Summary
Glucocorticoid dyshomeostasis is observed in a proportion of depressed individuals. As a result, glucocorticoid receptor (GR) antagonists are currently being tested as potential anti-depressants. The current study was designed to test the efficacy of mifepristone, a GR antagonist, in mitigating behavioral, neuroendocrine and central nervous system (CNS) responses to an acute stressor. Adult male rats were treated for five days with mifepristone (10 mg/kg) and then exposed to the forced swim test (FST). Treatment with mifepristone decreased immobility and increased swimming (but not climbing) behavior in the FST, consistent with antidepressant action. In addition, mifepristone dampened the ACTH response to FST exposure. In the CNS, mifepristone increased c-Fos expression in all subdivisions of the medial prefrontal cortex (mPFC) and decreased neuronal activity in some subdivisions of the hippocampus including the CA2, CA3, and hilus region of the dentate gyrus in animals exposed to FST. In contrast, mifepristone increased neuronal activity in the ventral subiculum (output region of the hippocampus) and decreased c-Fos expression in the central amygdala (CeA) in animals exposed to FST. These data suggest that antidepressant efficacy and perhaps HPA dampening properties of RU486 are related to alterations in key limbic circuits mediating CNS stress responses, resulting in enhanced stress inhibition (via the mPFC and ventral subiculum) as well as decreased stress excitation (central amygdala). Overall the data suggest that drugs targeting the glucocorticoid receptor may ameliorate stress dysfunction associated with depressive illness.
Keywords: glucocorticoids, forced swim test, depression, medial prefrontal cortex, RU486, HPA axis
Introduction
Dysregulation of the hypothalamic pituitary adrenal (HPA) axis, manifested by elevation in circulating glucocorticoids (hypercortisolemia), is associated with affective disorders including major depression (Gold and Chrousos, 1999). It is hypothesized that hypercortisolemia is caused by disruptions in glucocorticoid-mediated negative feedback. The glucocorticoid-mediated negative feedback protects against prolonged activation of this axis, thereby preventing many of the adverse psychological and physiological consequences of glucocorticoid hypersecretion. Notably, decreased hypercortisolemia is commonly observed with successful antidepressant treatment, (Delbende et al., 1991; Reul et al., 1994; Pariante, 2003) implicating aberrant glucocorticoid signaling in depressive pathology. As such, the glucocorticoid receptor (GR) is currently a target for development of new antidepressant therapies (Thomson and Craighead, 2008).
Mifepristone is a potent GR and progesterone receptor antagonist that is known to have anti-depressant effects following relatively brief treatment in humans (4-8 days) (Belanoff et al., 2001). The use of mifepristone in individuals with elevated glucocorticoids as a result of chronic stress or in the case of Cushing's syndrome may be particularly advantageous as preliminary blockade of GR can serve as a barrier against the deleterious effects of excess glucocorticoid production. An additional advantage of GR antagonists as discussed in, (Belanoff et al., 2001; Thomson and Craighead, 2008) is that prolonged antagonism of GR may actually lead to an up-regulation in GR in critical hypothalamic and limbic structures, thereby enhancing the GR negative feedback control of the HPA axis. Behavioral testing in rodents corroborates the efficacy of mifepristone or other GR antagonists (ORG34116) to ameliorate depression-like behaviors in the forced swim test (FST) (De Kloet et al., 1988; Bachmann et al., 2005). The FST, described originally by (Porsolt et al., 1977) is among the most widely used model for assessing pharmacological antidepressant efficacy. An effective antidepressant decreases immobility and increases active behaviors such as swimming and/or climbing in the FST (Cryan et al., 2005a). Previous studies have found that mifepristone (10 mg/kg, sc) administered 60 minutes prior to FST exposure, reverses the stress-induced increase in immobility normally observed in both male and female rats exposed to maternal separation in early life (Aisa et al., 2007, 2008). In addition, four-day treatment of mifepristone normalizes the stress-induced reductions in hippocampal neurogenesis (Oomen et al., 2007). More importantly, this study demonstrated that mifepristone alone has no effect on neurogenesis, suggesting that the normalizing effect of the drug is evident during conditions of elevated glucocorticoids. Collectively, these findings suggest that mifepristone prevents stress-induced decreases in neuroplasticity as well as stress-induced increases in depression-like behaviors.
To our knowledge, there are no published studies that have extensively examined the effects of mifepristone on stress-induced neuronal activation in depression-related and stress-sensitive brain regions. Thus, the aim of the present study was to assess the neuroendocrine and central impact of mifepristone treatment in rats exposed to an acute stressor. This was accomplished by measuring adrenocorticotropin hormone (ACTH) and corticosterone concentrations as well as Fos induction following FST exposure. Based on the reported effects of antidepressants on specific neural regions in depressed patients, (Arce et al., 2008; Ishizaki et al., 2008; Kukolja et al., 2008) we predicted that the antidepressant-like effect of mifepristone would be associated with alterations in forebrain regions responsible for regulation of stress responding.
