Abstract
Aim
Mast cells are important in experimental diabetes. Plasma levels of immunoglobulin E (IgE), tryptases, and chymases are inflammatory markers of human diabetes. Whether they also correlate with the risk of pre-diabetes, however, remains unknown.
Methods and results
A total of 260 subjects 55–75 years of age were grouped as normal glucose tolerance (NGT), isolated impaired fasting glucose (I-IFG), isolated impaired glucose tolerance (I-IGT), and mixed IFG/IGT. There were significant differences in plasma levels of high-sensitivity C-reactive protein (hsCRP) (P < 0.001) and IgE (P=0.003) among all subgroups of pre-diabetes, and chymase in I-IGT (P=0.043) and mixed IFG/IGT (P=0.037) subgroups compared with NGT group. High-sensitivity CRP was a risk factor in all subgroups of pre-diabetes; IgE was a risk factor of mixed IFG/IGT; and chymase was a risk factor of I-IGT and mixed IFG/IGT. Interactions between hsCRP and high waist circumference (WC), waist-to-hip ratio (WHR), or HOMA-β index, and interactions between IgE and high WC or tryptase levels all increased further the risk of developing I-IFG, I-IGT, or mixed IFG/IGT.
Conclusion
Plasma hsCRP, IgE, and chymase levels associate with pre-diabetes status. While hsCRP, IgE, and chymase are individual risk factors of pre-diabetes, interactions with metabolic parameters increased further the risk of pre-diabetes.
Keywords: Chymase, C-reactive protein, immunoglobulin E, pre-diabetes, tryptase
Introduction
Pre-diabetes refers to the intermediate states between normal glucose tolerance (NGT) and type 2 diabetes mellitus (type 2 DM) and is considered the precursor of type 2 DM (1). Isolated impaired fasting glucose (I-IFG), isolated impaired glucose tolerance (I-IGT), and mixed IFG/IGT are three categories of pre-diabetes (2). Increasing evidence suggests that I-IFG and I-IGT represent different populations with more or less overlapping subclinical characteristics and pathophysiological basis, and that, compared with I-IFG and I-IGT, mixed IFG/IGT seems to represent a more advanced stage of pre-diabetes that bears a distinctly higher risk of conversion to diabetes and other co-morbid diseases—although the precise mechanism for this conversion is unknown (2–7). Recent studies have focused on the inflammatory risk factors of pre-diabetes (8–12). Elevated plasma levels of inflammatory high-sensitivity C-reactive protein (hsCRP), a common risk factor and biomarker of cardiovascular diseases, may predict the development of type 2 DM (13,14). Mast cells are essential components of asthma and allergic responses (15,16), and we have shown that these cells play important roles in diet-induced obesity and type 2 DM in mice (17). One of the most popular mechanisms of mast cell activation is their release of histamine, the mast cell-specific serine proteases chymase, tryptase, proteoglycan, cytokines, and chemokines, by binding immunoglobulin E (IgE) to its high-affinity receptor FcεR1 on the cell surface (18–21). Many of these inflammatory mediators associate with diabetes (22 – 25). The current study examines whether IgE and mast cell proteases associate with inflammation and pre-diabetes status in a Chinese population from a pre-diabetes study.
Methods
Study population
The study is part of the Pre-Diabetes Intervention Project (PDIP), begun in 2008 at the School of Medicine, Huzhou Teachers College, Zhejiang, China. From July to August 2008, 3163 Chinese volunteers, 55 – 75 years of age, from three neighborhood communities in the city of Huzhou, were recruited to participate in a health-related risk factor survey. Subjects on medications, and subjects who had established DM (either type 1 or type 2 DM), cardiovascular disease, cerebrovascular disease, malignant disease, chronic liver disease, or kidney failure, were excluded. From September to December 2008, 1500 volunteers were invited for a fasting glucose test and a 2-hour oral glucose tolerance test (2h OGTT) as part of the pre-diabetes screening. Of the invited subjects, 1197 accepted the invitation and participated in both the fasting glucose test and 2h OGTT. Among 1197 volunteers, 807 (67.42%) were defined as NGT subjects, 267 (22.30%) as pre-diabetes subjects, and 123 (10.28%) as DM subjects. One year after the initial visit, 267 pre-diabetes subjects and 100 randomly-selected NGT subjects were invited for anthropometric measurements and clinical tests. A total of 260 subjects participated in the final study, among whom 71 subjects were NGT subjects and 189 were pre-diabetic, including 93 I-IFG subjects, 49 I-IGT subjects, and 47 mixed IFG/IGT subjects. This study was approved by the Huzhou City Ethics Committee, and all subjects gave written, informed consent prior to participating in the study.
Data collection
Demographic data (age and sex), anthropometric measurements (body weight and height, waist and hip circumferences), and blood pressure were collected from each participant when testing fasting glucose and 2h OGTT. The biochemical parameters were measured in the Clinical Biochemistry Unit of Huzhou First Hospital, a teaching hospital of the School of Medicine. Plasma chymase and tryptase levels were determined as described previously (26). Details of the study data collection have been reported elsewhere (25).
Clinical criteria
Pre-diabetes patients were grouped according to American Diabetes Association 2003 (ADA 2003) criteria (1). Pre-diabetes was defined as fasting plasma glucose (FPG) ≥5.6 and <7.0 mmol/L, or 2h OGTT ≥7.8 and <11.1 mmol/L. I-IGT classification indicates that the 2h OGTT level was between 7.8 and 11.0 mmol/L and the FPG level was less than 5.6 mmol/L; I-IFG classification indicates that the 2h OGTT level was less than 7.8 mmol/L and the FPG level was between 5.6 and 7.0 mmol/L; mixed IFG/IGT classification indicates that the 2h OGTT level was between 7.8 and 11.0 mmol/L and the fasting plasma glucose (FPG) level was between 5.6 and 7.0 mmol/L. Subjects were classified as having a normal glucose profile if FPG < 5.6 mmol/L and 2h OGTT <7.8 mmol/L.
Based on China 2006 Blood Pressure Control Criteria and China Prevention and Treatment Classification Recommendation on Dyslipidemia (27), hypertension was defined as systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) ≥ 140/90 mmHg, or as receiving blood pressure-lowering medications; high triglyceride (TG) was defined as a fasting plasma TG ≥ 1.70 mmol/L; low HDL-c as a fasting HDL-c ≤ 0.9 mmol/L; high total cholesterol (TC) as TC ≥5.72 mmol/L; and low LDL-c as a fasting LDL-c ≤3.64 mmol/L. Based on the China Obesity Task Group Recommendation (28), general obesity was classified as body mass index (BMI) ≥28 kg/m2, ≥ 80 cm in females or 85 cm in males. Waist-to-hip ratio (WHR) was classified as normal or abnormal according to the upper quartile (P75 = 0.92). Homeostasis model assessment–insulin resistance (HOMA-IR = value of FPG × value of fasting insulin/22.5) was classified as normal or abnormal according to the upper quartile (P75 = 2.23), and homeostasis model assessment-β cell function (HOMA-β = 20 × value of fasting insulin/(FPG–3.5)) was classified as normal or abnormal according to the bottom quartile (P25 = 43.05). Hyperinsulinemia was classified as normal or abnormal according to the upper quartiles (P75 = 9.28 mIU/L). High-sensitivity CRP was classified as normal or abnormal according to the upper quartile (P75 = 8.0 mg/L). IgE was classified as normal or abnormal according to the upper quartile (P75 = 55 IU/L). Chymase was classified as normal or abnormal according to the upper quartile (P75 = 26.42 μg/mL). Tryptase was classified as normal or abnormal according to the upper quartile (P75 = 2.65 ng/mL).
Statistical analysis
The mean and standard deviation (mean ± SD) of continuous and normal distributional variables, and median and interquartile range of continuous but skewed distributional variables, were used. Data were analyzed using one-way analysis of covariance (ANOVA), chi-square test, Kruskal–Wallis test, Mann–Whitney U test, or binary logistic model. All statistical analysis was conducted using SPSS statistical software (version 11.0).
