Confidence interval (CI) for Cohen’s d.
The mean norm-based t score was 50 ± 10.
FIGURE 2.
Proportion of study subjects with minimal, mild, moderate, and severe anxiety.
FIGURE 3.
Proportion of study subjects with minimal, mild, moderate, and severe depressive symptoms.
Health Care Utilization and Medications
Patients in the JFM group had significantly more outpatient visits, emergency department visits, and used more medications compared with control subjects (Table 1). The most commonly used medications by JFM patients were those used to treat FM and related symptoms and included antidepressants, pain medications, and anticonvulsants.
Marital Status, Living Situation, and Financial Support
Patients with JFM were more than twice as likely to be married, separated, or divorced compared with healthy control subjects and also more than twice as likely to have ≥1 child (Table 2). Consistent with the higher rates of marriage in the JFM group, they were more likely to live with a spouse/significant other than in a dormitory or with roommates. A greater proportion of healthy control subjects were enrolled in or had completed college (75.8%) than JFM patients (61.7%).
TABLE 2.
Living Situation, Education, Marital Status, and Sources of Financial Support of Healthy Control Subjects Versus JFM Patients
| Variable | Control (n = 33) | JFM (n = 94) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Living situation | ||||
| With parents | 13 | 39.4 | 39 | 41.5 |
| With roommate/in dormitory | 14 | 42.4 | 31 | 33.0 |
| With spouse/significant other | 3 | 9.1 | 17 | 18.1 |
| Alone | 3 | 9.1 | 7 | 7.4 |
| Years of education | ||||
| Seventh to eighth grade | 0 | 0.0 | 1 | 1.1 |
| High school/GED | 5 | 15.2 | 24 | 25.5 |
| Vocational, trade, associate’s degree | 3 | 9.1 | 11 | 11.7 |
| Some college/bachelor’s/graduate degree | 25 | 75.8 | 58 | 61.7 |
| Marital status | ||||
| Single | 30 | 90.9 | 73 | 77.7 |
| Married/divorced/separated | 3 | 9.1 | 21 | 22.3 |
| Primary source of income | ||||
| Job/scholarship/student loans | 17 | 51.5 | 33 | 35.1 |
| Parents | 13 | 39.4 | 46 | 48.9 |
| Spouse/partner | 2 | 6.1 | 11 | 11.7 |
| Public assistance | 1 | 3.0 | 4 | 4.3 |
| Children | 3 | 9.1 | 20 | 22.2 |
GED, general equivalency diploma.
Years of Education: Please choose the highest grade of school you completed. │6th grade or less than sixth grade│7th to 8th grade│9th to 11th grade│High school diploma or GED. │Vocational, trade school, or associate’s courses after high school│Vocational, trade school, or associate’s degree│Courses toward 4-year college degree│Bachelor’s or 4-year college degree│Master’s or professional degree│
Marital Status: What is your marital status? │Single│Married│Divorced│Separated│Remarried│Widowed│
Primary Source of Income: What is your primary source of financial support? Job│Parents│Husband or wife’s job│Scholarship│Student loans│Disability│Other│
Comparisons Between the FM Group, the sc-FM Group, and Control Subjects
Within the sample of JFM patients, the FM group had significantly higher pain intensity and number of pain locations, poorer physical functioning, and greater anxiety and depressive symptoms compared with the sc-FM group (Table 3). However, there were no significant differences between the FM and sc-FM groups in terms of outpatient visits, emergency department visits, or medication use (Table 4). Healthy control subjects had significantly less pain and emotional symptoms, physical impairment, and health care use compared with both the FM and the sc-FM groups.
TABLE 3.
Comparisons of Patients With Active FM (n = 48), sc-FM (n = 44), and Control Subjects (n = 33) on Pain, Physical Functioning, and Mood
| Variable | Group | Group | P | 95% CI (Lower to Upper)a |
|---|---|---|---|---|
| Pain | ||||
| Pain intensity, NRS (0–10) | FM | Control | <.001 | 1.57 to 2.67 |
| FM | sc-FM | <.001 | 0.76 to 1.64 | |
| sc-FM | Control | <.001 | 0.35 to 1.50 | |
| No. of pain locations | FM | Control | <.001 | 2.22 to 6.75 |
| FM | sc-FM | <.05 | 1.03 to 2.58 | |
| sc-FM | Control | <.01 | 1.34 to 4.19 | |
| Physical functioning | ||||
| Physical functioning, SF-36 | FM | Control | <.001 | 0.77 to 1.74 |
| FM | sc-FM | <.001 | 0.47 to 1.32 | |
| sc-FM | Control | NS | –0.09 to 0.81 | |
| Mood | ||||
| Anxiety, BAI (0–60) | FM | Control | <.001 | 0.95 to 2.19 |
| FM | sc-FM | <.001 | 0.48 to 1.52 | |
| sc-FM | Control | <.001 | 0.02 to 1.13 | |
| Depression, BDI (0–60) | FM | Control | <.001 | 0.52 to 1.68 |
| FM | sc-FM | .001 | 0.19 to 1.21 | |
| sc-FM | Control | NS | –0.15 to 0.95 |
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; NRS, numeric rating scale; SF-36, 36-item Short-Form Health Survey, version 2.