Materials and Methods
Subjects
Male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN; 250-300 G) were housed individually in standard rat shoebox cages and acclimated to the laboratory conditions for 1wk before initiation of the experiment. Rats were maintained in a temperature- and humidity-controlled room (lights on 0600-1800) with food and water available ad libitum. All experimental procedures and protocols were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Drug Treatment
In Experiment 1, twenty-six animals were matched by body weight and were divided into three groups, and administered a single daily injection of mifepristone (n=9) (Sigma-Aldrich 10mg/kg s.c. dissolved in propylene glycol), Imipramine (n=9) (Sigma-Aldrich10mg/kg i.p. dissolved in saline) or vehicle (n=8) (propylene glycol s.c.) for 5 days. Imipramine is known to have significant anti-depressant effects in the FST and was included as a positive control (Morley-Fletcher et al., 2004). On the fifth day, one hour after the last injection, animals were exposed to the FST as described below. In Experiment 2, thirty-six animals were matched by body weight and divided into two groups and received mifepristone (10 mg/kg in propylene glycol) (n=20) or the vehicle control, propylene glycol (n=16) as described in Experiment 1. On the fifth day, one hour after last injection, animals were exposed to the FST (n=10 for mifepristone; n=10 for vehicle). The remaining rats mifepristone (n=10) and vehicle (n=6) served as non-stressed controls. The mifepristone dose was selected based on successful antidepressant effects previously observed in rats (Aisa et al., 2007, 2008).
Behavior
Forced swim test
The FST test was chosen as a way to assess the effects of mifepristone on depression-like behavior and as an acute stressor to activate the HPA axis. The modified FST was conducted according to the methodology described by, (Cryan et al., 2005b) which differs from the classical Porsolt FST (Porsolt et al., 1977), in that animals are only exposed to the FST once for 10 min. The behavioral apparatus was a Plexiglas cylinder 45cm high and 20cm in diameter filled with 31 cm of water (30-33°C). Rats were placed in the cylinder for 10 min and the session was videotaped. Scoring was done by two independent observers blind to the treatment conditions. The behavior was scored every 5 s based on the criteria listed below. The total counts of each behavior during the 10 min testing session was summed for each animal and averaged within each treatment group. The behaviors scored are defined as follows: (i) climbing – rapid movement of limbs in and out of the water with the body parallel to the apparatus with, (ii) diving, (iii) swimming – moving limbs in an active manner and making circular movements around the apparatus; and immobility – rat not making any active movements or floating in the water without struggling.
Blood and Organ Collection
Tail blood was collected by tail vein nick 15 and 90 minutes after the end of FST and in control animals not subjected to FST at the same time intervals. Blood samples were collected into tubes containing EDTA and were immediately placed on ice. Plasma was obtained by centrifugation (1500 × g, 15min, 4°C) and stored at -20°C for subsequent analysis of plasma ACTH and corticosterone.
Organ Collection
In order to determine the effects of mifepristone on stress-responsive and reproductive organs, testicles, adrenal and thymus glands were removed and cleaned from each animal for weight determination.
Radioimmunoassay
Plasma corticosterone levels were measured using 125I RIA kits (MP Biomedicals, Inc., Orangerburg, NY). Plasma ACTH concentrations were determined by an RIA that used a specific antiserum generously donated by Dr. William Engeland (University of Minesota, Minneapolis, MN) at a dilution of 1:120,000, with 125I ACTH (Amersham Biosciences, Piscataway, NJ) as labeled tracer (Jasper and Engeland, 1991).
Immunohistochemistry
Ninety minutes following FST exposure, animals were anesthetized with an overdose of sodium pentobarbital and intracardially perfused with 100 mL of 0.9% saline followed by 200 mL of 3.7% Formaldehyde in 0.1M phosphate buffer (PBS), pH 7.6. Brains were subsequently removed and post-fixed using the same fixative for 24 hours at 4°C before being transferred and stored for two days in 0.1M PBS containing 30% sucrose. Coronal sections were cut at 35μm with a freezing microtome and alternate sections collected into wells containing cryoprotectant solution. Brain sections were transferred from the cryoprotectant solution to 0.1M PBS solution. After this step and all subsequent incubations, sections were rinsed 3 × 5 minutes in PBS. Subsequent incubations were (1) rabbit polyclonal c-Fos antibody (1:5000, Santa Cruz Biotechnology) in 0.05M soulution of PBS containing 0.25% bovine serum albumin and 0.5% Triton X-100 overnight at room temperature; (2) biotinylated goat anti-rabbit (1:500; Vector laboratories, Burlingame, CA) in 0.05M solution of PBS containing 0.25% bovine serum albumin and 0.5% Triton X-100 for 1 hour at room temperature; (3) avidin-biotin horseradish peroxidase complex (1:800; Vectastain ABC elite Kit, Vector Laboratories) in 0.05M solution of PBS containing 0.25% bovine serum albumin for 1 hour at room temperature; (4) ABC-horseradish peroxidase complex was visualized with 3,3′-diaminobenzidine (Sigma) that was dissolved in a solution containing Tris-NaCl and 0.09% hydrogen peroxide for 15 minutes. Sections were mounted on gelatinized slides, allowed to dry, dehydrated with alcohol and Xylene and coverslipped.