Results
Population distribution
Basic characteristics of the 260 participants 55–75 years of age are shown in Table I. Of the participants, the average age of the 96 men was 68.05 ± 5.19 years, and the average age of the 164 women was 64.49 ± 6.04 years. There was a significant difference in age between men and women (t = 4.481, P <0.001); 71 (27.3%) were classified as NGT subjects, 93 (35.8%) as I-IFG subjects, 49 (18.8%) as I-IGT subjects, and 47 (18.1%) as mixed IFG/IGT subjects. One-way ANOVA, Kruskal–Wallis test, or chi-square test demonstrated that, compared with normoglycemic subjects, those with pre-diabetes generally had more adverse risk factor profiles with significantly worse fasting glucose, 2h OGTT, SBP, WC, WHR, fasting insulin, β-cell sensitivity (marked as HOMA-β index), and insulin resistance (marked as HOMA-IR index) (Table I). When the anthropometric and biochemical parameters were compared between pre-diabetes subgroups, only fasting glucose, 2h OGTT, and HOMA-β index were higher in mixed IFG/IGT than in IGT or IFG. No significant differences existed among the subgroups on other risk factor profiles (Table I).
Table I.
Biochemical and anthropometric parameters in 260 subjects with varying glucose status, grouped according to fasting and post-load glucose levels.
| Parameters | NG (n = 71) | I-IFG (n = 93) | I-IGT (n = 49) | Mixed IFG/IGT (n = 47) |
|---|---|---|---|---|
| Age (years)a | 65.13±6.17 | 65.29±6.13 | 66.9±5.64 | 64.63±5.89 |
| Sex (% male) | 21.1 | 28.6 | 31.3 | 39.1c |
| Fasting glucose (mmol/L)b | 4.98 (4.60–5.19) | 5.92 (5.86–5.99)c | 5.13 (5.02–5.23)c,d | 6.03 (5.93–6.12)c,e |
| 2h OGTT (mmol/L)b | 5.53 (5.25–5.83) | 5.93 (5.71–6.16)c | 8.84 (8.54–9.11)c, d | 9.01 (8.75–9.27)c, d |
| SBP (mmHg)a | 134.00±15.89 | 137.04±17.91 | 142.76±15.39c | 140.72±18.38c |
| DBP (mmHg)a | 77.93±7.41 | 77.48±9.25 | 80.85±9.28 | 80.63±9.16 |
| WC (cm)b | 79.61 (77.75–81.51) | 82.79 (80.73–84.91)c | 83.22 (80.77–85.74)c | 83.33 (81.10–85.61)c |
| HC (cm)b | 91.65 (90.05–93.28) | 93.07 (91.77–94.39) | 92.53 (91.15–93.93) | 93.25 (91.62–94.92) |
| WHR b | 0.87 (0.86–0.88) | 0.89 (0.87–0.91)c | 0.90 (0.88–0.92)c | 0.89 (0.88–0.91)c |
| BMI (kg/m2)a | 23.52±3.39 | 24.19±3.88 | 24.41±2.99 | 24.57±3.18 |
| TC (mmol/L)b | 4.80 (4.60–5.01) | 4.92 (4.73–5.13) | 5.10 (4.83–5.39) | 4.74 (4.42–5.09) |
| TG (mmol/L)b | 1.22 (0.79–1.79) | 1.44 (1.26–1.64) | 1.58 (1.33–1.87) | 1.41 (1.22–1.62) |
| HDL-c (mmol/L)b | 1.23 (1.06–1.42) | 1.26 (1.20–1.32) | 1.22 (1.16–1.29) | 1.13 (1.06–1.21)c, d |
| LDL-c (mmol/L)b | 2.43 (2.04–3.00) | 2.42 (2.29–2.56) | 2.53 (2.34–2.72) | 2.50 (2.32–2.70) |
| Fasting insulin (mU/L)b | 5.54 (4.82–6.37) | 6.00 (5.32–6.77) | 6.95 (5.99–8.05)c | 6.44 (5.69–7.29) |
| HOMA-β indexb | 83.86 (72.35–97.21) | 49.74 (43.98–56.26)c | 87.19 (74.11–102.59) | 51.21 (45.16–58.07)c, e |
| HOMA-IR indexb | 1.14 (0.75–1.88) | 1.58 (1.40–1.78)c | 1.58 (1.36–1.84)c | 1.72 (1.52–1.96)c |
2h OGTT = 2 hour oral glucose tolerance test; BMI = body mass index; DBP=diastolic blood pressure; FPG=fasting plasma glucose; HC = hip circumference; HDL-c=high-density lipoprotein cholesterol; HOMA=homeostasis model assessment; I-IFG=isolated impaired fasting glucose; I-IGT=isolated impaired glucose tolerance; LDL-c=low-density lipoprotein cholesterol; Mixed IFG/IGT = mixed impaired fasting glucose and impaired glucose tolerance; NG = normal glucose; SBP = systolic blood pressure; TC=total cholesterol; TG=triglyceride; WC=waist circumference; WHR=waist-to-hip ratio.
Variable is described using mean and standard deviation and tested using ANOVA.
Variable is described using median and interquartile range, and tested using the Kruskal –Wallis H test.
P < 0.05 compared with the normal glucosec, IFGd, and IGT groupse.
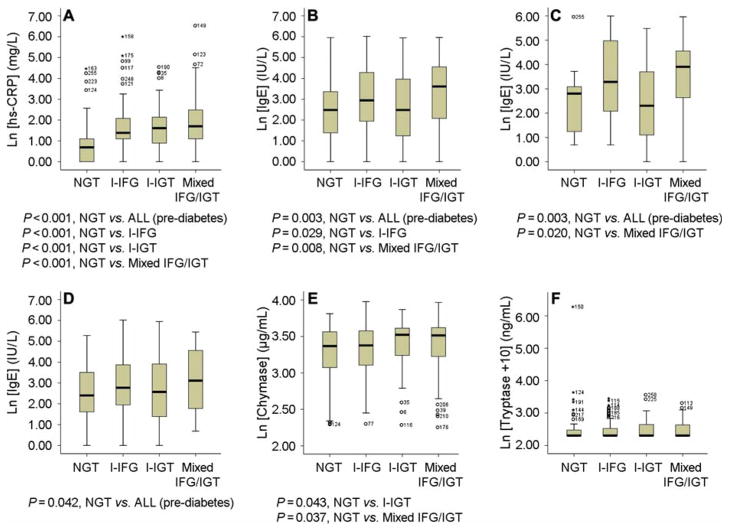
The Mann–Whitney U test was used to examine the differences in hsCRP, IgE, chymase, and tryptase between NGT subjects and those with different pre-diabetes status. High-sensitivity CRP was significantly increased in all hyperglycemia subgroups, versus NGT subjects (P < 0.001). Individual group comparison demonstrated that the I-IFG, I-IGT, and mixed IFG/IGT groups had a significantly higher hsCRP levels than the NGT group did (P <0.001, P < 0.001, and P <0.001, respectively) (Figure 1A). Plasma IgE levels also were increased significantly in all subgroups of hyperglycemia, versus the NGT group (P = 0.003). Individual group comparison showed that both the I-IFG and mixed IFG/IGT groups had significantly higher levels of IgE than did the NGT group (P = 0.029 and P = 0.008, respectively) (Figure 1B). Based on the upper quartile values (P75) of hsCRP (P75 = 8.0 mg/L), IgE (P75 = 55 IU/L), chymase (P75 = 26.42 μg/mL), and tryptase (P75 = 2.65 ng/mL) for normal and abnormal levels, we performed the chi-square test and found no significant differences in hsCRP distribution (chi-square = 0.517, P = 0.472), chymase distribution (chi-square = 2.235, P = 0.135), or tryptase distribution (chi-square = 1.622, P = 0.203), but significant differences in IgE distribution between men and women (18 IU/L (P25 = 5 IU/L, P75 = 95 IU/L) versus 14 IU/L (P25 = 5 IU/L, P75 = 48 IU/L)) (chi-square = 9.162, P = 0.002). This observation is consistent with the results of prior studies, that plasma IgE levels are offen significantly higher in men than in women—either in cases (e.g. glioma) or in controls (29)—although currently we have no explanation for these sex differences. We therefore compared plasma IgE levels according to sex. In men, plasma IgE levels were significantly increased in all subgroups of hyperglycemia versus the NGT group (P = 0.003). Among the hyperglycemia subjects, those with mixed IFG/IGT exhibited a significantly higher level of IgE than the NGT group (P = 0.020) (Figure 1C). In women, plasma IgE levels also were significantly increased in all subgroups of hyperglycemia, versus the NGT group (P = 0.042) (Figure 1D). I-IGT and mixed IFG/IGT groups exhibited a significantly higher level of plasma chymase than those in the NGT group (P = 0.043 and P = 0.037, respectively) (Figure 1E). Plasma tryptase levels were not significantly different, however, between any comparison groups (Figure 1F).
