Abstract
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that modulate key physiological processes ranging from neurotransmission to cancer signaling. These receptors are activated by the neurotransmitter, acetylcholine, and the tobacco alkaloid, nicotine. Recently, the gene cluster encoding the α3, α5 and β4 nAChR subunits received heightened interest after a succession of linkage analyses and association studies identified multiple single nucleotide polymorphisms (SNPs) in these genes that are associated with an increased risk for nicotine dependence and lung cancer. It is not clear whether the risk for lung cancer is direct or an effect of nicotine dependence, as evidence for both scenarios exist. Here, we summarize the body of work implicating nAChRs in the pathogenesis of lung cancer, with special focus on the clustered nAChR subunits and their emerging role in this disease state.
Keywords: Nicotine Addiction, Lung Cancer, Nicotinic Acetylcholine Receptors, CHRNA5/A3/B4 gene cluster
Introduction
Tobacco use is the leading cause of preventable mortality around the world, resulting in more than 5 million deaths per year (WHO 2009). Approximately 600,000 of these deaths are due to second-hand smoke, with one third of the adult population exposed to second-hand smoke globally. In the United States, overall tobacco use has been declining but approximately 46 million adults still smoked in 2008 (CDC 2009). If current trends persist, tobacco may kill a billion people by the end of this century.
The list of diseases caused by tobacco use is expanding, according to a recent Surgeon General’s report on the health effects of smoking (HHS 2004). A causal relationship was reported between active smoking and cardiovascular diseases, respiratory diseases, reproductive disorders, and several types of cancers including cancers of the lung, bladder, cervix, esophagus, kidney, larynx, mouth, pancreas, stomach as well as leukemia.
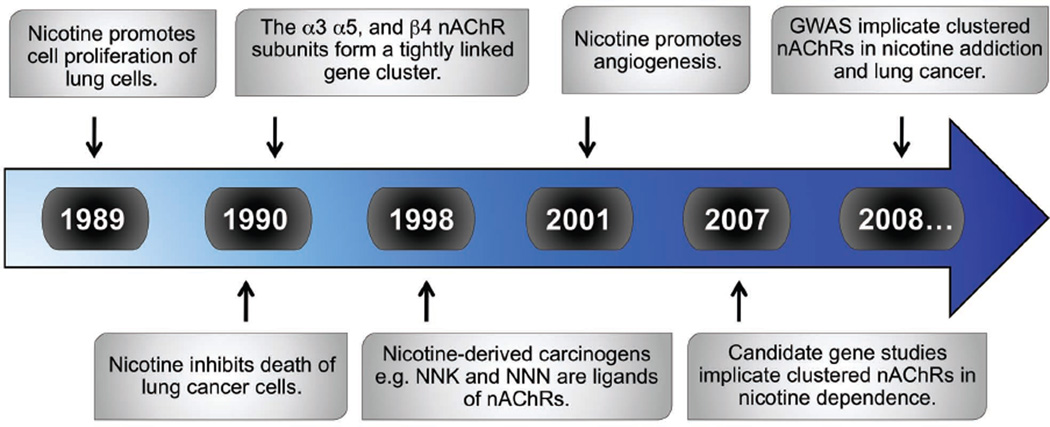
Cigarette smoke contains 4000 chemicals, 250 of which are known to be harmful, and at least 50 of which are carcinogens (Shields 2002). The most potent of these carcinogens are polycyclic aromatic hydrocarbons and nicotine metabolites such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitrosonornicotine (NNN). These nitrosamines form DNA adducts that cause mutations leading to cancer (Hecht and Hoffmann 1988). In the following sections, we review evidence accumulated through the years (see Timeline) showing that nicotine, itself, promotes lung cancer through its interaction with nAChRs.
Timeline. Key events implicating nAChRs in lung cancer etiology.
nAChR, nicotinic acetylcholine receptor; NNK [4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone]; NNN, [N-nitrosonornicotine] GWAS, genome wide association study.
Nicotinic acetylcholine receptors
nAChRs are a heterogeneous family of ligand-gated cation channels activated by the endogenous neurotransmitter acetylcholine (ACh) and exogenous chemicals such as nicotine and its metabolites. nAChRs were the first receptors to be characterized at the biochemical, biophysical, molecular, and pharmacological levels and have served as prototypes for all other ligand-gated ion channels including those activated by 5-hydroxytryptamine (5-HT3), γ-aminobutyric acid (GABAA and GABAC), and glycine (Le Novere and Changeux 1995, Taly et al 2009). Ligand binding induces a conformational change causing the channel to open thereby allowing the flow of Na+, K+, and Ca2+ ions down their electrochemical gradients. The propensity of nAChRs to flux intracellular calcium levels is important in the activation of downstream signaling cascades (Fucile 2004).
nAChRs can be classified into two main categories: muscle or neuronal receptors. Muscle nAChRs are expressed primarily in skeletal neuromuscular junctions and are composed of the α1, β1, δ, and ε or γ subunits (McGehee and Role 1995). In contrast, neuronal nAChRs were originally cloned from neuronal-like cell lines and brain cDNA libraries, hence their name, and are expressed throughout the nervous system where they increase neuronal excitability and facilitate synaptic transmission (Albuquerque et al 2009, Dani and Bertrand 2007, McGehee and Role 1995). Twelve neuronal nAChR subunits have been identified, namely α2-α10 and β2-β4 (Boyd 1997, Gotti et al 2006, Patrick et al 1993). Expression of these subunits has also been observed in many other cell types including endothelial cells, gastrointestinal tissue, glia, immune cells, keratinocytes, and lung tissue (Arredondo et al 2001, Battaglioli et al 1998, Gahring et al 2004, Gahring and Rogers 2006, Kawashima and Fujii 2003, Macklin et al 1998, Maus et al 1998, Nguyen et al 2000, Spindel 2003, Wang et al 2001, Wessler and Kirkpatrick 2008).