Data analysis
Cell counting
For analysis of Fos positive immunoreactive nuclei, digital images of regions of interest were captured and subjected to quantitative analysis of cell counts. The number of Fos-immunoreactive cell nuclei was determined from thresholded images using the Scion Image software. A uniform threshold (based on a pre-defined threshold function in Scion image) was applied to all images in a given brain region and the average optical density was automatically calculated and expressed as mean optical density. The final cell counts were expressed as the number of positive nuclei per unit area (mm2). The shape and size of each brain region studied were defined according to the boundaries outlined in Paxinos and Watson (1998) rat stereotaxic atlas as illustrated in Figure 1. A total of 2-3 images (including both right and left hemispheres) were analyzed for each region and averaged to produce a mean cell count/area for each region. We analyzed c-Fos activation in three areas of the medial prefrontal cortex (anterior cingulate, infralimbic and prelimbic), five subdivisions of the hippocampus (CA1, CA2, CA3, dentate gyrus, hilus and ventral subiculum), three amygdala subdivisions (central amygdala, medial amygdala and basolateral amygdala), three subdivisions of the bed nucleus of the stria terminalis (anteroventral, anterior lateral and posterior medial), ventral lateral septum, paraventricular nucleus of the hypothalamus, and paraventricular thalamic nucleus.
Figure 1.
Templates and relative sizes of each brain nucleus analyzed for c- Fos based on the Paxinos and Watson rat brain atlas. Abreviations: mPFC, medial prefrontal cortex; IL, infralimbic; PL, prelimbic; CG1, anterior cingulate; vLS, ventral lateral setum; hippocampal subdivisions include: CA1; CA2; CA3, DG, dentate gyrus; hil; hilus; VS, ventral subiculum; amygdaloid complex: CeA, central amygdala; MeA, medial amygdala; BLA, basolateral amygdala; BST, Bed nucleus of the stria terminalis: vBST, anteroventral BST; PMBNST, posterior medial BST; lBST, lateral BST; PVN paraventricular nucleus of the hypothalamus; and PVT, paraventricular nucleus of the thalamus.
Statistical Analysis
Behavioral data were analyzed with one-way ANOVA or t-test where applicable. Hormonal data were analyzed using a 3-way repeated measures ANOVA with stress and drug as between subjects factors and time as the within or repeating factor. For immunohistochemical analysis, differences in overall c-Fos content were analyzed as a 2-way ANOVA with drug and stress as between-subjects factors. Terminal measures including adrenals, thymus, body weight and testes were also analyzed using an independent sample t-test. Note, the terminal measures were analyzed regardless of stress because an acute 10 min swim stress is not sufficient to induce changes in these parameters; therefore the data were analyzed with respects to drug administration alone. When necessary data that violated Levene's homogeneity of variance were log transformed followed by the appropriate statistical analyses. Fisher's LSD post hoc tests were used to compare individual group differences. For all data p≤ 0.05 denotes statistical significance.
Results
Behavioral Analysis
Experiment 1
Mifepristone and imipramine significantly increased swimming in the FST (F(2,23)=6.18, p <0.01) relative to vehicle (Figure 2A). Imipramine significantly increased climbing in the FST (F(2,23)=4.35, p < 0.05) relative to both mifepristone and vehicle. There was however no significant difference in climbing between mifepristone and vehicle treated animals (Figure 2B). There was a trend for mifepristone to decrease time spent immobilie but imipramine significantly decreased time spent immobile in the FST (F(2,23)=4.45, p <0.05) compared with vehicle. There was no significant difference in immobility between imipramine and mifepristone treated animals (Figure 2C).
Figure 2.
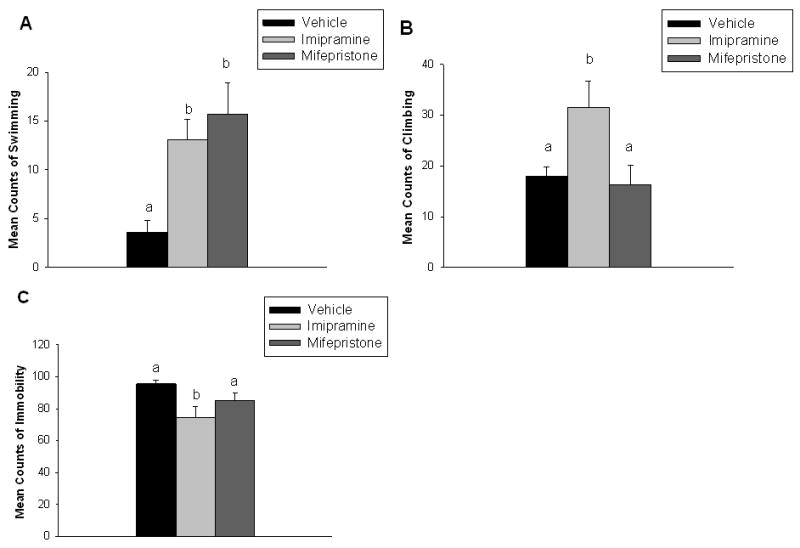
Mifepristone and imipramine significantly increased swimming relative to vehicle (A). Imipramine significantly increased climbing relative to mifepristone and vehicle (B). Imipramine significantly decreased immobility in the FST compared with mifepristone and vehicle (C). Data are represented as mean ± SEM, n=8-9 per group. Non-shared letters denotes statistical significance, p < 0.05.