Figure 1.
Box plots of hsCRP, IgE, chymase, and tryptase among NGT patients and patients with different categories of pre-diabetes. A: Plasma hsCRP levels from NGT, I-IFG, I-IGT, and mixed IFG/IGT patients. B: Plasma IgE levels from the same four categories. C: Plasma IgE levels in male subjects. D: Plasma IgE levels in female subjects. E: Plasma chymase levels. F: Plasma tryptase levels. All data are mean ± SD. P < 0.05 was considered statistically significant; non-parametric Mann–Whitney U test. Non-significant comparisons are not shown.
The associations of plasma hsCRP, IgE, chymase, or tryptase with pre-diabetes
Among all tested variables, most are risk factors of both cardiovascular events and metabolic diseases—including age, sex, hypertension, body weight (BMI, WC, WHR), lipid profiles (TC, LDL, TG, HDL), and hsCRP. Binary logistic regression analysis showed that, before adjustment for age, sex, and BMI, WC (odds ratio (OR)=2.687, P = 0.002), WHR (OR=3.853, P = 0.001), HOMA-β index (OR=3.135, P = 0.003), and hsCRP (OR=4.387, P = 0.001) were all significant risk factors of I-IFG; hypertension (OR=2.644, P = 0.011), WHR (OR=3.437, P = 0.009), hsCRP (OR=5.327, P = 0.001), and chymase (OR=2.862, P = 0.019) were significant risk factors of I-IGT; and HOMA-β index (OR=2.463, P = 0.043), hsCRP (OR=5.505, P = 0.001), IgE (OR=2.957, P = 0.01), and chymase (OR=3.142, P = 0.01) were significant risk factors of mixed IFG/IGT. But none of the cholesterol lipid molecules, including total cholesterol (TC), triglyceride (TG), HDL, and LDL, associated with any of the pre-diabetes subgroups, probably due to the exclusion of patients with cardiovascular events with and without established diabetes in this population. In contrast, among all variables, high hsCRP level was a common and significant (P = 0.001) risk factor for all three pre-diabetes subgroups (Table II), suggesting that pre-diabetes is also an inflammatory event.
Table II.
The associations of different variables with I-IFG, I-IGT, or mixed IFG/IGT—binary logistic regression model.
| Variable | I-IFG versus NGT |
I-IGT versus NGT |
Mixed IFG/IGT versus NGT
|
|||
|---|---|---|---|---|---|---|
| OR (95.0% CI)a | Sig | OR (95.0% CI)a | Sig | OR (95.0% CI)a | Sig | |
| Age | 0.999 (0.531–1.879) | 0.997 | 1.447 (0.664–3.152) | 0.353 | 0.524 (0.250–1.099) | 0.087 |
| Sex | 1.475 (0.721–3.017) | 0.287 | 1.676 (0.736–3.818) | 0.219 | 2.289 (1.021–5.129) | 0.044 |
| Hypertension | 1.237 (0.662–2.313) | 0.505 | 2.644 (1.253–5.578) | 0.011 | 1.888 (0.898–3.971) | 0.094 |
| BMI | 1.552 (0.497–4.853) | 0.449 | 2.356 (0.702–7.909) | 0.165 | 2.477 (0.736–8.331) | 0.143 |
| WC | 2.687 (1.426–5.064) | 0.002 | 1.664 (0.798–3.469) | 0.175 | 2.090 (0.989–4.418) | 0.053 |
| WHR | 3.853 (1.695–8.757) | 0.001 | 3.437 (1.359–8.695) | 0.009 | 2.588 (0.993–6.747) | 0.052 |
| TC | 1.908 (0.900–4.043) | 0.092 | 1.101 (0.430–2.816) | 0.842 | 0.978 (0.372–2.574) | 0.965 |
| TG | 1.328 (0.698–2.527) | 0.387 | 1.275 (0.597–2.725) | 0.531 | 1.204 (0.559–2.595) | 0.635 |
| HDL-c | 0.810 (0.271–2.421) | 0.705 | 0.422 (0.084–2.124) | 0.296 | 1.422 (0.447–4.522) | 0.551 |
| LDL-c | 2.523 (0.257–24.767) | 0.427 | 8.605 (0.973–76.095) | 0.053 | 6.884 (0.745–63.590) | 0.089 |
| Fasting insulin | 1.397 (0.681–2.867) | 0.362 | 1.676 (0.736–3.818) | 0.219 | 0.873 (0.351–2.176) | 0.771 |
| HOMA-β index | 3.135 (6.623–1.479) | 0.003 | 0.477 (0.144–1.577) | 0.225 | 2.463 (5.882–1.031) | 0.043 |
| HOMA-IR index | 2.012 (0.952–4.252) | 0.067 | 1.590 (0.656–3.854) | 0.305 | 2.023 (0.852–4.805) | 0.110 |
| HsCRP | 4.387 (1.789–10.757) | 0.001 | 5.327 (2.005–14.156) | 0.001 | 5.505 (2.067–14.659) | 0.001 |
| IgE | 1.389 (0.654–2.951) | 0.392 | 1.981 (0.853–4.599) | 0.112 | 2.957 (1.299–6.731) | 0.010 |
| Chymase | 1.584 (0.704–3.568) | 0.267 | 2.862 (1.186–6.907) | 0.019 | 3.142 (1.310–7.541) | 0.010 |
| Tryptase | 1.261 (0.589–2.699) | 0.551 | 1.942 (0.833–4.528) | 0.124 | 1.584 (0.667–3.764) | 0.298 |
Unadjusted odds ratio (95% confidence intervals).
After adjustment for age, sex, and BMI, WC (OR = 2.799, P = 0.002), WHR (OR = 3.723, P = 0.002), HOMA-β index (OR = 3.344, P = 0.003), and hsCRP (OR = 4.540, P = 0.001) remained significant risk factors of I-IFG; hypertension (OR = 2.662, P = 0.014), WHR (OR = 2.871, P = 0.044), hsCRP (OR = 5.215, P = 0.001), and chymase (OR = 3.057, P = 0.016) remained significant risk factors of I-IGT; and hypertension (OR = 2.494, P = 0.036), WC (OR = 2.317, P = 0.036), HOMA-β index (OR = 3.378, P = 0.014), hsCRP (OR = 5.145, P = 0.002), IgE (OR = 2.448, P = 0.047), and chymase (OR = 3.127, P = 0.018) became significant risk factors of mixed IFG/IGT (Table III). None of the cholesterol lipid markers associated with pre-diabetes, but higher hsCRP level remained a common risk factor of all three pre-diabetes subgroups after adjustment (Table III). These observations affirm the hypothesis that pre-diabetes is an inflammatory disease. High cholesterol may associate with cardiovascular events and diabetes (25,30) but did not reach statistical significance in pre-diabetic patients, at least in this Chinese population. In contrast, both IgE and chymase were significant risk factors of pre-diabetes before and after adjustment for age, sex, and BMI, whereas plasma tryptase was only a weak risk factor for I-IFG (OR = 2.071, P = 0.07) (Table II and Table III). We have previously shown that patients with coronary heart disease (CHD) had higher plasma chymase, tryptase, and IgE levels than those without CHD, and both tryptase (P = 0.002) and IgE (P <0.001) reached significant differences between CHD and non-CHD patients (26,31)—suggesting that IgE and mast cell proteases participate in the development of pre-diabetes, type 2 DM, and cardiovascular events.
Table III.