Per-comparison type I error was controlled with the appropriate post hoc follow-up test (least significant difference was used if homogeneity of variance was met; otherwise, the Games-Howell post hoc mean comparison technique was used). Family-wise type I error was controlled by using the false discovery rate.
TABLE 4.
Comparisons of Patients With Active FM (n = 48), sc-FM (n = 44), and Control Subjects (n = 33) on Health Care Utilization and Medication Use
| Variable | Group | Group | P | 95% CI (Lower–Upper)a |
|---|---|---|---|---|
| Health care utilization (within the past year) | ||||
| Outpatient visits | FM | Control | .001 | 1.41 to 3.61 |
| FM | sc-FM | NS | 0.44 to 1.03 | |
| sc-FM | Control | NS | 0.94 to 2.45 | |
| Emergency department | FM | Control | .001 | 1.73 to 9.84 |
| FM | sc-FM | NS | 0.92 to 3.17 | |
| sc-FM | Control | NS | 0.17 to 1.02 | |
| No. of current medications | FM | Control | <.001 | 2.11 to 9.89 |
| FM | sc-FM | NS | 0.70 to 2.02 | |
| sc-FM | Control | .001 | 1.76 to 8.43 |
Per-comparison type I error was controlled with the appropriate post hoc follow-up test (least significant difference was used if homogeneity of variance was met; otherwise, the Games-Howell post hoc mean comparison technique was used). Family-wise type I error was controlled by using the false discovery rate.
Discussion
The current study is the largest prospective longitudinal study of JFM patients to date, allowing for the first time an in-depth understanding of physical, psychological, and social/vocational outcomes in young adulthood. We found that the majority of adolescent patients (>80%) with JFM seen in a pediatric specialty care setting continued to report persistent pain and other FM symptoms as they transitioned into young adulthood (ie, in their early 20s). Moreover, one-half of these patients met the full criteria for FM at follow-up, and these patients were the most impaired in all domains. These findings signify that persistent widespread musculoskeletal pain in childhood and adolescence lasting >3 months and requiring medical attention may signal the onset of a chronic pain disorder and should be taken seriously. Furthermore, the impact of JFM seems to persist into early adulthood and is associated with significant impairment in physical functioning, lower perceived health status, and higher health care utilization compared with age-matched healthy peers. Results of this study are consistent with the findings of recent longitudinal studies in adults with FM41,42 that show continuing FM symptoms and a fluctuating course, with improvement in some patients (similar to our sc-FM group). With regard to health care use, the JFM group reported a mean of 11.5 outpatient visits annually, 0.8 emergency visit, and 1.4 medications, which was significantly higher than control subjects but not as high as reported for older adult patients with FM (mean age: 50.4 years) who reported a mean of 20.3 outpatient visits annually, 1 emergency visit, and an average of 2 medications.43
Emotional distress (anxiety and depressive symptoms) was markedly elevated in the JFM group, with mean scores more than twice as high compared with control subjects. Perhaps not surprisingly, those who had active FM at follow-up had significantly more mood difficulties and physical impairment than those in the sc-FM group. Although the active FM group fared the poorest overall in terms of their physical and psychological functioning, results for the sc-FM group indicated that a negative physical and psychosocial impact of JFM was seen even for those with relatively fewer symptoms in adulthood.
In addition to the findings regarding persistent problems with physical functioning and mood difficulties, this is the first study to document emerging differences in marital and educational/vocational outcomes of JFM patients compared with their matched healthy peers. The JFM group was more likely to marry and have children at an early age compared with the healthy control group. In contrast, healthy control subjects were more likely to move out of the home and attend college. Although the characteristics of the JFM group were not strikingly different from national norms based on 2011 census data44,45 (22.3% of JFM patients reported being married/separated/divorced compared with 19.3% of US 19- to 24-year-olds, and 17.8% of JFM patients reported living with a spouse compared with 14.9% of US 19- to 24-year-olds), they were clearly dissimilar to their healthy peers in the current study. It should be noted that the control group was a contemporaneous control group closely matched on demographic characteristics at initial enrollment (mostly white, middle-income families). Given their similar demographic characteristics, the JFM group may be showing some divergence from norms for matched peers. Overall, our results are consistent with findings from prospective studies (eg, National Longitudinal Study of Adolescent Health46), which have shown that youth with chronic illnesses are at increased risk for poorer educational/vocational outcomes in adulthood and that those with psychiatric disorders in adolescence (National Comorbidity Study47,48) tend to have poorer educational attainment and marry at an earlier age.