Experiment 2
Animals administered mifepristone displayed significantly more swimming (t(18)= 2.57, p=0.05) (Figure 3A) and less immobility (t(18)=2.55, p< 0.05) (Figure 3B) in the FST compared with vehicle treated animals, suggesting a decrease in depression-like behavior in the mifepristone group. Consistent with Experiment 1, there were no differences in climbing behaviors between animals treated with mifepristone and vehicle, p >0.05 (data not shown).
Figure 3.

Mifepristone significantly increased swimming (A) and decreased immobility significantly compared with vehicle (B). Data are represented as mean ± SEM, n=10 per group. *p < 0.05 versus vehicle treated groups.
Hormonal Analysis ACTH
There was a significant main effect of stress (F(1,32)=33.85, p<0.01), drug (F(1,32)=35.14, p<0.01) and time (F(1,71)=71.55, p<0.01) on ACTH responses. In addition, there was a significant stress × time interaction (F(1,71)=4.06, p=0.05, and drug × time interaction (F(1,71)=30.45, p <0.01). Specifically, at 15 minutes animals exposed to FST exhibited significantly higher ACTH concentrations relative to non-stressed controls. At the 15 minute time point, the FST mifepristone group had lower ACTH concentrations relative to the FST vehicle animals. There were no differences among groups at 90 minutes (Figure 4).
Figure 4.
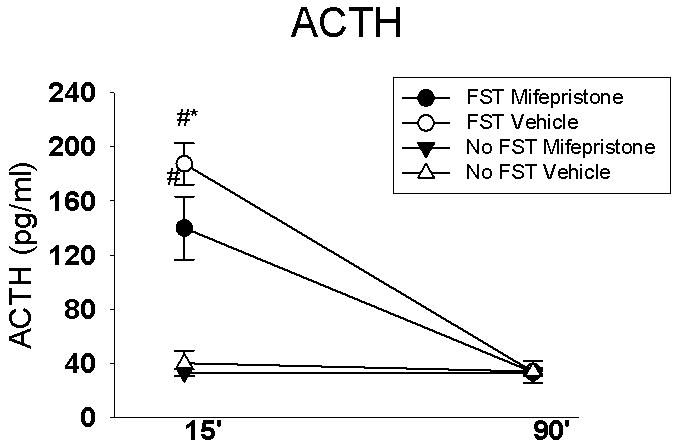
FST significantly increased ACTH levels at the 15 min time point, regardless of drug treatment. However, animals exposed to FST and treated with mifepristone displayed decreased ACTH concentrations in response to FST exposure relative to the FST vehicle treated animals, suggesting a dampening of the ACTH response in the FST mifepristone group. Data are represented as mean ± SEM, n=6-10 per group. # p < 0.05 vs. no FST group, * p < 0.05 vs. all other groups
Hormonal Analysis Corticosterone
There was a significant main effect of stress on corticosterone concentrations (F(1,32)=178.77, p<0.01) There was no significant main effect of drug on corticosterone responses; however, there was a significant main effect of time (F(1,71)=57.8, p<0.01) and significant stress × time (F(1,71)=5.46, p<0.05) drug × time (F(1,71)=38.64, p<0.01) and stress × drug × time interactions (F(1,71)=4.05, p=0.05). At 15 minutes, animals exposed to FST displayed higher corticosterone concentrations regardless of drug treatment. At 90 minutes the animals exposed to FST continued to mount a higher corticosterone response relative to their non-stressed counterparts. Of note, the FST mifepristone group displayed significantly higher corticosterone at 90 minutes compared with all other groups, (Figure 5) consistent with deficits in intermediate feedback regulation of the HPA axis.
Figure 5.

FST significantly increased corticosterone levels at the 15 min time point, regardless of drug treatment. There was however a strong trend for mifepristone to decrease the corticosterone response to FST exposure relative to the respective vehicle treated group. At the 90 min time point animals exposed to FST continued to display increased corticosterone levels relative to the animals not exposed to FST. At the 90 min time point the FST mifepristone group exhibited higher corticosterone relative to all other groups. Data are represented as mean ± SEM, n=6-10 per group. # p < 0.05 vs. no FST group, * p < 0.05 vs. all other groups.