The associations of different variables with I-IFG, I-IGT, or mixed IFG/IGT—binary logistic regression model.
| Variable | I-IFG versus NGT |
I-IGT versus NGT |
Mixed IFG/IGT versus NGT
|
|||
|---|---|---|---|---|---|---|
| OR (95.0% CI)a | Sig | OR (95.0% CI)a | Sig | OR (95.0% CI)a | Sig | |
| Age | 0.834 (0.430–1.619) | 0.592 | 1.310 (0.586–2.927) | 0.510 | 0.422 (0.189–0.943) | 0.035 |
| Sex | 1.590 (0.756–3.345) | 0.222 | 1.526 (0.656–3.549) | 0.326 | 2.861 (1.195–6.847) | 0.018 |
| Hypertension | 1.261 (0.667–2.387) | 0.476 | 2.662 (1.215–5.831) | 0.014 | 2.494 (1.062–5.852) | 0.036 |
| BMI | 1.597 (0.508–5.016) | 0.423 | 2.293 (0.678–7.756) | 0.182 | 2.860 (0.816–10.018) | 0.100 |
| WC | 2.799 (1.473–5.321) | 0.002 | 1.679 (0.797–3.534) | 0.173 | 2.317 (1.054–5.094) | 0.036 |
| WHR | 3.723 (1.599–8.668) | 0.002 | 2.871 (1.028–8.019) | 0.044 | 1.568 (0.527–4.664) | 0.418 |
| TC | 2.071 (0.942–4.551) | 0.070 | 1.096 (0.407–2.948) | 0.856 | 1.058 (0.362–3.088) | 0.918 |
| TG | 1.342 (0.698–2.579) | 0.377 | 1.182 (0.543–2.571) | 0.673 | 1.194 (0.526–2.709) | 0.672 |
| HDL-c | 0.810 (0.263–2.495) | 0.714 | 0.366 (0.071–1.889) | 0.230 | 1.337 (0.388–4.608) | 0.646 |
| LDL-c | 2.354 (0.235–23.603) | 0.467 | 8.521 (0.942–77.051) | 0.057 | 5.953 (0.569–62.287) | 0.136 |
| Fasting insulin | 1.428 (0.676–3.020) | 0.351 | 1.484 (0.616–3.579) | 0.379 | 0.831 (0.292–2.362) | 0.728 |
| HOMA-β index | 3.344 (7.407–1.513) | 0.003 | 0.501 (0.145–1.730) | 0.274 | 3.378 (8.929–1.282) | 0.014 |
| HOMA-IR index | 2.094 (0.961–4.561) | 0.063 | 1.335 (0.519–3.434) | 0.549 | 1.898 (0.718–5.019) | 0.197 |
| HsCRP | 4.540 (1.821–11.318) | 0.001 | 5.215 (1.912–14.224) | 0.001 | 5.145 (1.829–14.473) | 0.002 |
| IgE | 1.337 (0.617–2.899) | 0.461 | 2.011 (0.847–4.773) | 0.113 | 2.448 (1.012–5.925) | 0.047 |
| Chymase | 1.559 (0.685–3.549) | 0.290 | 3.057 (1.231–7.590) | 0.016 | 3.127 (1.218–8.030) | 0.018 |
| Tryptase | 2.071 (0.942–4.551) | 0.070 | 1.096 (0.407–2.948) | 0.856 | 1.058 (0.362–3.088) | 0.918 |
Odds ratio (95% confidence interval) after adjustment for age, sex, and BMI.
The associations of the interactions between plasma hsCRP, IgE, chymase or tryptase and common cardiovascular risk factors with pre-diabetes
Binary logistic regression analysis demonstrated that interactions between hsCRP and high WC increased further the relationships to pre-diabetes before (I-IFG: OR = 10.580, P = 0.002; I-IGT: OR=12.514, P = 0.001; mixed IFG/IGT: OR=10.139, P = 0.004) and after (I-IFG: OR = 10.571, P = 0.002; I-IGT: OR=12.843, P = 0.001; mixed IFG/IGT: OR=8.491, P = 0.010) adjustment for age, sex, and BMI (Table IV). These data suggest that patients with high hsCRP levels have increased risk of developing I-IFG, I-IGT, or mixed IFG/IGT with an OR between 4.387 (P = 0.001) and 5.327 (P = 0.001), and that patients with high CR values have an increased risk of developing I-IFG, I-IGT, or mixed IFG/IGT with an OR between 1.664 (P = 0.175) and 2.687 (P = 0.002) (Table II). After adjustment for age, sex, and BMI, the risk of developing IFG, I-IGT, or mixed IFG/IGT remained, with an OR between 4.540 (P = 0.001) and 5.215 (P = 0.001) for those with high hsCRP, and between 1.679 (P = 0.173) and 2.799 (P = 0.002) for those with high WC (Table III). But the risk of developing IFG, I-IGT, or mixed IFG/IGT greatly increased to an OR between 10.139 and 12.514 among patients with high hsCRP and high WC before adjustment—a nearly 2-fold higher risk than that among patients with only high hsCRP, and about a 4-fold higher risk than among patients with only high WC. After adjustment for age, sex, and BMI, the risk of developing all three subgroups of pre-diabetes remained high, with an OR between 8.491 and 12.843 (Table IV). Similarly, interactions between hsCRP and WHR further increased the relationships to I-IFG before (OR = 10.436, P = 0.027) and after (OR=11.065, P = 0.024) adjustment for age, sex, and BMI. Interactions between hsCRP and HOMA-β index further increased the relationships to I-IGT before (OR = 6.364, P = 0.001) and after (OR = 6.844, P = 0.001) adjustment for age, sex, and BMI (Table IV).
Table IV.
The associations of interactions between hsCRP and different variables with I-IFG, I-IGT, or mixed IFG/IGT—binary logistic regression model.
| Variable | I-IFG versus NGT |
I-IGT versus NGT |
Mixed IFG/IGT versus NGT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before adjustment |
After adjustmenta
|
Before adjustment |
After adjustment a
|
Before adjustment |
After adjustmenta
|
|||||||
| OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | |
| Age | 4.529 (1.622–12.646) | 0.004 | 4.433 (1.565–12.554) | 0.005 | 3.684 (1.174–11.564) | 0.025 | 3.416 (1.072–10.888) | 0.038 | 2.450 (0.729–8.229) | 0.147 | 2.176 (0.942–5.029) | 0.069 |
| Sex | 4.562 (0.967–21.517) | 0.055 | 5.287 (1.092–25.606) | 0.039 | 3.318 (0.584–11.869) | 0.176 | 3.135 (0.545–18.040) | 0.201 | 5.341 (1.031–27.685) | 0.046 | 6.759 (1.257–36.332) | 0.026 |
| Hypertension | 2.877 (0.993–8.336) | 0.052 | 3.264 (1.097–9.710) | 0.033 | 4.162 (1.345–12.881) | 0.013 | 3.874 (1.220–12.301) | 0.022 | 3.889 (1.236–12.237) | 0.020 | 4.747 (1.360–16.570) | 0.015 |
| WC | 10.580 (2.384–46.959) | 0.002 | 10.571 (2.359–47.361) | 0.002 | 12.514 (2.656–58.975) | 0.001 | 12.843 (2.561–64.417) | 0.002 | 10.139 (2.110–48.723) | 0.004 | 8.491 (1.682–42.865) | 0.010 |
| WHR | 10.436 (1.315–82.841) | 0.027 | 11.065 (1.374–89.137) | 0.024 | 8.810 (0.996–77.945) | 0.050 | 8.635 (0.840–88.810) | 0.070 | 9.024 (1.019–79.887) | 0.048 | 4.748 (0.474–47.510) | 0.185 |
| BMI | 2.576 (0.262–25.307) | 0.417 | 2.877 (0.290–28.512) | 0.366 | 5.636 (0.719–61.287) | 0.095 | 7.056 (0.758–65.633) | 0.086 | 3.318 (0.292–37.669) | 0.333 | 3.790 (0.312–45.997) | 0.295 |
| TC | 4.353 (0.497–38.106) | 0.184 | 4.484 (0.496–40.538) | 0.182 | 10.571 (1.231–90.809) | 0.032 | 11.066 (1.240–98.734) | 0.031 | 8.810 (0.996–77.945) | 0.050 | 7.974 (0.830–76.579) | 0.072 |
| TG | 3.342 (0.896–3.342) | 0.072 | 3.485 (0.924–13.140) | 0.065 | 2.791 (0.635–12.264) | 0.174 | 2.415 (0.532–10.958) | 0.253 | 1.636 (0.316–8.467) | 0.557 | 1.658 (0.309–8.888) | 0.555 |
| Lower HDL-c | 1.259 (0.205–7.737) | 0.804 | 1.158 (0.183–7.329) | 0.876 | 1.587 (0.216–11.661) | 0.650 | 1.424 (0.178–11.371) | 0.739 | 2.489 (0.400–15.481) | 0.328 | 2.176 (0.341–13.907) | 0.411 |
| Higher LDL-c | – | – | – | – | 4.933 (0.498–48.877) | 0.173 | 5.055 (0.490–52.159) | 0.174 | 0.166 (0.063–0.440) | 0.000 | 4.219 (0.351–50.754) | 0.257 |
| Hyperinsulinemia | 3.078 (0.620–15.287) | 0.169 | 3.252 (0.629–16.825) | 0.160 | 5.214 (1.007–27.007) | 0.049 | 4.397 (0.811–23.839) | 0.086 | 1.622 (0.221–11.925) | 0.635 | 1.395 (0.158–12.283) | 0.764 |
| HOMA-β index | 3.500 (1.232–9.942) | 0.019 | 3.754 (1.298–10.860) | 0.015 | 6.364 (2.132–18.993) | 0.001 | 6.844 (2.175–21.537) | 0.001 | 3.316 (1.037–10.602) | 0.043 | 3.275 (0.975–11.004) | 0.055 |
| HOMA-IR index | 3.561 (0.733–17.310) | 0.115 | 3.652 (0.727–18.347) | 0.116 | 4.244 (0.789–22.831) | 0.092 | 3.178 (0.557–18.143) | 0.193 | 3.395 (0.597–19.319) | 0.168 | 3.138 (0.510–19.320) | 0.218 |
| IgE | 7.220 (0.882–59.102) | 0.065 | 7.267 (0.847–62.343) | 0.071 | 8.605 (0.973–76.095) | 0.053 | 8.294 (0.910–75.578) | 0.061 | 6.884 (0.745–63.590) | 0.089 | 8.763 (0.851–90.209) | 0.068 |
| Tryptase | 2.272 (0.580–8.897) | 0.239 | 2.284 (0.573–9.114) | 0.242 | 2.140 (0.457–10.026) | 0.334 | 1.748 (0.357–8.559) | 0.491 | 4.718 (1.182–18.824) | 0.028 | 4.446 (1.052–18.783) | 0.042 |
| Chymase | – | – | – | – | – | – | – | – | – | – | – | – |
Odds ratio after adjustment for age, sex, and BMI.