Although the course of JFM seems chronic for many patients, not all patients are as severely affected or impaired by their symptoms. More research on identifying risk and resilience factors is needed. Meanwhile, early detection and appropriate referrals for multidisciplinary pain management (including medication, physical therapy, and behavioral treatments) can help minimize the impact of JFM and allow patients to engage in their everyday lives without debilitating effects. Much progress has been made over the last decade in the proper management of JFM. There is good evidence that cognitive-behavioral therapy focused on coping skills training, in addition to usual medical care, is a safe and effective intervention for adolescents with JFM.14,15 Exercise-based treatments are also beneficial,49 and pharmacologic trials testing the safety and efficacy of medications approved by the US Food and Drug Administration for adult FM in pediatric patients with JFM are underway (clinicaltrials.gov identifiers: pregabalin, NCT 01020474; duloxetine, NCT 01237587). These developments will undoubtedly enhance the treatment options currently available for adolescents with JFM.
Conclusions
This study highlights the chronic course of JFM for many adolescents who seek medical care for this chronic pain condition, and the functional, emotional, and social effects that can persist into adulthood. It also sheds more light on the variability in how JFM symptoms affect youth and underscores the need for greater study into early risk and resilience factors that might explain why some patients with JFM do better or worse than others during the crucial transition from adolescence to adulthood. Such research is critical to providing targeted, effective, and early treatment to youth affected by this chronic pain disorder.
Glossary
- FM
adult fibromyalgia
- JFM
juvenile fibromyalgia
- sc-FM
subclinical fibromyalgia
- SS
symptom severity
- WPI
Widespread Pain Index
Footnotes
Dr Kashikar-Zuck (principal investigator) designed and conceptualized the study, drafted the manuscript, supervised all aspects of the implementation of the study, consolidated manuscript revisions from all coauthors, and submitted the final document; Dr Cunningham (postdoctoral fellow) conducted the study assessments, assisted in the interpretation of findings, drafted portions of the initial manuscript, and critically reviewed/revised the manuscript; Dr Sil (postdoctoral fellow) conducted the study assessments, assisted in the interpretation of findings, drafted portions of the initial manuscript, and critically reviewed/revised the manuscript; Dr Bromberg (psychology resident) conducted the literature review for the manuscript, drafted portions of the manuscript, and critically reviewed/revised the manuscript; Dr Lynch-Jordan (coinvestigator) provided clinical supervision for the psychological assessments, monitored safety and risk of study participants, assisted with conceptualizing the focus of the manuscript, and critically reviewed/revised the manuscript; Mr Strotman (clinical research coordinator) managed all aspects of data collection, data entry, data cleaning, initial analysis, and preparation of tables and figures for the manuscript; Dr Peugh (study biostatistician) completed all statistical analyses for the manuscript, wrote the analysis plan and assisted with the interpretation of data for the results section, and critically reviewed/revised the manuscript; Dr Noll (coinvestigator and developmental psychologist) assisted in the conceptualization and design of the study and selection of instruments, provided guidance on retention strategies for this long-term study, and critically reviewed/revised the manuscript; Dr Ting (coinvestigator and pediatric rheumatologist expert in the assessment of juvenile fibromyalgia) initially diagnosed the patients by using standard criteria during adolescence, assisted in the conceptualization and design of the study, trained staff in the medical assessment of fibromyalgia, and critically reviewed/revised the manuscript; Dr Powers (coinvestigator) assisted in the conceptualization and design of the study and selection of instruments, and critically reviewed/revised the manuscript; Dr Lovell (coinvestigator) assisted in the conceptualization and design of the study and critically reviewed/revised the manuscript; and Dr Arnold (coinvestigator and adult fibromyalgia researcher) assisted in the conceptualization and design of the study, trained study staff in clinical examinations to confirm the diagnosis of adult fibromyalgia, and critically reviewed/revised the manuscript. All authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (grants R01AR054842 and K24AR056687 to Dr Kashikar-Zuck). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Buskila D, Press J, Gedalia A, et al. Assessment of nonarticular tenderness and prevalence of fibromyalgia in children. J Rheumatol. 1993;20(2):368–370 [PubMed] [Google Scholar]