Immunohistochemical analyses
Medial Prefrontal Cortex
Similar patterns of Fos activation were observed in all subdivisions of the medial prefrontal cortex. There was a significant main effect of stress on c-Fos expression in the AC (F(1,28)=19.42, p<0.01), PL (F(1,28)=23.69, p<0.01), and IL (F(1,28)=19.78, p<0.01), a significant main effect of drug only within the AC (F(1,28)=6.347, p < 0.01) and a significant stress × drug interaction in the AC: (F(1,28)=7.62, p <0.01); PL: (F(1,28)=10.24, p <0.01), IL (F(1,28)=10.53, p< 0.01). In all cases, post hoc analyses revealed greater numbers of c-Fos labeled neurons in the FST mifepristone group relative to all other groups. Within the infralimbic and prelimbic regions, the FST vehicle group had greater c-Fos activity relative to the No FST mifepristone treated group only (Figure 6).
Figure 6.

Animals exposed to FST and treated with mifepristone had significantly higher c-Fos activation in the anterior cingulate (A), prelimbic (B) and infralimbic (C) relative to all other groups. Data are represented as mean ± SEM, n=6-10 per group. Non-shared letters denote statistical significance, p < 0.05.
Hippocampus
We examined five regions of the hippocampus: CA1, CA2, CA3, dentate gyrus (DG), and the hilus (i.e., CA4). There was a significant main effect of stress on c-Fos expression in the CA1 (F(1,29)=10,56, p<0.01); CA2 (F(1,30)=10.99, p <0.01); CA3 (1,31)=13.18, p<0.01;DG (F(1,30)=34.59, p <0.01); and hilus, (F(1,30)=12.52, p <0.01) with animals exposed to FST displaying higher levels of c-Fos activation compared with No FST group. There was no significant main effect of drug on c-Fos expression within any of the subdivisions. However, there was a significant stress × drug interaction in the CA1 (F(1,29)=4.66, p< 0.05); CA2 (F(1,30)=9.12, p<0.01); CA3 (F(1,31)=8.61, p<0.01) and hilus (F(1,33)=4.52, p < 0.05) regions. Specifically, the FST mifepristone group had significantly lower c-Fos expression in the CA2, CA3 and hilus regions compared with the respective vehicle treated group and within the CA1, the FST vehicle group had significantly higher c-Fos expression relative to all other groups. Of note, there was a trend for the FST mifepristone group to have lower c-Fos expression within the CA1 Refer to Figure 7.1 for data illustration of CA1, CA2 and CA3 regions and Figure 7.2 for the dentate gyrus and hilus subdivisions of the hippocampus. For a representative photomicrograph of c-Fos immunohistochemistry of the CA3 region, refer to Figure 8.
Figure 7.


Figure 7.1 (A) Animals exposed to FST and treated with vehicle displayed greater c-Fos activation in the CA1 relative to the no FST group. (B) Vehicle treated animals exposed to FST displayed greater c-Fos activation relative to all other animals in the CA2. Within the CA2, mifepristone decreased c-Fos activation in animals exposed to FST relative to their FST vehicle counterparts and the no FST vehicle group. (C) Vehicle treated animals exposed to FST displayed greater c-Fos activation in the CA3 compared to all other groups. In particular, mifepristone decreased c-Fos activation in FST exposed animals compared with their FST vehicle counterparts as well as the no FST vehicle group. Data are represented as mean ± SEM, n=6-10 per group. Non-shared letters denote statistical significance, p < 0.05.
Figure 7.2 (D) Animals exposed to FST displayed greater c-Fos activation in the dentate gyrus relative to non-stressed animals regardless of drug treatment. (E) Within the hilus area, animals exposed to FST and treated with vehicle displayed greater c-Fos activity relative to all other groups. Furthermore, animals exposed to FST and treated with mifepristone displayed decreased c-Fos activity relative to the vehicle treated animals. Data are represented as mean ± SEM, n=6-10 per group. Non-shared letters denote statistical significance, p < 0.05.
Figure 8.
Representative image of c-Fos immunoreactivity in the CA3 region of the hippocampus at the level of -3.30 mm (bregma). (A) No FST vehicle (B) No FST mifepristone (C) FST vehicle (D) FST mifepristone. Arrows indicate examples of c-Fos positive neurons. Immunohistochemistry results demonstrate that FST-mifepristone group displayed less neuronal activation within this region relative to the FST-vehicle group. Scale bar 100μm.
Ventral Subiculum
There was a significant main effect of stress on c-Fos activation in the Vsub (F(1,34)=4.75, p < 0.01), a significant main effect of drug (F(1,34)=15.03, p < 0.01) and significant stress × drug interaction (F(1,34)=3.84, p < 0.05). Unlike the dampened c-Fos response in the FST mifepristone group in observed in the CA2, CA3 and hilus, the FST mifepristone group had the greatest amount of c-Fos activity relative to all other groups in the Vsub (Figure 9).
Figure 9.

FST mifepristone treated animals displayed greater c-Fos activation relative to all other groups within the ventral subiculum. Data are represented as mean ± SEM, n=6-10 per group. Non-shared letters denote statistical significance, p < 0.05.