Using the same binary logistic regression analysis, we demonstrated that interactions between IgE and high WC further increased the relationship to I-IGT and mixed IFG/IGT before (OR= 4.204, P = 0.023 and OR=6.265, P = 0.003, respectively) and after (OR=4.321, P = 0.024 and OR=4.858, P = 0.018, respectively) adjustment for age, sex, and BMI (Table V). Compared with the risks of developing I-IGT and mixed IFG/IGT when only high IgE was concerned—with OR values at 1.981 (P = 0.112) and 2.957 (P = 0.010) before adjustment (Table II), and 2.011 (P = 0.113) and 2.448 (P = 0.047) after adjustment (Table III)—IgE interaction with WC doubled the risk of I-IGT and mixed IFG/ IGT before (P = 0.023, P = 0.003) and after (P = 0.024, P = 0.018) adjustment (Table V). When high tryptase was considered as the only variable, the OR values for I-IGT and IFG/IGT were 1.942 (P = 0.124) and 1.584 (P = 0.298), respectively, before adjustment (Table II) and 1.096 (P = 0.858) and 1.078 (P = 0.918) after adjustment; combined consideration with IgE and tryptase increased the OR values to 7.179 (P = 0.016) and 5.122 (P = 0.052), more than 3-fold before adjustment, and to 7.303 (P = 0.016) and 5.722 (P = 0.048), more than 5-fold increase, after adjustment (Table V). When chymase was considered as an independent risk factor, the risks of developing I-IGT and mixed IFG/IGT had OR values at 2.862 (P = 0.019) and 3.142 (P = 0.010) before adjustment (Table II), and 3.057 (P = 0.016) and 3.127 (P = 0.018) after adjustment (Table III). When hypertension was considered alone, the risks of having I-IGT and mixed IFG/IGT had OR values at 2.644 (P = 0.011) and 1.888 (P = 0.094) before adjustment (Table II), and 2.662 (P = 0.014) and 2.494 (P = 0.036) after adjustment (Table III). Interactions between chymase and hypertension, however, increased the risk of developing I-IGT and mixed IFG/IGT—with OR values of 3.662 (P = 0.028) and 3.722 (P = 0.025) before adjustment, and 3.775 (P = 0.026) and 5.355 (P = 0.009) after adjustment (Table VI). In contrast, interactions between tryptase and different variables did not further increase the relation to pre-diabetes (Table VII).
Table V.
The associations of interactions between IgE and different variables with I-IFG, I-IGT, or mixed IFG/IGT.
| Variable | I-IFG versus NGT |
I-IGT versus NGT |
Mixed IFG/IGT versus NGT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before adjustment |
After adjustmenta
|
Before adjustment |
After adjustmenta
|
Before adjustment |
After adjustmenta
|
|||||||
| OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | |
| Age | 1.787 (0.719–4.440) | 0.212 | 0.821 (0.719–4.440) | 0.212 | 2.204 (0.802–6.059) | 0.126 | 2.316 (0.822–6.529) | 0.112 | 2.559 (0.945–6.933) | 0.065 | 2.029 (0.711–5.793) | 0.186 |
| Sex | 2.441 (0.744–8.008) | 0.141 | 2.565 (0.774–8.498) | 0.123 | 2.536 (0.676–9.506) | 0.168 | 2.498 (0.661–9.449) | 0.177 | 4.204 (1.214–14.555) | 0.023 | 4.358 (1.187–15.997) | 0.027 |
| Hypertension | 1.575 (0.504–4.921) | 0.435 | 1.470 (0.463–4.661) | 0.513 | 2.800 (0.858–9.139) | 0.088 | 2.881 (0.859–9.662) | 0.087 | 4.941 (1.611–15.157) | 0.005 | 4.361 (1.341–14.176) | 0.014 |
| WC | 3.550 (1.124–11.208) | 0.031 | 3.404 (1.070–10.831) | 0.038 | 4.204 (1.214–14.555) | 0.023 | 4.321 (1.213–15.394) | 0.024 | 6.265 (1.881–20.865) | 0.003 | 4.858 (1.310–18.014) | 0.018 |
| WHR | 6.241 (0.750–51.921) | 0.090 | 5.432 (0.641–46.018) | 0.121 | 10.829 (1.260–93.072) | 0.030 | 10.813 (1.193–98.010) | 0.034 | 11.100 (1.291–95.450) | 0.028 | 5.949 (0.612–57.797) | 0.124 |
| BMI | 0.830 (0.051–13.494) | 0.896 | 0.723 (0.044–11.949) | 0.821 | 1.553 (0.095–25.438) | 0.758 | 1.707 (0.096–30.489) | 0.716 | 8.902 (1.005–78.820) | 0.049 | 8.783 (0.961–80.266) | 0.054 |
| TC | 3.042 (0.613–15.103) | 0.174 | 3.104 (0.619–15.570) | 0.169 | 2.433 (0.391–15.129) | 0.340 | 2.797 (0.439–17.799) | 0.276 | 2.489 (0.400–15.481) | 0.328 | 1.879 (0.266–13.290) | 0.527 |
| TG | 2.963 (0.785–11.188) | 0.109 | 2.881 (0.749–11.076) | 0.124 | 6.316 (1.639–24.332) | 0.007 | 6.041 (1.536–23.761) | 0.010 | 4.200 (1.029–17.145) | 0.046 | 3.286 (0.733–14.738) | 0.120 |
| Lower HDL-c | 1.663 (0.148–18.704) | 0.680 | 1.337 (0.111–16.122) | 0.819 | 1.574 (0.096–25.783) | 0.750 | 1.315 (0.073–23.682) | 0.853 | 3.289 (0.290–37.316) | 0.337 | 2.140 (0.167–23.470) | 0.559 |
| Higher LDL-c | – | – | – | – | – | – | – | – | – | – | – | – |
| Hyperinsulinemia | 1.103 (0.239–5.092) | 0.900 | 1.061 (0.226–4.977) | 0.940 | 4.098 (1.005–16.713) | 0.049 | 4.621 (1.095–19.504) | 0.037 | 1.636 (0.316–8.467) | 0.557 | 1.430 (0.241–8.500) | 0.694 |
| HOMA-β index | 0.970 (0.407–2.311) | 0.970 | 0.985 (0.409–2.368) | 0.972 | 2.645 (1.092–6.403) | 0.031 | 2.643 (1.073–6.512) | 0.035 | 2.468 (1.009–6.037) | 0.048 | 1.971 (0.739–5.255) | 0.175 |
| HOMA-IR index | 1.103 (0.239–5.092) | 0.900 | 1.061 (0.226–4.977) | 0.940 | 4.098 (1.005–16.713) | 0.049 | 4.621 (1.095–19.504) | 0.037 | 2.857 (0.650–12.565) | 0.165 | 2.103 (0.420–10.538) | 0.366 |
| Tryptase | 2.059 (0.388–10.937) | 0.397 | 1.719 (0.309–9.573) | 0.536 | 7.179 (1.452–35.497) | 0.016 | 7.303 (1.442–36.982) | 0.016 | 5.122 (0.988–26.565) | 0.052 | 5.722 (1.015–32.257) | 0.048 |
| Chymase | 5.988 (0.719–49.842) | 0.098 | 5.493 (0.643–46.930) | 0.120 | 8.452 (0.955–74.822) | 0.055 | 9.066 (1.002–81.985) | 0.050 | 6.605 (0.715–61.042) | 0.096 | 3.445 (0.334–35.478) | 0.299 |
Odds ratio after adjustment for age, sex, and BMI.