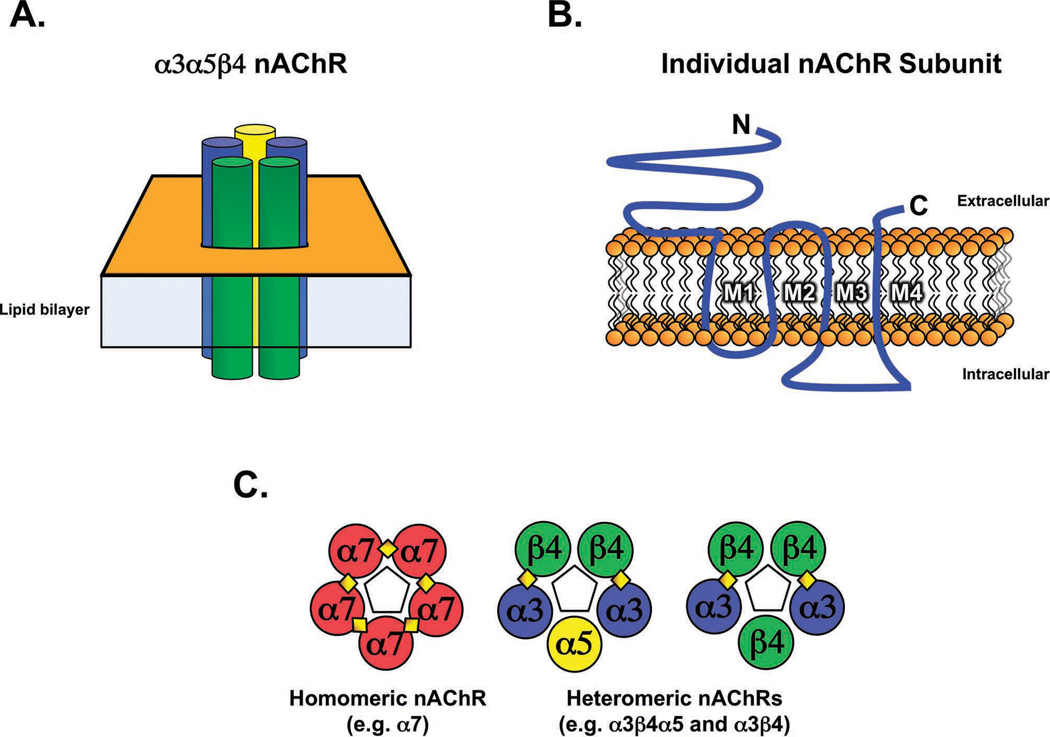
nAChRs are integral membrane proteins composed of five subunits symmetrically arranged around a central pore (Figure 1A) (Corringer et al 2000). Each nAChR subunit consists of a large extracellular amino-terminal domain, four transmembrane domains, a cytoplasmic loop of variable length between the third and fourth transmembrane domains, and a short extracellular carboxy-terminal domain (Figure 1B) (Unwin 2005). The large extracellular domain of α subunits contain adjacent cysteines important for ligand binding whereas β subunits lack these residues (Albuquerque et al 2009). Unlike other α subunits, however, α5 does not contribute to ligand binding as it is missing a key tyrosine residue (Karlin 2002). Importantly though, incorporation of the α5 subunit into a mature receptor does alter receptor biophysical properties such as increasing the calcium conductance (Gerzanich et al 1998).
Figure 1. Structure of the nAChR.
A.) Schematic representation illustrating the pentameric arrangement of subunits in an assembled nAChR. B.) Conserved domains of a nAChR subunit including the amino (N) and carboxy (C) terminals, transmembrane segments (M1–M4), and the intracellular loop. C.) Assembly of heteromeric and homomeric nAChR subtypes. Individual nAChR subunits are represented as colored circles, with diamonds representing ligand-binding sites. Pentagons in the center of each pentamer represent the pore region.
The combination of different nAChR subunits can lead to the formation of a vast array of nAChR subtypes. The α2 – α6 subunits can form heteromeric receptors with the β2 -β4 subunits while the α7, α8 and α9 subunits can form homomeric receptors that are blocked by α-bungarotoxin (Couturier et al 1990, Elgoyhen et al 1994, Schoepfer et al 1990). In addition, α9 can form a heteromeric receptor with α10 (Elgoyhen et al 2001, Lustig et al 2001) and α7 can form a heteromeric receptor with β2 (Liu et al 2009). Each of these receptor subtypes has distinct electrophysiological and pharmacological properties (Albuquerque et al 2009, Boyd 1997, Gerzanich et al 1997, Role and Berg 1996).
The functional diversity by the nAChR family offers abundant prospects for the design of novel therapeutics. As such, nAChRs are being actively investigated as drug targets for nervous system disorders including Alzheimer’s disease, anxiety, attention deficit hyperactivity disorder (ADHD), depression, epilepsy, pain, Parkinson’s disease, schizophrenia, Tourette’s syndrome, and nicotine addiction (Arneric et al 2007, Lloyd and Williams 2000, Romanelli et al 2007).
The α3/α5/β4 nAChR subunit gene cluster
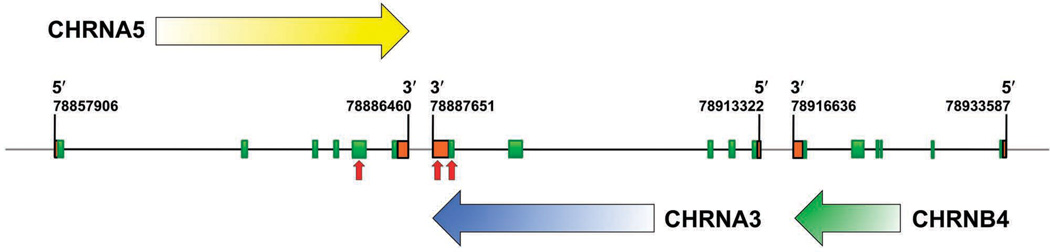
In recent years, a series of linkage analyses, candidate-gene association studies, and genome-wide association studies (GWAS) pointed to a possible role for the α3, α5, and β4 nAChR subunits in nicotine addiction as well as lung cancer (Amos et al 2008, Berrettini et al 2008, Bierut et al 2008, Caporaso et al 2009, Freathy et al 2009, Hung et al 2008, Pillai et al 2009, Portugal and Gould 2008, Saccone et al 2009a, Sasaki et al 2009, Schlaepfer et al 2007, Spitz et al 2008, Stevens et al 2008, Thorgeirsson et al 2008, Wacholder et al 2008, Weiss et al 2008). The genes that encode the α3, α5, and β4 nAChR subunits lie in a genomic cluster in strong linkage disequilibrium with each other (Figure 2) (Boulter et al 1990). These three genes encode a predominant nAChR subtype expressed in the peripheral nervous system (PNS) (Leonard and Bertrand 2001).
Figure 2. The human nAChR α3/α5/β4 gene cluster.
Green boxes represent exons and oragne boxes represent untranslated regions. Black lines located between green boxes represent introns while gray lines represent intragenic regions. The boundaries for each gene are labeled with corresponding Genbank annotations. Horizontal arrows indicate the direction of transcription. Vertical red arrows indicate SNPs associated with nicotine dependence and lung cancer.
The function of the clustered subunits can be gleaned from knockout (KO) animal studies. These studies have shown that the α3 subunit is necessary for survival, with homozygous KO mice dying perinatally due to multiorgan dysfunction (Xu et al 1999b). α3 heterozygous (+/−) mice have enlarged bladders, causing bladder infection, dribbling urination, and urinary stones – a phenotype resembling that of a rare human condition called megacystis-microcolon-intestinal hypoperistalsis syndrome. Patients with this disease also do not appear to express α3 mRNA (Richardson et al 2001). α3 heterozygous mice also display extreme mydriasis and lack of pupil contraction in response to light, with loss of bladder contraction in response to nicotine (Xu et al 1999c). Furthermore, α3 heterozygous mice are partially resistant to nicotine-induced seizures compared to wild-type littermates (Salas et al 2004a). In contrast, α5 and β4 KO mice are both viable and lack any gross abnormalities (Wang et al 2002, Wang et al 2003, Xu et al 1999c). However, α5 and β4 KO mice do exhibit autonomic dysfunction and are less sensitive to nicotine. Mice lacking α5 are also more susceptible to experimentally induced inflammatory bowel disease (Orr-Urtreger et al 2005) while β4 KO mice display less anxiety in behavioral tests (Salas et al 2003).