- 2.Clark P, Burgos-Vargas R, Medina-Palma C, Lavielle P, Marina FF. Prevalence of fibromyalgia in children: a clinical study of Mexican children. J Rheumatol. 1998;25(10):2009–2014 [PubMed] [Google Scholar]
- 3.Gerloni V, Ghirardini M, Fantini F. Assessment of nonarticular tenderness and prevalence of primary fibromyalgia syndrome in healthy Italian schoolchildren. Arthritis Rheum. 1998;41(9):1405 [Google Scholar]
- 4.Mikkelsson M, Salminen JJ, Kautiainen H. Non-specific musculoskeletal pain in preadolescents. Prevalence and 1-year persistence. Pain. 1997;73(1):29–35 [DOI] [PubMed] [Google Scholar]
- 5.Konijnenberg AY, De Graeff-Meeder ER, Kimpen JL, van der Hoeven J, Buitelaar JK, Uiterwaal CS, Pain of Unknown Origin in Children Study Group . Children with unexplained chronic pain: do pediatricians agree regarding the diagnostic approach and presumed primary cause? Pediatrics. 2004;114(5):1220–1226 [DOI] [PubMed] [Google Scholar]
- 6.Perquin CW, Hunfeld JA, Hazebroek-Kampschreur AA, et al. Insights in the use of health care services in chronic benign pain in childhood and adolescence. Pain. 2001;94(2):205–213 [DOI] [PubMed] [Google Scholar]
- 7.Holm S, Ljungman G, Söderlund A. Pain in children and adolescents in primary care; chronic and recurrent pain is common. Acta Paediatr. 2012;101(12):1246–1252 [DOI] [PubMed] [Google Scholar]
- 8.Kashikar-Zuck S, Johnston M, Ting TV, et al. Relationship between school absenteeism and depressive symptoms among adolescents with juvenile fibromyalgia. J Pediatr Psychol. 2010;35(9):996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashikar-Zuck S, Parkins IS, Graham TB, et al. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clin J Pain. 2008;24(7):620–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. J Pain. 2002;3(5):412–419 [DOI] [PubMed] [Google Scholar]
- 11.Reid GJ, Lang BA, McGrath PJ. Primary juvenile fibromyalgia: psychological adjustment, family functioning, coping, and functional disability. Arthritis Rheum. 1997;40(4):752–760 [DOI] [PubMed] [Google Scholar]
- 12.Zernikow B, Gerhold K, Bürk G, et al. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften . Definition, diagnosis and therapy of chronic widespread pain and so-called fibromyalgia syndrome in children and adolescents. Systematic literature review and guideline [in German]. Schmerz. 2012;26(3):318–330 [DOI] [PubMed] [Google Scholar]
- 13.American Pain Society Guideline for the Management of Fibromyalgia Syndrome Pain in Adults and Children. Glenview, IL: American Pain Society; 2005 [Google Scholar]
- 14.Degotardi PJ, Klass ES, Rosenberg BS, Fox DG, Gallelli KA, Gottlieb BS. Development and evaluation of a cognitive-behavioral intervention for juvenile fibromyalgia. J Pediatr Psychol. 2006;31(7):714–723 [DOI] [PubMed] [Google Scholar]
- 15.Kashikar-Zuck S, Ting TV, Arnold LM, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012;64(1):297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walco GA, Ilowite NT. Cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. J Rheumatol. 1992;19(10):1617–1619 [PubMed] [Google Scholar]
- 17.Buskila D, Neumann L, Hershman E, Gedalia A, Press J, Sukenik S. Fibromyalgia syndrome in children—an outcome study. J Rheumatol. 1995;22(3):525–528 [PubMed] [Google Scholar]
- 18.Mikkelsson M. One year outcome of preadolescents with fibromyalgia. J Rheumatol. 1999;26(3):674–682 [PubMed] [Google Scholar]
- 19.Gedalia A, García CO, Molina JF, Bradford NJ, Espinoza LR. Fibromyalgia syndrome: experience in a pediatric rheumatology clinic. Clin Exp Rheumatol. 2000;18(3):415–419 [PubMed] [Google Scholar]
- 20.Kashikar-Zuck S, Parkins IS, Ting TV, et al. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford). 2010;49(11):2204–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel DM, Janeway D, Baum J. Fibromyalgia syndrome in children and adolescents: clinical features at presentation and status at follow-up. Pediatrics. 1998;101(3 pt 1):377–382 [DOI] [PubMed] [Google Scholar]