Table VI.
The associations of interactions between chymase and different variables with I-IFG, I-IGT, or mixed IFG/IGT.
| Variable | I-IFG versus NGT |
I-IGT versus NGT |
Mixed IFG/IGT versus NGT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before adjustment |
After adjustmenta
|
Before adjustment |
After adjustmenta
|
Before adjustment |
After adjustmenta
|
|||||||
| OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | |
| Age | 0.889 (0.325–2.434) | 0.819 | 0.836 (0.300–2.329) | 0.732 | 2.743 (1.024–7.344) | 0.045 | 2.836 (1.031–7.804) | 0.044 | 1.895 (0.674–5.326) | 0.226 | 1.647 (0.549–4.947) | 0.374 |
| Sex | 1.940 (0.483–7.785) | 0.350 | 2.050 (0.508–8.278) | 0.313 | 2.738 (0.622–12.051) | 0.183 | 2.561 (0.562–11.669) | 0.224 | 4.718 (1.182–18.824) | 0.028 | 5.436 (1.273–23.218) | 0.022 |
| Hypertension | 1.718 (0.559–5.276) | 0.345 | 1.655 (0.533–5.141) | 0.384 | 3.662 (1.151–11.394) | 0.028 | 3.775 (1.172–12.159) | 0.026 | 3.722 (1.182–11.725) | 0.025 | 5.355 (1.508–19.022) | 0.009 |
| WC | 2.088 (0.700–6.233) | 0.187 | 2.060 (0.679–6.246) | 0.202 | 3.259 (1.017–10.445) | 0.047 | 3.359 (1.023–11.026) | 0.046 | 3.722 (1.182–11.725) | 0.025 | 3.954 (1.175–13.304) | 0.026 |
| WHR | 3.341 (0.365–30.575) | 0.286 | 2.646 (0.274–25.574) | 0.400 | 6.762 (.731–62.526) | 0.092 | 7.128 (0.739–70.470) | 0.089 | 3.227 (0.284–36.648) | 0.345 | 2.101 (0.172–25.693) | 0.561 |
| BMI | – | – | – | – | – | – | – | – | – | – | – | – |
| TC | – | – | – | – | – | – | – | – | – | – | – | – |
| TG | – | – | – | – | 2.140 (0.457–10.026) | 0.334 | 2.320 (0.462–10.758) | 0.318 | 4.025 (0.985–16.445) | 0.052 | 4.818 (1.015–22.878) | 0.048 |
| Lower HDL-c | 0.798 (0.049–12.979) | 0.874 | 0.778 (0.046–13.144) | 0.862 | – | – | – | – | 3.156 (0.278–35.820) | 0.354 | 2.972 (0.221–39.951) | 0.441 |
| Higher LDL-c | – | – | – | – | – | – | – | – | – | – | – | – |
| Hyperinsulinemia | 2.059 (0.388–10.937) | 0.397 | 2.066 (0.382–11.186) | 0.400 | 4.167 (0.774–22.443) | 0.097 | 4.665 (0.846–25.704) | 0.077 | 2.386 (0.383–14.854) | 0.351 | 3.001 (0.400–22.492) | 0.285 |
| HOMA-β index | 1.022 (0.433–2.411) | 0.961 | 1.013 (0.426–2.412) | 0.976 | 2.599 (1.070–6.317) | 0.035 | 2.798 (1.120–6.989) | 0.028 | 1.901 (0.759–4.760) | 0.170 | 1.695 (0.633–4.540) | 0.294 |
| HOMA-IR index | 2.500 (0.489–12.778) | 0.271 | 2.610 (0.500–13.616) | 0.255 | 0.387 (0.181–0.829) | 0.015 | 2.467 (0.388–15.703) | 0.339 | 4.167 (0.774–22.443) | 0.097 | 4.326 (0.706–26.526) | 0.113 |
Odds ratio after adjustment for age, sex, and BMI.
Table VII.
The associations of interactions between tryptase and different variables with I-IFG, I-IGT, or mixed IFG/IGT—binary logistic regression model.
| Variable | I-IFG versus NGT |
I-IGT versus NGT |
Mixed IFG/IGT versus NGT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before adjustment |
After adjustmenta
|
Before adjustment |
After adjustmenta
|
Before adjustment |
After adjustmenta
|
|||||||
| OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | OR (95.0% CI) | Sig | |
| Age | 1.199 (0.518–2.775) | 0.672 | 1.037 (0.435–2.470) | 0.935 | 1.499 (0.580–3.871) | 0.403 | 1.414 (0.541–3.694) | 0.480 | 0.970 (0.347–2.713) | 0.954 | 0.803 (0.274–2.384) | 0.688 |
| Sex | 1.353 (0.312–5.862) | 0.686 | 1.337 (0.309–6.129) | 0.675 | 3.366 (0.798–14.189) | 0.098 | 3.146 (0.741–13.358) | 0.120 | 4.718 (1.182–18.824) | 0.028 | 0.704 (1.595–28.173) | 0.009 |
| Hypertension | 1.718 (0.559–5.276) | 0.345 | 1.924 (0.609–6.076) | 0.265 | 4.094 (1.320–12.701) | 0.015 | 3.660 (1.161–11.539) | 0.027 | 2.821 (0.862–9.237) | 0.087 | 2.788 (0.766–10.145) | 0.120 |
| WC | 1.882 (0.677–5.228) | 0.225 | 1.909 (0.647–5.632) | 0.242 | 1.974 (0.619–6.298) | 0.250 | 1.809 (0.551–5.994) | 0.329 | 1.650 (0.498–5.466) | 0.413 | 1.391 (0.388–4.992) | 0.613 |
| WHR | 2.272 (0.580–8.897) | 0.239 | 2.228 (0.551–9.009) | 0.261 | 1.605 (0.310–8.314) | 0.573 | 1.355 (0.239–7.683) | 0.731 | 2.190 (0.467–10.272) | 0.320 | 1.398 (0.265–7.367) | 0.692 |
| BMI | 1.643 (0.292–9.239) | 0.573 | 1.793 (0.314–10.255) | 0.511 | 0.750 (0.066–8.513) | 0.816 | 0.729 (0.064–8.284) | 0.799 | 0.767 (0.068–8.705) | 0.830 | 1.115 (0.092–13.511) | 0.932 |
| TC | 1.940 (0.483–7.785) | 0.350 | 2.214 (0.529–9.262) | 0.276 | 1.022 (0.164–6.361) | 0.981 | 0.907 (0.142–5.792) | 0.918 | 0.500 (0.050–4.956) | 0.554 | 0.668 (0.062–7.210) | 0.740 |
| TG | 1.239 (0.474–3.525) | 0.616 | 1.358 (0.489–3.774) | 0.557 | 1.239 (0.474–3.525) | 0.616 | 0.996 (0.290–3.424) | 0.995 | 0.864 (0.238–3.130) | 0.824 | 0.854 (0.224–3.251) | 0.817 |
| Lower HDL-c | 0.258 (0.026–2.539) | 0.246 | 0.277 (0.027–2.800) | 0.277 | – | – | – | – | 1.568 (0.303–8.120) | 0.592 | 1.560 (0.263–9.248) | 0.624 |
| Higher LDL-c | – | – | – | – | – | – | – | – | – | – | – | – |
| Hyperinsulinemia | 1.000 (0.258–3.869) | 1.000 | 0.975 (0.222–4.269) | 0.973 | 2.488 (0.662–9.343) | 0.177 | 2.260 (0.589–8.678) | 0.235 | 0.370 (0.040–3.413) | 0.380 | 0.221 (0.018–2.668) | 0.235 |
| HOMA-β index | 0.696 (0.288–1.686) | 0.422 | 0.704 (0.278–1.785) | 0.460 | 1.912 (0.785–4.657) | 0.154 | 1.824 (0.740–4.497) | 0.192 | 0.875 (0.317–2.413) | 0.796 | 0.885 (0.304–2.576) | 0.822 |
| HOMA-IR index | 1.214 (0.329–4.478) | 0.771 | 1.264 (0.307–5.199) | 0.745 | 2.488 (0.662–9.343) | 0.177 | 2.193 (0.553–8.698) | 0.264 | 1.581 (0.376–6.658) | 0.532 | 1.194 (0.255–5.594) | 0.822 |
Odds ratio after adjustment for age, sex, and BMI.