The observation that the α3, α5, and β4 genes are co-expressed and co-regulated in many cell types in the nervous system is consistent with the hypothesis that their expression is due to coordinated transcriptional regulation. The three subunits are highly expressed in the PNS as well as in several regions of the brain including the brain stem, cerebellum, hippocampus, interpeduncular nucleus, medial habenula, pineal gland, and the ventral tegmental area (VTA) (Flora et al 2000b, Gahring et al 2004, Klink et al 2001, Perry et al 2002, Quick et al 1999, Salas et al 2003, Salminen et al 2004, Turner and Kellar 2005, Xu et al 1999b, Zoli et al 2002). Furthermore, mRNA levels of the three genes are coordinately up-regulated during neural development and differentiation (Corriveau and Berg 1993, Levey et al 1995, Levey and Jacob 1996, Zhou et al 1998).
Efforts have been made to understand the regulatory mechanisms governing the expression of the clustered nAChR subunit genes. Sequencing of the individual gene promoters has revealed that each promoter lacks classical CAAT and TATA boxes (Boulter et al 1990). Instead, the promoters are GC-rich and contain several binding sites for the transcription factor, Sp1. Sp1 regulates transcription of each of the clustered subunit genes through multiple binding sites in each individual promoter (Bigger et al 1997, Campos-Caro et al 1999, Campos-Caro et al 2001, Flora et al 2000a, Melnikova et al 2000, Melnikova and Gardner 2001, Terzano et al 2000, Valor et al 2002, Yang et al 1995). Chromatin immunoprecipitation (ChIP) experiments have confirmed Sp1 binding in the context of native chromatin for all three promoters (Benfante et al 2007, Scofield et al 2008). It is likely that Sp1 is involved in tethering the basal transcription machinery to the TATA-less nAChR subunit gene promoters (Pugh and Tjian 1991). In addition to the Sp1 regulation common to all three promoters, other transcription factors have been found to govern expression of the clustered genes either independently or coordinately including ASCL1, Brn-3a-c, c-Jun, hnRNPK, PHOX2A, Purα, Sox10, Sp3, Tst-1/Oct6/SCIP (Benfante et al 2007, Bigger et al 1997, Du et al 1997, Du et al 1998, Improgo et al 2010, Liu et al 1999, Melnikova et al 2000, Milton et al 1996, Yang et al 1994). Two regulatory elements have also been found that direct the expression of the clustered nAChR genes in a tissue-specific manner: β43´, found at the β4 3´-untranslated region, and CNR4, a conserved noncoding region located 20 kb upstream of β4 (Xu et al 2006). Recently, we showed that a 2.3-kb fragment of the β4 gene promoter directs spatially and developmentally regulated expression of a reporter gene in vivo (Bruschweiler-Li et al 2010). Whether this region also regulates expression of the α3 and α5 genes remains to be determined.
Role of nAChRs in nicotine addiction
Nicotine is one of the most widely consumed psychoactive drugs in the world and is the primary reinforcing chemical in tobacco (Stolerman and Jarvis 1995). Nicotine addiction is initiated upon nicotine-mediated activation of nAChRs in the mesolimbic dopaminergic (DAergic) pathway, known as the reward circuitry of the brain (Corrigall et al 1992, Dani and De Biasi 2001, Di Chiara 2000). DAergic neurons in this pathway originate in the VTA and project to the nucleus accumbens (NAc) and the prefrontal cortex. Activation of nAChRs expressed in the VTA ultimately causes an increase in the firing of DAergic neurons, resulting in an increase of DA release in the NAc (Calabresi et al 1989, Nisell et al 1994, Pidoplichko et al 1997, Pontieri et al 1996). Expression of α4- and β2-containing receptors in the VTA is necessary and sufficient for nicotine- mediated DA elevation in the NAc (Marubio et al 2003, Maskos et al 2005, Picciotto et al 1998, Pons et al 2008). α4β2* nAChRs are also critical for nicotine reward/reinforcement, sensitization, and tolerance (Picciotto et al 1998, Pons et al 2008, Tapper et al 2004, Tapper et al 2007). Elevation of DA levels in the NAc reinforces drug use and is critical for the onset and maintenance of nicotine dependence (Di Chiara and Imperato 1988). Conversely, inhibiting DA elevation via lesions or pharmacological blockade attenuates the rewarding effects of nicotine (Corrigall and Coen 1991).
Nicotine dependence is a consequence of both positive reinforcement as well as avoidance of the aversive effects of cessation (Kenny and Markou 2001). Smoking cessation produces withdrawal symptoms, which account for the high incidence of relapse in people attempting to quit smoking (Corrigall et al 1989, Kenny and Markou 2001). The withdrawal syndrome involves both mood-oriented (affective) as well as physical (somatic) symptoms (De Biasi and Salas 2008). α5- and β4-containing nAChRs as well as α7 nAChRs appear to be involved in the physical symptoms of withdrawal as somatic signs are diminished in α5, α7, and β4 KO mice (Jackson et al 2008, Salas et al 2004b, Salas et al 2007). Conversely, affective symptoms are absent in β2 KO mice but are readily observable in α5 and α7 KO mice (Jackson et al 2008, Portugal et al 2008).
Results of the aforementioned genetic studies also support the role of the α3, α5, and β4 subunits in nicotine dependence. In a candidate-gene study targeting 348 genes, smokers of European descent who developed nicotine dependence were compared to smokers who were not dependent (Saccone et al 2007). In this study, several SNPs associated with nicotine dependence were found within the α5/α3/β4 gene cluster. Of particular interest is the non-synonymous SNP, rs16969968, found in the fifth exon of the α5 gene. This polymorphism changes an aspartic acid residue into asparagine at position 398 (D398N) in the second intracellular loop of α5. Receptors expressing the aspartic acid variant show greater maximal response to nicotine, causing higher intracellular calcium levels (Bierut et al 2008). Individuals with one copy of the minor allele were found to have a 1.3-fold increased risk for nicotine dependence while individuals with two copies of this risk variant have almost a 2-fold increase in risk (Saccone et al 2007). rs16969968 was also found to be associated with pleasurable responses during smoking initiation among Caucasians (Sherva et al 2008).
Other SNPs highly correlated with rs16969968 also influence the risk for nicotine dependence such as rs1051730 found in exon 5 of α3 and rs578776 found in the α3 3´-untranslated region (Saccone et al 2007). The latter had an even stronger association with nicotine dependence. These same SNPs were associated with increased smoking intake in an independent study analyzing 219 European American families (Bierut et al 2008). Furthermore, these SNPs were associated with early onset smoking, a phenotype associated with more severe nicotine dependence in adults (Weiss et al 2008). rs1051730 was also found to be strongly associated with smoking quantity in an Icelandic population (Thorgeirsson et al 2008) and was associated with decreased likelihood of quitting during pregnancy in women of European descent (Freathy et al 2009). These studies provide compelling evidence for the role of the α5/α3/β4 gene cluster in nicotine dependence.