Amygdala
There was a significant main effect of stress (F(1,31)=63.15, p < 0.01), significant main effect of drug (F(1,31)=4.37, p <0.05) and a significant stress × drug interaction (F(1,31)=5.41, p < 0.05) in the central amygdala (CeA). The FST mifepristone group displayed less activity in the CeA relative to the FST vehicle treated group indicating that the drug dampened c-Fos activation in response to FST in this region (Figure 10). In the basolateral amygdala, there was a significant main effect of stress (F(1,31)=24.06, p <0.01, no significant main effect of drug (F(1,31)=.54, p > 0.05) and no significant stress × drug interaction (F(1,31)=1.10, p > 0.05). Animals exposed to FST exhibited greater c-Fos activation in the BLA relative to non-stressed controls (Table 1). There was no main effect of stress, drug or significant stress × drug interaction on c-Fos activation in the medial amygdala (Table 1).
Figure 10.

Animals exposed to FST and treated with vehicle displayed the greatest degree of c-Fos activation in the central amygdala relative to all other groups. The FST mifepristone group displayed less c-Fos activation relative to the FST vehicle treated group. Data are represented as mean ± SEM, n=6-10 per group. Non-shared letters denote statistical significance, p < 0.05.
Table 1.
Effects of FST stress and drug treatment on Fos expression in male rats. Fos expression is corrected for area (mm2). φ indicates significant main effect of stress, p <0.05.
| No-FST | FST | |||
|---|---|---|---|---|
| Vehicle | Mifepristone | Vehicle | Mifepristone | |
| Amygdala | ||||
| MeA | 2.44±0.40 | 1.81±0.30 | 2.33±0.21 | 1.97±0.17 |
| BLAφ | 1.76±0.11 | 2.23±0.23 | 3.26±0.22 | 3.42±0.14 |
| Hypothalamus-Thalamus | ||||
| PVNφ | 2.14±0.15 | 2.24±0.24 | 5.16±0.36 | 5.50±0.28 |
| PVTφ | 1.60±0.36 | 2.00±0.23 | 2.52±0.19 | 2.85±0.15 |
| BNST | ||||
| Anteroventralφ | 4.46±0.55 | 3.25±0.60 | 7.07±0.98 | 6.34±1.15 |
| Ant. Lateralφ | 2.23±0.39 | 2.01±0.51 | 3.43±0.53 | 3.14±0.60 |
| Post. Medial | 2.67±0.64 | 2.52±0.32 | 3.06±0.28 | 2.82±0.83 |
| Septal Area | ||||
| vLSφ | 7.20±1.54 | 5.81±1.12 | 10.58±1.40 | 11.87±2.13 |
Abrevations: MeA, medial amygdala; BLA, basolateral amygdala; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; BNST, bed nucleus of the stria terminalis; vLS, ventral lateral septum. Counts are reported in number of positive nuclei per unit area (mm2). Data are represented as mean ± SEM, n=8-10 per group (FST-mifepristone, FST-Vehicle and No FST-mifepristone) and n=6 per group (No FST-Vehicle).
Bed Nucleus of Stria Terminalis
There was a significant main effect of stress on c-Fos expression in the anteroventral (F(1,20)=9.11, p<0.01) and lateral (F(1,20)=4.2, p=0.05) subdivisions of the BNST but no significant main effect of drug (F(1,20)=.22, p>0.05) and no significant stress × drug interaction (F(1,20)=.01, p >0.05) (Table 1). In both cases, exposure to FST significantly increased c-Fos expression within the anteroventral and anterolateral subdivisions of the BNST compared with non-stressed controls. There was no significant main effect of stress or drug and no significant stress × drug interaction on c-Fos expression in the posterior medial BNST (Table 1).
Lateral Septum
There was a significant main effect of stress on c-Fos expression in the ventral lateral septum (F(1,34)=6.11, p < 0.05) but no significant main effect of drug or significant stress × drug interaction. Animals exposed to FST exhibited significantly increased c-Fos expression within the ventral lateral septum compared with non-stressed controls (Table 1).
Paraventricular Nucleus of Hypothalamus
There was a significant main effect of stress on c-Fos activation within the PVN (F(1,30)=103.08, p < 0.01) but no significant main effect of drug and no significant stress × drug interaction on c-Fos activation within this region. Animals exposed to FST exhibited greater c-Fos activation within the PVN relative to non-stressed controls (Table 1).
Paraventricular Thalamic Nucleus
There was a significant main effect of stress on c-Fos activation in the PVT (F(1,30)=14.24, p < 0.01) but no significant main effect of drug and no significant stress × drug interaction on c-Fos activation within this region. Animals exposed to FST exhibited greater c-Fos activation within the PVT relative to non-stressed controls (Table 1).
Terminal Measures
There were no differences in body, adrenal, thymus and testes weights between animals treated with mifepristone and vehicle (data not shown), suggesting that five days of mifepristone were not sufficient to produce gross changes in reproductive and stress-responsive organs.