Discussion
Accumulating evidence indicates that obesity, sedentary lifestyle, high-fat and saturated fatty acid-rich diets, age, sex, hypertension, dyslipidemia, insulin resistance, decreased β-cell sensitivity, hyperinsulinemia, hemoglobin A1c, and hsCRP are all risk factors for type 2 DM and pre-diabetes (6,32–40). Inflammatory cells and pro-inflammatory mediators from these cells are important players in the pathogenesis of pre-diabetes and type 2 DM (40–42). Increased plasma hsCRP levels predict newly developed metabolic syndrome (43) and correlate with type 2 DM (44–47) and pre-diabetes (15,48). In a follow-up study of European-American patients with type 2 DM, baseline CRP levels were significantly higher in deceased patients than in surviving patients (9.37 ± 15.94 mg/L versus 5.36 ± 7.91 mg/L, P < 0.0001), therefore predicting type 2 DM-associated mortality (49). IgE often associates with allergic responses. Several small human population studies indicate an association between plasma IgE levels and coronary heart diseases (50,51). Our prior study of two independent Chinese populations (982 patients from Central China and 240 patients from Eastern China) demonstrated that plasma IgE levels were highest among patients with acute myocardial infarction, followed by those with unstable angina pectoris —nearly twice as high as in patients with stable angina pectoris and in normal subjects (31). IgE is the most popular activator of mast cells (52), which are essential in type 2 DM (17). Increased levels of IgE in plasma or tissues may activate mast cells and increase mast cell mediators in the extracellular milieu. But mast cell functions in diabetes are complicated, depending on the subtypes of this metabolic disease. Although there has been no direct examination of how mast cells contribute to human type 1 DM, they can be either beneficial or detrimental in experimental type 1 DM. Alloxan- or streptozotocin-induced type 1 DM in rats associates with reduction of both total and activated pleural mast cells and overexpression of corticosteroids, which inhibits tissue cytokine and stem cell factor expression, thereby reducing mast cell population. These diabetic animals are resistant to allergic inflammatory responses. IgE levels, and possibly chymase and tryptase levels, are suppressed in these rats. This beneficial role of mast cells is consistent with the observation that children with type 1 DM are partially protected from asthma. But mast cell functions can be different in biobreeding (BB) rats, non-obese diabetic (NOD) mice, and DRlyp/lyp rats—all of which develop spontaneous type 1 DM. Development of diabetes in BB rats associates with increased mast cells in the pancreatic islets. In DRlyp/lyp rats, mast cell stabilization with cromolyn delays the onset of type 1 DM. Mechanistically, mast cells play a detrimental role by activating T cells in these animals. Our recent review summarized various functions of mast cells in type 1 DM (53). In contrast, relatively less information is available regarding the role of mast cells in type 2 DM. We have recently demonstrated that in both diet-induced (17) and genetically generated ob/ob (Shi, unpublished observation) obese mice, development of type 2 DM associates with increased mast cells in white adipose tissue, the liver, and the gastrointestinal tract, although we did not measure plasma or tissue IgE, chymase, or tryptase levels. Mast cell deficiency or stabilization with cromolyn or ketotifen (Zaditor) prevents the onset of diet-induced type 2 DM. In this experimental type 2 DM, mast cells release interleukin 6 and interferon-γ to stimulate cysteinyl protease cathepsin expression and promote angiogenesis (17). In humans, kidneys from patients with type 2 DM contain high levels of chymase (54). Due to the complexity of mast cell functions in type 1 DM, and as all current available evidence suggests a detrimental role of mast cells in type 2 DM, this study focuses on only patients with pre-diabetes, a precursor to type 2 DM (1).
We recently reported that plasma levels of hsCRP, IgE, and chymase associate with diabetes status. Interactions of hsCRP and IgE with mast cell chymase increased the relationships to diabetes and pre-diabetes (25). We have not examined, however, whether these mast cell-associated molecules are also important risk factors for different pre-diabetes subsets in humans. This study examined the hypothesis that patients with pre-diabetes have higher plasma hsCRP, IgE, and mast cell chymase and tryptase levels than those in non-diabetic controls, and that, therefore, these inflammatory molecules are important risk factors for pre-diabetes. As discussed, hsCRP is a well-known risk factor of diabetes and pre-diabetes; this study demonstrated that plasma IgE and chymase are also higher in patients with pre-diabetes than in non-diabetic subjects. IgE and chymase were both significant risk factors for pre-diabetes (Figure 1). Interactions of these individual risk factors with other known diabetic risk factors—such as WC, WHC, HOMA-β index, hypertension, and mast cell tryptase—greatly increased the impact of these mast cell-associated molecules on pre-diabetes I-IFG, I-IGT, or mixed IFG/IGT.
High-sensitivity CRP is a common inflammatory biomarker (55), and diabetes is considered a chronic inflammatory disease (56). Increased hsCRP in diabetic and pre-diabetic patients may reflect the degree of inflammation among these patients. High plasma levels of chymase and tryptase in pre-diabetic and diabetic patients (25) suggest an activation of mast cells among these patients. This hypothesis is consistent with the observation that plasma IgE levels decreased from diabetic patients, to pre-diabetic patients, to normal control subjects (25). Therefore, one potential role of increased IgE in pre-diabetic and diabetic patients is mast cell activation. Activated mast cells may use surface LFA-1 to enhance T cell proliferation (57), which is essential to the pathogenesis of experimental type 2 DM in mice (58). In human atherosclerotic lesions, we found that increased IgE localized not only to mast cells, but also to macrophages, smooth muscle cells, and endothelial cells. In vitro, IgE induced expression of inflammatory cytokines and apoptosis in macrophages, smooth muscle cells, and endothelial cells, along with reduced extracellular pH (31). All of these events require interaction between IgE and its receptor FcεR1. Absence of FcεR1 protected mice from diet-induced atherosclerosis, and macrophages from FcεR1-deficient mice did not respond to IgE stimulation (31). In human atherosclerotic lesions, we detected acidic pH in areas rich in macrophages and IgE (Shi, unpublished observation), suggesting that IgE affects macrophages in human atherogenesis. Therefore, as a second possible mechanism, IgE may participate in type 2 DM by directly activating macrophages or other inflammatory cells without the involvement of mast cells — a hypothesis that merits further investigation.
Although larger studies, or independent population studies, may be required to affirm our observations, this study provides evidence that elevated plasma levels of mast cell proteases and IgE may serve as important risk factors for human I-IFG, I-IGT, and mixed IFG/IGT and biomarkers for monitoring human pre-diabetes treatment.
Key messages.
Plasma levels of hsCRP, IgE, and mast cell protease chymase are significantly higher in patients with pre-diabetes than in those with normal blood glucose levels.
Plasma levels of hsCRP, IgE, and chymase are significant risk factors of human pre-diabetes before and after adjustment for common diabetes risk factors.
Interactions between plasma hsCRP and IgE levels with metabolic parameters increase further the risk of pre-diabetes.
Acknowledgments
We thank all the volunteers who participated in this study, and the clinic staff members from the Huzhou First Hospital, Huzhou, Zhejiang, China, for their assistance with clinical data collection. We also thank Ms Sara Karwacki for her editorial assistance.