Role of nAChRs in lung cancer
Smoking is the major risk factor associated with lung cancer, the leading cause of cancer-related deaths for both men and women (ACS 2009). Lung cancer is also the second most common form of cancer in both sexes, with an overall five-year survival rate of 15%. The two major histopathological types of lung cancer are small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC). NSCLC can be subdivided into adenocarcinoma, squamous cell, bronchioalveolar, and large cell lung carcinoma. Greater than 95% of patients with SCLC have a history of cigarette smoking and five-year survival rates for these patients can reach as low as 2% (Jackman and Johnson 2005).
Several lines of evidence indicate that nAChRs play a role in lung carcinogenesis as discussed in the following sections. nAChRs are expressed in both normal and lung cancer cells (Improgo et al 2010, Lam et al 2007, Maneckjee and Minna 1990, Maus et al 1998, Sartelet et al 2008, Schuller 1989, Song et al 2003, Wang et al 2001). The clustered nAChR subunits, in particular, are over-expressed in SCLC (Improgo et al 2010). This over-expression appears to be regulated by achaete-scute complex homolog-1 (ASCL1)(Improgo et al 2010), a basic helix-loop-helix transcription factor that is also over-expressed in SCLC (Ball et al 1993). Transgenic mice that constitutively express ASCL1 and the SV40 large T antigen develop aggressive lung tumors with SCLC features (Linnoila et al 2000). Up-regulation of the clustered nAChRs by ASCL1 provides a mechanism by which the effects of nicotine and other nAChR ligands are potentiated in SCLC, contributing to the aggressiveness of this type of lung cancer (Improgo et al 2010). Additional evidence for a role of the clustered nAChR genes in lung cancer comes from the recent demonstration that the α3 subunit gene is a frequent target of aberrant DNA hypermethylation and silencing in lung cancer (Paliwal et al 2010).
nAChRs and cell proliferation
The various ligands that activate nAChRs promote the development and progression of lung cancer via different mechanisms. First, ACh is synthesized by and acts as an autocrine growth factor for SCLC (Song et al 2003). ACh has also been shown to activate signaling pathways vital for growth and differentiation of human epithelial cells (Grando 2008). Similarly, nicotine can induce cell proliferation in a manner reminiscent of classical growth factors activating cancer signaling pathways. Specifically, nicotine treatment has been shown to cause physical interactions between the retinoblastoma protein (Rb) and the signaling kinase Raf-1, leading to downstream events such as inactivation of cyclins and cyclin-dependent kinases, dissociation of the transcription factor E2F1 from Rb, binding of E2F1 to proliferative promoters causing their transcription, and entry into S-phase (Dasgupta and Chellappan 2006, Egleton et al 2008). In addition, nicotine treatment can increase the levels of growth factors such as brain-derived neurotrophic factor (BDNF), hepatocyte growth factor (HGF), plateletderived growth factor (PDGF), transforming growth factor alpha (TGF-α), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and vascular endothelial growth factor C (VEGF-C) as well as the corresponding growth factor receptors such as EGFR, HGFR, PDGFR, and VEGFR-2 (Conti-Fine et al 2000). Moreover, nicotine activation of EGFR appears to involve increases in intracellular calcium levels (Sher et al 1998). Nicotine also stimulates NSCLC cell proliferation by up-regulating fibronectin expression while down-regulating epithelial markers such as E-cadherin and β-catenin (Davis et al 2009, Zheng et al 2007b). Nicotine-induced fibronectin expression is associated with activation of the extracellular signal-regulated kinase (ERK) and the phosphoinositide 3-kinase (PI3-K)/mammalian target of rapamycin (mTOR) signaling pathways and is abrogated by treatment with the α7 nAChR antagonist, α-bungarotoxin (Zheng et al 2007a). This group also showed that nicotine induces NSCLC cell proliferation by stimulating the expression of the nuclear hormone receptor, peroxisome proliferator- activated receptor β/δ (PPARβ/δ), an effect that can be blocked by α-bungarotoxin, α7 nAChR short interfering RNA (siRNA), and PI3-K inhibitors (Sun et al 2009). Taken together, these results suggest that nicotine increases PPARβ/ gene expression through α7 nAChR–mediated activation of PI3K/mTOR signals leading to cell proliferation (Sun et al 2009, Zheng et al 2007a). Nicotine also promotes cell proliferation in other types of cancers: Nicotine promotes growth of gastric tumors by activating ERK and cyclooxygenase-2 and promotes growth of colon cancer via EGFR, c-Src, and 5-lipooxygenase-mediated signaling pathways (Shin et al 2004, Ye et al 2004).
nAChRs and apoptosis
John Minna’s group first showed that low concentrations of nicotine confer resistance to apoptosis in lung cancer cells (Maneckjee and Minna 1994). Since then, nicotine has been shown to inhibit apoptosis induced by various stress stimuli including UV radiation, oxidative stress, and exposure to opioids, Ca2+ ionophores, neurotoxins, and anticancer drugs (Egleton et al 2008, Zeidler et al 2007). This apoptotic inhibition appears to involve several signaling pathways. One mechanism involves phosphorylation and consequent activation of the anti-apoptotic protein, B cell lymphoma gene 2 (BCL2) by protein kinase Cα and phospholipase C (Mai et al 2003). Consistently, nicotine inactivates the pro-apoptotic functions of Bax and Bad (Jin et al 2004, Xin and Deng 2005). Another mechanism involves nicotine-mediated activation of Akt (also called protein kinase B), a serine-threonine kinase whose activation leads to apoptotic inhibition and tumorigenesis (Scheid and Woodgett 2001). Nicotine exposure causes site-specific phosphorylation of Akt at Thr308 and Ser473 as well as phosphorylation of downstream Akt substrates such as mTOR, FKHR, elf-4, GSK3B, tuberin, and S6K (West et al 2003). The use of pharmacological agents suggests that this process involves α3-containing nAChRs. In the same study, increased Akt activation was observed in lung cancer tissue from smokers. Further evidence implicating α3 in Akt signal transduction is a recent report demonstrating that small hairpin RNA-mediated depletion of the α3 subunit leads to a dramatic Ca2+ influx in a NSCLC cell line that was followed by activation of the Akt pathway (Paliwal et al 2010). In this study, NSCLC cells in which the α3 subunit was depleted were resistant to apoptosis-inducing drugs.
nAChRs and angiogenesis
Endothelial cells express nAChRs as well as key molecules for cholinergic signaling such as choline acetyltransferase and acetylcholinesterase (Macklin et al 1998, Wang et al 2001). In these cells, ACh is thought to act in an autocrine or paracrine manner to stimulate angiogenesis (Cooke and Ghebremariam 2008). Nicotine also functions as a pro-angiogenic agent, activating both physiologic and pathologic angiogenesis via the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways (Heeschen et al 2001). Analogous to angiogenic cytokines, nicotine promotes endothelial cell migration, proliferation, survival, tube formation, and nitric oxide production and can be as potent as fibroblast growth factor (Cooke and Ghebremariam 2008). Nicotine and its metabolite, cotinine, have also been shown to up-regulate the expression of VEGF in endothelial cells (Conklin et al 2002). In addition, second-hand smoke increases VEGF expression and elevates levels of circulating endothelial progenitor cells, promoting angiogenesis and tumor growth - an effect reduced by the non-selective nAChR antagonist mecamylamine (Zhu et al 2003). Even in the absence of exogenous nicotine, angiogenic processes stimulated by VEGF or FGF can be blocked by nAChR antagonists such as mecamylamine and hexamethonium and the α7-selective antagonist α-bungarotoxin (Cooke and Ghebremariam 2008). In lung cancer cells, nicotine also induces the expression of hypoxia-inducible factor-1 alpha (HIF-1α), a transcription factor that promotes hypoxia-induced angiogenesis (Zhang et al 2007).