- 22.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Rheum. 2007;57(3):474–480 [DOI] [PubMed] [Google Scholar]
- 23.Kashikar-Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. J Rheumatol. 2005;32(8):1594–1602 [PubMed] [Google Scholar]
- 24.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28(2):138–145 [DOI] [PubMed] [Google Scholar]
- 25.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138 [PubMed] [Google Scholar]
- 26.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318 [DOI] [PubMed] [Google Scholar]
- 27.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62(5):600–610 [DOI] [PubMed] [Google Scholar]
- 28.Swain NF, Kashikar-Zuck S, Graham TB, Prahalad S. Tender point assessment in juvenile primary fibromyalgia syndrome. Arthritis Rheum. 2005;53(5):785–787 [DOI] [PubMed] [Google Scholar]
- 29.Wolfe F, Smythe HA, Yunus MB, et al. Report of the Multicenter Criteria Committee . The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33(2):160–172 [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483 [PubMed] [Google Scholar]
- 31.Wittink H, Turk DC, Carr DB, Sukiennik A, Rogers W. Comparison of the redundancy, reliability, and responsiveness to change among SF-36, Oswestry Disability Index, and Multidimensional Pain Inventory. Clin J Pain. 2004;20(3):133–142 [DOI] [PubMed] [Google Scholar]
- 32.Beck A, Steer R. Manual for the Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation; 1990 [Google Scholar]
- 33.Beck A, Steer R, Brown G. BDI-II Manual. San Antonio, TX: The Psychological Corporation; 1996 [Google Scholar]
- 34.Ho IK, Kashikar-Zuck S, Kotagal U, Tessman C, Jones BA. Healthcare utilization and indirect burden among families of pediatric patients with chronic pain. J Musculoskeletal Pain. 2008;16(3):155–164 [Google Scholar]
- 35.Enders CK. Applied Missing Data Analysis. Guilford Press; 2010 [Google Scholar]
- 36.Graham JW. Missing Data: Analysis and Design. New York, NY: Springer; 2012 [Google Scholar]
- 37.Anderson NH. Empirical Direction in Design and Analysis. Mahwah, NJ: Lawrence Erlbaum Associates; 2001 [Google Scholar]
- 38.Keppel G, Wickens TD. Design and Analysis: A Researcher's Handbook. Upper Saddle River, NJ: Pearson Prentice Hall; 2004
- 39.Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. New York, NY: Taylor & Francis; 2004 [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57(1):289–300 [Google Scholar]
- 41.Fitzcharles MA, Costa DD, Pöyhiä R. A study of standard care in fibromyalgia syndrome: a favorable outcome. J Rheumatol. 2003;30(1):154–159 [PubMed] [Google Scholar]
- 42.Walitt B, Fitzcharles MA, Hassett AL, Katz RS, Häuser W, Wolfe F. The longitudinal outcome of fibromyalgia: a study of 1555 patients. J Rheumatol. 2011;38(10):2238–2246 [DOI] [PubMed] [Google Scholar]
- 43.Robinson RL, Kroenke K, Williams DA, et al. Longitudinal observation of treatment patterns and outcomes for patients with fibromyalgia: 12-month findings from the Reflections Study. Pain Med. 2013;14(9):1400–1415 [DOI] [PubMed]
- 44.US Census Bureau. Marital status of the population by sex and age: 2011. In: America's Families and Living Arrangements: 2011 Table 1A. Available at: www.census.gov/hhes/families/data/cps2011.html. Accessed December 23, 2013 [Google Scholar]
- 45.US Census Bureau. Living arrangements of persons 15 years old and over by age and sex: 2011. In: America's Families and Living Arrangements: 2011 Table A2. Available at: www.census.gov/hhes/families/data/cps2011.html. Accessed December 23, 2013 [Google Scholar]
- 46.Maslow GR, Haydon A, McRee AL, Ford CA, Halpern CT. Growing up with a chronic illness: social success, educational/vocational distress. J Adolesc Health. 2011;49(2):206–212 [DOI] [PMC free article] [PubMed]
- 47.Forthofer MS, Kessler RC, Story AL, Gotlib IH. The effects of psychiatric disorders on the probability and timing of first marriage. J Health Soc Behav. 1996;37(2):121–132 [PubMed] [Google Scholar]
- 48.Kessler RC, Heeringa S, Lakoma MD, et al. Individual and societal effects of mental disorders on earnings in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2008;165(6):703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens S, Feldman BM, Bradley N, et al. Feasibility and effectiveness of an aerobic exercise program in children with fibromyalgia: results of a randomized controlled pilot trial. Arthritis Rheum. 2008;59(10):1399–1406 [DOI] [PubMed] [Google Scholar]