Discussion
The aim of the present study was to determine if the potential antidepressant effects of mifepristone were due to modulation of neuroendocrine and central HPA axis responsiveness to stress. The results illustrate that repeated administration of mifepristone (5days, 10mg/kg) decreases immobility and increases swimming behavior in the modified FST, consistent with an antidepressant effect. In addition, patterns of c-Fos induction in the CNS demonstrate that mifepristone induces an increase in neuronal activation in the medial prefrontal cortex and ventral subiculum and inactivation of the central amygdaloid nucleus, all of which are consistent with mifepristone-induced inhibition of central stress activation. Stress-dampening actions of mifepristone pretreatment are corroborated by decreased ACTH concentrations after FST exposure.
Modified Forced Swim Test
In the present study, we utilized the modified version of the FST to assess depression-like behavior and neuroendocrine and central responses to an acute stressor. The modified version of the FST used in this experiment obviates any potential confounds of learning and memory associated with the pretest in the traditional Porsolt FST and allows us to assess the rodent's reactivity to the novel stress environment. In Experiment 1, consistent with previous reports, imipramine was effective in decreasing immobility and increasing climbing and swimming behaviors (Barros and Ferigolo, 1998) indicative of an antidepressant effect of a positive control compound in the modified FST. In Experiment 1, mifepristone was also effective in increasing swimming, but not climbing behaviors. Although there was a strong trend for decreased immobility in the mifepristone treated group versus those treated with vehicle, it did not reach significance. However, in Experiment 2, mifepristone decreased immobility and increased swimming behavior illustrating an antidepressant-like effect of this compound. These findings are consistent with previous reports demonstrating decreased immobility in mifepristone treated animals in the traditional FST (De Kloet et al., 1988; Aisa et al., 2007, 2008). Of note, these studies only measured the impact of mifepristone on immobility; thus the effects of the compound on active behaviors were not discussed.
Neuroendocrine Responses
Mifepristone modestly decreased stress-induced ACTH and although not significantly, reduced corticosterone concentrations when compared to stressed animals treated with vehicle at the15 minute time-point. The lack of a significant effect of mifepristone on stress-induced corticosterone concentrations may be due to timing issues as peak corticosterone levels typically occur at later time points (e.g., 30 min). The decreased stress-responsiveness with mifepristone treatment is consistent with a previous report by (Ratka et al., 1989) in which animals treated systemically with mifepristone prior to exposure to novel environment displayed decreased stress-responsiveness (i.e., corticosterone) compared with vehicle treated animals. However, there are other reports whereby mifepristone increases neuroendocrine responsiveness to restraint stress in females prenatally exposed to ethanol (Glavas et al., 2006).
Our basal ACTH and corticosterone concentrations following mifepristone treatment are consistent with (De Kloet et al., 1988; Ratka et al., 1989; Spencer et al., 1998; Bachmann et al., 2003) which report no difference in tonic HPA axis activity with acute GR antagonism. On the other hand, others find increased basal ACTH and/or corticosterone concentrations following prolonged antagonism of GR with either mifepristone or selective GR antagonists (van Haarst et al., 1996; Bachmann et al., 2003). An increase in basal corticosterone following chronic treatment with mifepristone is not surprising since mifepristone acts by blocking GR which would increase the amount of freely circulating corticosterone. Belanoff and associates (2000) report that increased glucocorticoids following mifepristone treatment may be due to partial disruption of the GR mediated feedback. At the 90 min time-point, animals exposed to FST and treated with mifepristone had significantly higher corticosterone concentrations relative to all other groups, suggesting the possibility of a partial decrease in GR mediated feedback efficacy. These findings are also consistent with (Ratka et al., 1989) which report increased circulating corticosterone levels well after termination of novel environmental stress exposure (i.e., 240 min). Taken together, the present data suggest that mifepristone modulates the feed forward and the feedback components of the HPA axis specifically under periods of elevated glucocorticoids as seen during the FST.
c-Fos Induction
Medial Prefrontal Cortex
Mifepristone increased activity in all regions of the mPFC. The rodent mPFC is divided into three main regions including the anterior cingulate, prelimbic (located in the dorsal mPFC) and the infralimbic (located in the ventral mPFC) cortices. Lesions studies in rats have demonstrated an inhibitory role of the anterior cingulate and prelimbic structures on HPA axis activation (Diorio et al., 1993; Figueiredo et al., 2003). In contrast, the infralimbic subdivision has an excitatory effect on HPA axis activation as bilateral lesions of this region decrease corticosterone response to restraint stress (Sullivan and Gratton, 1999). The infralimbic region in particular projects to many regions associated with HPA axis activation including the anterolateral BNST, CeA and the nucleus of the solitary tract, for review (Herman et al., 2003). The global increase in mPFC activation with mifepristone treatment is notable given that decreased activity in the mPFC is routinely observed in severely depressed patients (Drevets et al., 1997; Siegle et al., 2007).