nAChRs and the immune system
The function of nAChRs in immunity and cancer has two aspects. The first involves the complex interplay between the inflammatory effects of irritants in cigarette smoke and the anti-inflammatory effects of nicotine (Gahring and Rogers 2006). Chronic inflammation triggered by tobacco smoke has been shown to promote lung carcinogenesis (Takahashi et al 2010). Inflammation induced by cigarette smoke also promotes COPD, a disease associated with increased lung cancer risk (Grivennikov et al 2010, Punturieri et al 2009). Chronic inflammation increases cancer risk by influencing every stage of cancer from initiation, promotion, invasion, and metastasis via induction of oncogenic mutations and genomic instability, local immunosuppression, and angiogenesis (reviewed in (Grivennikov et al 2010). In contrast, nicotine itself appears to suppress immune function and has been shown to be protective against inflammatory diseases such as pneumonia and ulcerative colitis (Blanchet et al 2004, Rubin and Hanauer 2000, Shivji et al 2005). Suppression of the immune response by nicotine may impact immune surveillance, preventing the clearance of nascent tumor cells (Gahring and Rogers 2006, Grivennikov et al 2010).
The second aspect of nAChR function in immunity and cancer involves the production of autoantibodies against nAChRs in cancer patients with paraneoplastic syndromes (Gahring and Rogers 2006). In particular, antibodies against α3 nAChRs have been detected in the serum of SCLC patients that display autonomic neuropathy (Vernino et al 1998, Vernino et al 2000). Dysautonomia caused by these autoantibodies is characterized by symptoms such as impaired papillary light reflex, gastrointestinal dysmotility, and bladder dysfunction that are reminiscent of those observed in α3 heterozygous KO mice (McKeon et al 2009, Xu et al 1999a).
Carcinogenic nitrosamines as nAChR ligands
Nicotine-derived nitrosamines such as NNK and NNN activate nAChRs with varying affinities (Schuller and Orloff 1998). NNK preferentially activates α7 nAChRs while NNN has higher affinity for heteromeric nAChRs. Activation of nAChRs by these ligands promotes cell proliferation, apoptotic inhibition, and angiogenesis (Schuller 2009). NNK and NNN appear to stimulate distinct proliferative pathways in bronchial epithelial cells. NNK causes activation of the transcription factors GATA-3, NF-KB, and STAT-1 while NNN predominantly activates GATA-3 and STAT-1, effects that can be abolished by the nAChR antagonists α-bungarotoxin and mecamylamine, respectively (Arredondo et al 2006a). In SCLC cells, NNK promotes calcium influx, serotonin release, and activation of the PKC and Raf-1/MAPK pathway (Arredondo et al 2006b, Jull et al 2001, Schuller 1992). NNK has also been shown to activate the Akt pathway in vitro and inhibit apoptosis (West et al 2003). In the same study, increased Akt phosphorylation was found in the lungs of NNK-treated mice. These studies suggest that carcinogenic nitrosamines can initiate lung cancer via their genotoxic effects but can also promote lung cancer via nAChR-mediated mechanisms (Arredondo et al 2006a).
Risk alleles in lung cancer
Several SNPs found in the α5/α3/β4 gene cluster appear to influence the risk for lung cancer. In a large-scale GWAS involving approximately 317,000 SNPs in samples of European origin, the non-synonymous SNP, rs16969968, was found to be strongly associated with lung cancer (Hung et al 2008). This SNP was also found to increase the risk for lung adenocarcinoma in an Italian population (Falvella et al 2009). Hung and colleagues also showed that the increased risk for lung cancer was observed even in non-smokers, suggesting that the association is not simply a consequence of nicotine dependence. Another evidence for direct association is that rs16969968 did not increase the risk for other smoking-related cancers such as head and neck cancer.
The α3 exon 5 SNP, rs1051730, was also found to be associated with lung cancer (Hung et al 2008). Furthermore, in an independent GWAS, rs1051730 was found to be associated with lung cancer and was only weakly associated with nicotine dependence (Amos et al 2008). rs1051730 was also found to be associated with familial lung cancer even after adjustment for pack-years of cigarette exposure (Liu et al 2008). Another group also found rs1051730 to be associated with lung cancer and peripheral arterial disease (Thorgeirsson et al 2008). Taken together, these studies represent a strong convergence of genetic data implicating the α3/α5/β4 gene cluster in lung cancer.
One report, however, showed that the rs1051730 SNP was associated with both nicotine dependence and lung cancer but that there was no increased risk for lung cancer in lifetime never-smokers, suggesting that the association with lung cancer was an effect of nicotine dependence (Thorgeirsson et al 2008). Reasons for the conflicting data may include differences in populations, sample sizes, phenotypes used to assess nicotine dependence and instruments used to measure phenotypes (Greenbaum and Lerer 2009). For example, most of the studies were done in populations of European origins where the frequency of the rs16969968 allele is 37% whereas in African populations the frequency of this allele is significantly lower (Bierut et al 2008, Saccone et al 2009b).
Conclusions and perspectives
Given the number of carcinogens found in cigarettes, it is not surprising that smoking is the major risk factor associated with lung cancer. Hence, many mechanisms leading to cancer can be envisaged. One such mechanism involves the activation of nAChRs by nicotine and its metabolites, which subsequently engage cancer signaling pathways associated with cell proliferation, apoptotic inhibition and angiogenesis. Previous studies investigating the link between nAChRs and these pathways have implicated primarily the α7 nAChR. The recent deluge of genetic studies, however, suggests that other subtypes should be investigated, in particular, the α3/α5/β4 nAChR subtype. Our work demonstrating the over-expression of the clustered nAChR genes in SCLC and their regulation by ASCL1, a critical player in the pathogenesis of lung cancer, provides evidence for the role of the clustered nAChR genes in this disease (Improgo et al 2010). This is further substantiated by the recent finding of aberrant DNA hypermethylation and silencing of the α3 subunit gene in NSCLC (Paliwal et al 2010). The use of genetic approaches to investigate the non-synonymous SNP found in α5 as well as other SNPs found in the cluster should be fertile areas for future investigations.