Footnotes
Declaration of interest: This work was supported by awards from the Huzhou Municipal Science and Technology Agency (2008GS09) (H.Z.), the Zhejiang Province Department of Health (2008B178) (Z.W.), and the Zhejiang Province Department of Education (20070470) (X.H.S.); by grants from the National Institutes of Health HL60942, HL81090, HL88547 (G.P.S.); and by an EIA award (0840118N) from the American Heart Association (G.P.S.). The authors report no conflicts of interest.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;33:S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 3.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA. 2001;285:2109–13. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 4.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T, et al. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care. 2003;26:868–74. doi: 10.2337/diacare.26.3.868. [DOI] [PubMed] [Google Scholar]
- 5.Valensi P, Schwarz EH, Hall M, Felton AM, Maldonato A, Mathieu C. Pre-diabetes essential action: a European perspective. Diabetes Metab. 2005;31:606–20. doi: 10.1016/s1262-3636(07)70239-2. [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55:1430–5. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 7.Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52:1714–23. doi: 10.1007/s00125-009-1443-3. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–52. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 11.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–9. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 12.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 13.Sabanayagam C, Shankar A, Lim SC, Lee J, Tai ES, Wong TY. Serum C-reactive protein level and prediabetes in two Asian populations. Diabetologia. 2011;54:767–75. doi: 10.1007/s00125-011-2052-5. [DOI] [PubMed] [Google Scholar]
- 14.Ong KL, Tso AW, Xu A, Law LS, Li M, Wat NM, et al. Evaluation of the combined use of adiponectin and C-reactive protein levels as biomarkers for predicting the deterioration in glycaemia after a median of 5.4 years. Diabetologia. 2011;54:2552–60. doi: 10.1007/s00125-011-2227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann N Y Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 16.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–82. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, et al. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108:1316–27. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacGlashan D, Jr, Lavens-Phillips S, Katsushi M. IgE-mediated desensitization in human basophils and mast cells. Front Biosci. 1998;3:d746–56. doi: 10.2741/a318. [DOI] [PubMed] [Google Scholar]
- 21.Gruber B. Activation of rheumatoid synovial mast cells. Role of IgE-associated antiglobulins. Monogr Allergy. 1989;26:120–34. [PubMed] [Google Scholar]
- 22.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 23.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: the central role of maternal obesity. J Clin Endocrinol Metab. 2003;88:3507–12. doi: 10.1210/jc.2003-030186. [DOI] [PubMed] [Google Scholar]
- 24.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Zhang H, Shen XH, Jin KL, Ye GF, Qian L, et al. Immunoglobulin e and mast cell proteases are potential risk factors of human pre-diabetes and diabetes mellitus. PLoS One. 2011;6:e28962. doi: 10.1371/journal.pone.0028962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang M, Sun J, Lin Y, Zhang J, Chen H, Yang D, et al. Usefulness of serum tryptase level as an independent biomarker for coronary plaque instability in a Chinese population. Atherosclerosis. 2011;215:494–9. doi: 10.1016/j.atherosclerosis.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Huxley R, Li L, Anna V, Xie G, Yao C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from the China National Nutrition and Health Survey 2002. Circulation. 2008;118:2679–86. doi: 10.1161/CIRCULATIONAHA.108.788166. [DOI] [PubMed] [Google Scholar]
- 28.Zhang PH, Jiao SF, Zhou Y, Wang HB, Wu F, Jiang Y, et al. Study on chronic disease related behavior and lifestyle in adults in Beijing, 2005. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;28:1162–6. [PubMed] [Google Scholar]
- 29.Calboli FC, Cox DG, Buring JE, Gaziano JM, Ma J, Stampfer M, et al. Prediagnostic plasma IgE levels and risk of adult glioma in four prospective cohort studies. J Natl Cancer Inst. 2011;103:1588–95. doi: 10.1093/jnci/djr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krentz AJ. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab. 2003;5(Suppl 1):S19–27. doi: 10.1046/j.1462-8902.2003.0310.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Cheng X, Xiang MX, Alanne-Kinnunen M, Wang JA, Chen H, et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe−/− mice. J Clin Invest. 2011;121:3564–77. doi: 10.1172/JCI46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Ndumele CE, Pradhan AD, Ridker PM. Interrelationships between inflammation, C-reactive protein, and insulin resistance. J Cardiometab Syndr. 2006;1:190–6. doi: 10.1111/j.1559-4564.2006.05538.x. [DOI] [PubMed] [Google Scholar]
- 34.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–84. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 36.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55:3536–49. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 37.Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vänttinen M, Stancáková A, et al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;51:502–11. doi: 10.1007/s00125-007-0899-2. [DOI] [PubMed] [Google Scholar]
- 38.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. Baltimore Longitudinal Study of Aging. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–84. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 39.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–7. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 40.Wasada T, Kuroki H, Katsumori K, Arii H, Sato A, Aoki K. Who are more insulin resistant, people with IFG or people with IGT? Diabetologia. 2004;47:758–9. doi: 10.1007/s00125-004-1339-1. [DOI] [PubMed] [Google Scholar]
- 41.Sommer P, Sweeney G. Functional and mechanistic integration of infection and the metabolic syndrome. Korean Diabetes J. 2010;34:71–6. doi: 10.4093/kdj.2010.34.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JK. Inflammation and insulin resistance: an old story with new ideas. Korean Diabetes J. 2010;34:137–45. doi: 10.4093/kdj.2010.34.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onat A, Can G, Hergenç G. Serum C-reactive protein is an independent risk factor predicting cardiometabolic risk. Metabolism. 2008;57:207–14. doi: 10.1016/j.metabol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Nabipour I, Vahdat K, Jafari SM, Beigi S, Assadi M, Azizi F, et al. Elevated high sensitivity C-reactive protein is associated with type 2 diabetes mellitus: the Persian Gulf Healthy Heart Study. Endocr J. 2008;55:717–22. doi: 10.1507/endocrj.k08e-026. [DOI] [PubMed] [Google Scholar]
- 45.Hu G, Jousilahti P, Tuomilehto J, Antikainen R, Sundvall J, Salomaa V. Association of serum C-reactive protein level with sex-specific type 2 diabetes risk: a prospective Finnish study. J Clin Endocrinol Metab. 2009;94:2099–105. doi: 10.1210/jc.2008-2260. [DOI] [PubMed] [Google Scholar]
- 46.Lee CC, Adler AI, Sandhu MS, Sharp SJ, Forouhi NG, Erqou S, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;52:1040–7. doi: 10.1007/s00125-009-1338-3. [DOI] [PubMed] [Google Scholar]
- 47.Asegaonkar SB, Marathe A, Tekade ML, Cherekar L, Bavikar J, Bardapurkar J, et al. High-sensitivity C-reactive protein: a novel cardiovascular risk predictor in type 2 diabetics with normal lipid profile. J Diabetes Complications. 2011;25:368–70. doi: 10.1016/j.jdiacomp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Lin J, Zhang M, Song F, Qin J, Wang R, Yao P, et al. Association between C-reactive protein and pre-diabetic status in a Chinese Han clinical population. Diabetes Metab Res Rev. 2009;25:219–23. doi: 10.1002/dmrr.923. [DOI] [PubMed] [Google Scholar]
- 49.Cox AJ, Agarwal S, Herrington DM, Carr JJ, Freedman BI, Bowden DW. C-reactive protein concentration predicts mortality in type 2 diabetes: the Diabetes Heart Study. Diabet Med. 2012;29:767–70. doi: 10.1111/j.1464-5491.2011.03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovanen PT, Mänttäri M, Palosuo T, Manninen V, Aho K. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch Intern Med. 1998;158:1434–9. doi: 10.1001/archinte.158.13.1434. [DOI] [PubMed] [Google Scholar]
- 51.Shahzad F, Tawwab S, Afzal N. Association of interleukin-4 and IgE levels with LDL oxidation in atherosclerosis. Iran J Immunol. 2010;7:109–16. [PubMed] [Google Scholar]
- 52.Xu J, Shi GP. Emerging role of mast cells in cardiovascular and metabolic diseases. Endocr Rev. 2012;33:71–108. doi: 10.1210/er.2011-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi MA, Shi GP. Different roles of mast cells in obesity and diabetes: lessons from experimental animals and humans. Front Immunol. 2012;3:7. doi: 10.3389/fimmu.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang XR, Chen WY, Truong LD, Lan HY. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol. 2003;14:1738–47. doi: 10.1097/01.asn.0000071512.93927.4e. [DOI] [PubMed] [Google Scholar]
- 55.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107:370–1. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
- 56.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15:846–7. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 57.Sayed BA, Brown MA. Mast cells as modulators of T-cell responses. Immunol Rev. 2007;217:53–64. doi: 10.1111/j.1600-065X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 58.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]