Acknowledgments
Work in the authors’ laboratories is supported in part by grants NS030243 (PDG) and AA017656 (ART) from the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- ACS. Cancer Facts and Figures 2009. American Cancer Society. 2009:1–68. [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol. 2007;74:1092–1101. doi: 10.1016/j.bcp.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Nguyen VT, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. A receptor-mediated mechanism of nicotine toxicity in oral keratinocytes. Lab Invest. 2001;81:1653–1668. doi: 10.1038/labinvest.3780379. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Grando SA. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J Cancer Res Clin Oncol. 2006a;132:653–663. doi: 10.1007/s00432-006-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006b;20:2093–2101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- Ball DW, Azzoli CG, Baylin SB, Chi D, Dou S, Donis-Keller H, et al. Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc Natl Acad Sci U S A. 1993;90:5648–5652. doi: 10.1073/pnas.90.12.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglioli E, Gotti C, Terzano S, Flora A, Clementi F, Fornasari D. Expression and transcriptional regulation of the human alpha3 neuronal nicotinic receptor subunit in T lymphocyte cell lines. J Neurochem. 1998;71:1261–1270. doi: 10.1046/j.1471-4159.1998.71031261.x. [DOI] [PubMed] [Google Scholar]
- Benfante R, Flora A, Di Lascio S, Cargnin F, Longhi R, Colombo S, et al. Transcription factor PHOX2A regulates the human α3 nicotinic receptor subunit gene promoter. J Biol Chem. 2007;282:13290–13302. doi: 10.1074/jbc.M608616200. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger CB, Melnikova IN, Gardner PD. Sp1 and Sp3 regulate expression of the neuronal nicotinic acetylcholine receptor β4 subunit gene. J Biol Chem. 1997;272:25976–25982. doi: 10.1074/jbc.272.41.25976. [DOI] [PubMed] [Google Scholar]
- Blanchet MR, Israël-Assayag E, Cormier Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am J Respir Crit Care Med. 2004;169:903–909. doi: 10.1164/rccm.200210-1154OC. [DOI] [PubMed] [Google Scholar]
- Boulter J, O'Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, et al. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptorrelated gene family form a gene cluster. J Biol Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- Boyd RT. The molecular biology of neuronal nicotinic acetylcholine receptors. Crit Rev Toxicol. 1997;27:299–318. doi: 10.3109/10408449709089897. [DOI] [PubMed] [Google Scholar]
- Bruschweiler-Li L, Fuentes Medel YF, Scofield MD, Trang EBT, Binke SA, Gardner PD. Temporally- and spatially-regulated transcriptional activity of the nicotinic acetylcholine receptor β4 subunit gene promoter region. Neurosci. 2010;166:864–877. doi: 10.1016/j.neuroscience.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Caro A, Carrasco-Serrano C, Valor LM, Viniegra S, Ballesta JJ, Criado M. Multiple functional Sp1 domains in the minimal promoter region of the neuronal nicotinic receptor alpha5 subunit gene. J Biol Chem. 1999;274:4693–4701. doi: 10.1074/jbc.274.8.4693. [DOI] [PubMed] [Google Scholar]
- Campos-Caro A, Carrasco-Serrano C, Valor LM, Ballesta JJ, Criado M. Activity of the nicotinic acetylcholine receptor alpha5 and alpha7 subunit promoters in muscle cells. DNA Cell Biol. 2001;20:657–666. doi: 10.1089/104454901753340640. [DOI] [PubMed] [Google Scholar]
- Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Cigarette smoking among adults and trends in smoking cessation - United States, 2008. Centers for Disease Control and Prevention. 2009 [Google Scholar]
- Conklin BS, Zhao W, Zhong DS, Chen C. Nicotine and cotinine up-regulate vascular endothelial growth factor expression in endothelial cells. Am J Pathol. 2002;160:413–418. doi: 10.1016/S0002-9440(10)64859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Fine BM, Navaneetham D, Lei S, Maus AD. Neuronal nicotinic receptors in non-neuronal cells: new mediators of tobacco toxicity? Eur J Pharmacol. 2000;393:279–294. doi: 10.1016/s0014-2999(00)00036-4. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Ghebremariam YT. Endothelial nicotinic acetylcholine receptors and angiogenesis. Trends Cardiovasc Med. 2008;18:247–253. doi: 10.1016/j.tcm.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Herling S, Coen KM. Evidence for a behavioral deficit during withdrawal from chronic nicotine treatment. Pharmacol Biochem Behav. 1989;33:559–562. doi: 10.1016/0091-3057(89)90387-0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux J-P. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Berg DK. Coexpression of multiple acetylcholine receptor genes in neurons: quantification of transcripts during development. J Neurosci. 1993;13:2662–2671. doi: 10.1523/JNEUROSCI.13-06-02662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, et al. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–2328. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med. 2008;233:917–929. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Du Q, Tomkinson AE, Gardner PD. Transcriptional regulation of neuronal nicotinic acetylcholine receptor genes. A possible role for the DNA-binding protein Puralpha. J Biol Chem. 1997;272:14990–14995. doi: 10.1074/jbc.272.23.14990. [DOI] [PubMed] [Google Scholar]
- Du Q, Melnikova IN, Gardner PD. Differential effects of heterogeneous nuclear ribonucleoprotein K on Sp1- and Sp3-mediated transcriptional activation of a neuronal nicotinic acetylcholine receptor promoter. J Biol Chem. 1998;273:19877–19883. doi: 10.1074/jbc.273.31.19877. [DOI] [PubMed] [Google Scholar]
- Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–158. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvella FS, Galvan A, Frullanti E, Spinola M, Calabro E, Carbone A, et al. Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clin Cancer Res. 2009;15:1837–1842. doi: 10.1158/1078-0432.CCR-08-2107. [DOI] [PubMed] [Google Scholar]
- Flora A, Schulz R, Benfante R, Battaglioli E, Terzano S, Clementi F, et al. Transcriptional regulation of the human alpha5 nicotinic receptor subunit gene in neuronal and non-neuronal tissues. Eur J Pharmacol. 2000a;393:85–95. doi: 10.1016/s0014-2999(00)00040-6. [DOI] [PubMed] [Google Scholar]
- Flora A, Schulz R, Benfante R, Battaglioli E, Terzano S, Clementi F, et al. Neuronal and extraneuronal expression and regulation of the human alpha5 nicotinic receptor subunit gene. J Neurochem. 2000b;75:18–27. doi: 10.1046/j.1471-4159.2000.0750018.x. [DOI] [PubMed] [Google Scholar]
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Dunn D, Weiss R, Meyer EL, Rogers SW. Mouse strain-specific nicotinic acetylcholine receptor expression by inhibitory interneurons and astrocytes in the dorsal hippocampus. J Comp Neurol. 2004;468:334–346. doi: 10.1002/cne.10943. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS Journal. 2006;7:E885–E894. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Kuryatov A, Anand R, Lindstrom J. "Orphan" alpha6 nicotinic AChR subunit can form a functional heteromeric acetylcholine receptor. Mol Pharmacol. 1997;51:320–327. [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grando SA. Basic and clinical aspects of non-neuronal acetylcholine: biological and clinical significance of non-canonical ligands of epithelial nicotinic acetylcholine receptors. J Pharmacol Sci. 2008;106:174–179. doi: 10.1254/jphs.fm0070087. [DOI] [PubMed] [Google Scholar]
- Greenbaum L, Lerer B. Differential contribution of genetic variation in multiple brain nicotinic cholinergic receptors to nicotine dependence: recent progress and emerging open questions. Mol Psychiatry. 2009;14:912–945. doi: 10.1038/mp.2009.59. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- HHS. 2004 Surgeon General's Report - The Health Consequences of Smoking. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General. 2004 [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Improgo MRD, Schlichting NA, Cortes RY, Zhao-Shea R, Tapper AR, Gardner PD. ASCL1 regulates the expression of the CHRNA5/A3/B4 lung cancer susceptibility locus. Mol Cancer Res. 2010;8:194–203. doi: 10.1158/1541-7786.MCR-09-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman DM, Johnson B. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837–23844. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- Jull BA, Plummer HK, 3rd, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–717. doi: 10.1007/s004320100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DC, Girard L, Ramirez R, Chau W, Suen W, Sheriden S, et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638–4647. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Changeux JP. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3:203–223. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- Levey MS, Brumwell CL, Dryer SE, Jacob MH. Innervation and target tissue interactions differentially regulate acetylcholine receptor subunit mRNA levels in developing neurons in situ. Neuron. 1995;14:153–162. doi: 10.1016/0896-6273(95)90249-x. [DOI] [PubMed] [Google Scholar]
- Levey MS, Jacob MH. Changes in the regulatory effects of cell-cell interactions on neuronal AChR subunit transcript levels after synapse formation. J Neurosci. 1996;16:6878–6885. doi: 10.1523/JNEUROSCI.16-21-06878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila RI, Zhao B, DeMayo JL, Nelkin BD, Baylin SB, DeMayo FJ, et al. Constitutive achaete-scute homologue-1 promotes airway dysplasia and lung neuroendocrine tumors in transgenic mice. Cancer Res. 2000;60:4005–4009. [PubMed] [Google Scholar]
- Liu P, Vikis HG, Wang D, Lu Y, Wang Y, Schwartz AG, et al. Familial aggregation of common sequence variants on 15q24-25.1 in lung cancer. J Natl Cancer Inst. 2008;100:1326–1330. doi: 10.1093/jnci/djn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Melnikova IN, Hu M, Gardner PD. Cell type-specific activation of neuronal nicotinic acetylcholine receptor subunit genes by Sox10. J Neurosci. 1999;19:9747–9755. doi: 10.1523/JNEUROSCI.19-22-09747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, et al. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–929. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd GK, Williams M. Neuronal nicotinic acetylcholine receptors as novel drug targets. J Pharmacol Exp Ther. 2000;292:461–467. [PubMed] [Google Scholar]
- Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA. Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10) Genomics. 2001;73:272–283. doi: 10.1006/geno.2000.6503. [DOI] [PubMed] [Google Scholar]
- Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–439. [PubMed] [Google Scholar]
- Mai H, May WS, Gao F, Jin Z, Deng X. A functional role for nicotine in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 2003;278:1886–1891. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci U S A. 1990;87:3294–3298. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ. 1994;5:1033–1040. [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, et al. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol. 1998;54:779–788. doi: 10.1124/mol.54.5.779. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McKeon A, Lennon VA, Lachance DH, Fealey RD, Pittock SJ. Ganglionic acetylcholine receptor autoantibody: oncological, neurological, and serological accompaniments. Arch Neurol. 2009;66:735–741. doi: 10.1001/archneurol.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova IN, Lin HR, Blanchette AR, Gardner PD. Synergistic transcriptional activation by Sox10 and Sp1 family members. Neuropharm. 2000;39:2615–2623. doi: 10.1016/s0028-3908(00)00125-8. [DOI] [PubMed] [Google Scholar]
- Melnikova IN, Gardner PD. The signal transduction pathway underlying ion channel gene regulation by SP1-C-Jun interactions. J Biol Chem. 2001;276:19040–19045. doi: 10.1074/jbc.M010735200. [DOI] [PubMed] [Google Scholar]
- Milton NG, Bessis A, Changeux JP, Latchman DS. Differential regulation of neuronal nicotinic acetylcholine receptor subunit gene promoters by Brn-3 POU family transcription factors. Biochem J. 1996;317(Pt 2):419–423. doi: 10.1042/bj3170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Hall LL, Gallacher G, Ndoye A, Jolkovsky DL, Webber RJ, et al. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res. 2000;79:939–949. doi: 10.1177/00220345000790040901. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Kedmi M, Rosner S, Karmeli F, Rachmilewitz D. Increased severity of experimental colitis in alpha 5 nicotinic acetylcholine receptor subunit-deficient mice. NeuroReport. 2005;16:1123–1127. doi: 10.1097/00001756-200507130-00018. [DOI] [PubMed] [Google Scholar]
- Paliwal A, Vaissière T, Krais A, Cuenin C, Cros MP, Zaridze D, et al. Aberrant DNA Methylation Links Cancer Susceptibility Locus 15q25.1 to Apoptotic Regulation and Lung Cancer. Cancer Res. 2010;70:2779–2788. doi: 10.1158/0008-5472.CAN-09-4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J, Sequela P, Vernino S, Amador M, Luetje C, Dani JA. Functional diversity of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1993;98:113–120. doi: 10.1016/s0079-6123(08)62387-0. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5:e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav Brain Res. 2008;193:1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh BF, Tjian R. Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev. 1991;5:1935–1945. doi: 10.1101/gad.5.11.1935. [DOI] [PubMed] [Google Scholar]
- Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunitcontaining nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Richardson CE, Morgan JM, Jasani B, Green JT, Rhodes J, Williams GT, et al. Megacystis-microcolon-intestinal hypoperistalsis syndrome and the absence of the alpha3 nicotinic acetylcholine receptor subunit. Gastroenterology. 2001;121:350–357. doi: 10.1053/gast.2001.26320. [DOI] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Romanelli MN, Gratteri P, Guandalini L, Martini E, Bonaccini C, Gualtieri F. Central nicotinic receptors: structure, function, ligands, and therapeutic potential. ChemMedChem. 2007;2:746–767. doi: 10.1002/cmdc.200600207. [DOI] [PubMed] [Google Scholar]
- Rubin DT, Hanauer SB. Smoking and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:855–862. doi: 10.1097/00042737-200012080-00004. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009a;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster afects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009b;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The α3 and β4 nicotinic receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004a;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004b;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Sartelet H, Maouche K, Totobenazara JL, Petit J, Burlet H, Monteau M, et al. Expression of nicotinic receptors in normal and tumoral pulmonary neuroendocrine cells (PNEC) Pathol Res Pract. 2008;204:891–898. doi: 10.1016/j.prp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hikosaka Y, Okuda K, Kawano O, Yukiue H, Yano M, et al. CHRNA5 gene D398N polymorphism in Japanese lung adenocarcinoma. J Surg Res. 2009 doi: 10.1016/j.jss.2009.01.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, et al. The CHRNA5/A3/B4 Gene Cluster Variability as an Important Determinant of Early Alcohol and Tobacco Initiation in Young Adults. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain alphabungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Cell type specific, receptor-mediated modulation of growth kinetics in human lung cancer cell lines by nicotine and tobacco-related nitrosamines. Biochem Pharmacol. 1989;38:3439–3442. doi: 10.1016/0006-2952(89)90112-3. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Nitrosamine-induced lung carcinogenesis and Ca2+/calmodulin antagonists. Cancer Res. 1992;52:2723s–2726s. [PubMed] [Google Scholar]
- Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol. 1998;55:1377–1384. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Bruschweiler-Li L, Mou Z, Gardner PD. Transcription factor assembly on the nicotinic receptor beta4 subunit gene promoter. Neuroreport. 2008;19:687–690. doi: 10.1097/WNR.0b013e3282fbcef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E, Codignola A, Passafaro M, Tarroni P, Magnelli V, Carbone E, et al. Nicotinic receptors and calcium channels in small cell lung carcinoma. Functional role, modulation, and autoimmunity. Ann N Y Acad Sci. 1998;841:606–624. doi: 10.1111/j.1749-6632.1998.tb10993.x. [DOI] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with 'pleasurable buzz' during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields PG. Molecular epidemiology of smoking and lung cancer. Oncogene. 2002;21:6870–6876. doi: 10.1038/sj.onc.1205832. [DOI] [PubMed] [Google Scholar]
- Shin VY, Wu WK, Ye YN, So WH, Koo MW, Liu ES, et al. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis. 2004;25:2487–2495. doi: 10.1093/carcin/bgh266. [DOI] [PubMed] [Google Scholar]
- Shivji M, Burger S, Moncada CA, Clarkson ABJ, Merali S. Effect of nicotine on lung S-adenosylmethionine and development of Pneumocystis pneumonia. J Biol Chem. 2005;280:15219–15228. doi: 10.1074/jbc.M413946200. [DOI] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, et al. Acetycholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- Spindel ER. Neuronal nicotinic acetylcholine receptors: not just in brain. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1201–L1202. doi: 10.1152/ajplung.00251.2003. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Amos CI, Dong Q, Lin J, Wu X. The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst. 2008;100:1552–1556. doi: 10.1093/jnci/djn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Sun X, Ritzenthaler JD, Zhong X, Zheng Y, Roman J, Han S. Nicotine stimulates PPARbeta/delta expression in human lung carcinoma cells through activation of PI3K/mTOR and suppression of AP-2alpha. Cancer Res. 2009;69:6445–6453. doi: 10.1158/0008-5472.CAN-09-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Marks MJ, Lester HA. Nicotine responses in hypersensitive and knockout alpha 4 mice account for tolerance to both hypothermia and locomotor suppression in wild-type mice. Physiol Genomics. 2007;31:422–428. doi: 10.1152/physiolgenomics.00063.2007. [DOI] [PubMed] [Google Scholar]
- Terzano S, Flora A, Clementi F, Fornasari D. The minimal promoter of the human alpha 3 nicotinic receptor subunit gene. Molecular and functional characterization. J Biol Chem. 2000;275:41495–41503. doi: 10.1074/jbc.M006197200. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Kellar KJ. Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci. 2005;25:9258–9265. doi: 10.1523/JNEUROSCI.2112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Valor LM, Campos-Caro A, Carrasco-Serrano C, Ortiz JA, Ballesta JJ, Criado M. Transcription factors NF-Y and Sp1 are important determinants of the promoter activity of the bovine and human neuronal nicotinic receptor beta 4 subunit genes. J Biol Chem. 2002;277:8866–8876. doi: 10.1074/jbc.M110454200. [DOI] [PubMed] [Google Scholar]
- Vernino S, Adamski J, Kryzer TJ, Fealey RD, Lennon VA. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. 1998;50:1806–1813. doi: 10.1212/wnl.50.6.1806. [DOI] [PubMed] [Google Scholar]
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- Wacholder S, Chatterjee N, Caporaso N. Intermediacy and gene-environment interaction: the example of CHRNA5-A3 region, smoking, nicotine dependence, and lung cancer. J Natl Cancer Inst. 2008;100:1488–1491. doi: 10.1093/jnci/djn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Orr-Urtreger A, Chapman J, Rabinowitz R, Nachman R, Korczyn AD. Autonomic function in mice lacking alpha5 neuronal nicotinic acetylcholine receptor subunit. J Physiol. 2002;542:347–354. doi: 10.1113/jphysiol.2001.013456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Orr-Urtreger A, Chapman J, Rabinowitz R, Korczyn AD. Deficiency of nicotinic acetylcholine receptor beta 4 subunit causes autonomic cardiac and intestinal dysfunction. Mol Pharmacol. 2003;63:574–580. doi: 10.1124/mol.63.3.574. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, et al. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:1201–1209. doi: 10.1124/mol.60.6.1201. [DOI] [PubMed] [Google Scholar]
- Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for agedependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO report on the global tobacco epidemic, 2009: implementing smoke-free environments. World Health Organization. 2009 [Google Scholar]
- Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou C-N, et al. Megacystis, mydriasis, and ion channel defect in mice lacking the α3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1999a;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, et al. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1999b;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, et al. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999c;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Scott MM, Deneris ES. Shared long-range regulatory elements coordinate expression of a gene cluster encoding nicotinic receptor heteromeric subtypes. Mol Cell Biol. 2006;26:5636–5649. doi: 10.1128/MCB.00456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, McDonough J, Fyodorov D, Morris M, Wang F, Deneris ES. Characterization of an acetylcholine receptor alpha 3 gene promoter and its activation by the POU domain factor SCIP/Tst-1. J Biol Chem. 1994;269:10252–10264. [PubMed] [Google Scholar]
- Yang X, Fyodorov D, Deneris ES. Transcriptional analysis of acetylcholine receptor alpha 3 gene promoter motifs that bind Sp1 and AP2. J Biol Chem. 1995;270:8514–8520. doi: 10.1074/jbc.270.15.8514. [DOI] [PubMed] [Google Scholar]
- Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH. Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway. J Pharmacol Exp Ther. 2004;308:66–72. doi: 10.1124/jpet.103.058321. [DOI] [PubMed] [Google Scholar]
- Zeidler R, Albermann K, Lang S. Nicotine and apoptosis. Apoptosis. 2007;12:1927–1943. doi: 10.1007/s10495-007-0102-8. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tang X, Zhang ZF, Velikina R, Shi S, Le AD. Nicotine induces hypoxiainducible factor-1alpha expression in human lung cancer cells via nicotinic acetylcholine receptor-mediated signaling pathways. Clin Cancer Res. 2007;13:4686–4694. doi: 10.1158/1078-0432.CCR-06-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Ritzenthaler JD, Roman J, Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol. 2007a;37:681–690. doi: 10.1165/rcmb.2007-0051OC. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Ritzenthaler JD, Roman J, Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol. 2007b;37:681–690. doi: 10.1165/rcmb.2007-0051OC. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Deneris E, Zigmond RE. Differential regulation of levels of nicotinic receptor subunit transcripts in adult sympathetic neurons after axotomy. J Neurobiol. 1998;34:164–178. doi: 10.1002/(sici)1097-4695(19980205)34:2<164::aid-neu6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Zhu BQ, Heeschen C, Sievers RE, Karliner JS, Parmley WW, Glantz SA, et al. Second hand smoke stimulates tumor angiogenesis and growth. Cancer Cell. 2003;4:191–196. doi: 10.1016/s1535-6108(03)00219-8. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]