Hippocampus
In the hippocampus, mifepristone had region specific effects in animals exposed to the FST, as neuronal activity was decreased in certain ventral regions of the hippocampus (CA2, CA3 and hilus) along with a trend for decreased activation in the CA1 region. In contrast, mifepristone increased neuronal activity within the ventral subiculum (the principle output region). The hippocampus is highly stress-responsive and similar to the mPFC, contains an abundance of GR. Both clinical and preclinical data involving depressed individuals or chronic stress regimes demonstrates that the hippocampus undergoes marked alterations in volume size and synaptic plasticity (i.e., neurogenesis) presumably as a result of elevated glucocorticoids (Schmidt and Duman, 2007). The decrease in neuronal excitability within certain divisions of the hippocampus may serve as a protective mechanism to prevent the deleterious effects of glucocorticoids within this neural region. Consistent with this fact, mifepristone prevents stress-induced impairment in hippocampal LTP and corticosterone-induced decreases in hippocampal neurogenesis (Mayer et al., 2006; Mailliet et al., 2008). In contrast, mifepristone increased neuronal activity within the ventral subiculum in animals exposed to the FST. The ventral subiculum functions as the prime outflow area of the hippocampal formation (Herman and Mueller, 2006). Our laboratory has consistently demonstrated that the inhibitory effect of the hippocampus on HPA axis activity is via the ventral subiculum (Herman et al., 1995; Herman et al., 1998; Mueller et al., 2004). Therefore by increasing neuronal activity of this inhibitory output region, mifepristone may inhibit stressed-induced HPA axis outflow.
Amygdala
Mifepristone decreased neuronal activity in the CeA in animals exposed to the FST. Contrary to the inhibitory effects of the mPFC and hippocampus on HPA axis responses, the CeA has an excitatory effect as lesions to this region decrease neuroendocrine and central responses (c-fos) to a number of stressors (Beaulieu et al., 1986; Feldman et al., 1994; Xu et al., 1999). Consistent with its excitatory role in HPA axis activity, the CeA plays an integral role in the expression of fear responses. For example, lesions of the CeA blocks fear potentiated startle reflexes to either auditory or visual stimuli (Kim and Davis, 1993; Campeau and Davis, 1995). Together these findings suggest that the decreased neuronal activation in the CeA in animals exposed to FST and treated with mifepristone may underlie both the decreased neuroendocrine responses and decreased depression-like behavior.
Paraventricular Nucleus of the Hypothalamus
To our surprise, we did not find a significant difference in PVN activation in animals treated with mifepristone and exposed to the FST. The aforementioned regions do not project directly to the PVN but most likely via the BNST, (Herman et al., 2003) an area in which we also did not see a mifepristone-mediated effect on stress responses. This suggests that mifepristone (at least within the context of the current study) dampens the neuroendocrine responses to stress in other areas outside of the PVN. The most likely target area outside of the PVN by which mifepristone modulates the neuroendocrine responses to stress is via direct action at the level of the anterior pituitary. The possibility that mifepristone modulates the neuroendocrine response to stress via interactions with the anterior pituitary is raised by (De Kloet et al., 1988). However, we did not assess c-Fos activation in the pituitary so we can't definitely state whether this is indeed the case. We do want to emphasize that c-Fos expression in the aforementioned brain regions does not necessarily indicate that these are the only brain regions involved in depression and/or stress-related behaviors. For example, there are many other regions that are involved in the stress-induced HPA axis activity and psychopathology and importantly, not all cells express c-Fos. It is also important to note that c-Fos activation may not be solely restricted to HPA axis function as other molecular mechanisms underlying mifepristone treatment may be associated with c-Fos expression.
We administered mifepristone for 5 days to be consistent with preclinical and clinical studies which reported protective effects against stress-induced decreases in neurogenesis (Flores et al., 2006; Mayer et al., 2006; Oomen et al., 2007; Mailliet et al., 2008) and therapeutic effects for the treatment of psychotic depression (Flores et al., 2006) with 4-8 day treatment. However, there are some shortcomings to this treatment regimen. First, prolonged antagonism of GR with mifepristone may permit glucocorticoids to signal through the mineralocorticoid receptor (MR) or the membrane-bound GR. Although MR is mainly viewed as being primarily important for regulating basal HPA axis activity, there are reports that MR is also important for stress-induced HPA axis activation (Pace and Spencer, 2005). Thus, we can't completely rule out the role of MR or the membrane-bound GR on the behavioral, neuroendocrine or central stress responses in the present study. Second, it is difficult to discern whether the reported effects are due to acute or chronic effects of the drug because all of the animals were also injected one hour prior to FST exposure. Finally, there may have been some adaptation due to repeated administration of the drug. The last point is less likely given that we see clear effects of the mifepristone only within the animals that are exposed to the FST.
Concluding remarks
Mifepristone decreased depression-like behavior and modulated the neuroendocrine and central stress responsiveness to the FST. As such, the cognitive appraisal of the FST may have been different in the animals treated with mifepristone versus vehicle. For example, animals treated with mifepristone may not have deemed the FST as “stressful” as those treated with vehicle. This difference in perception and behavior during FST exposure may subsequently alter both the neuroendocrine and central stress responsiveness and vice versa. However, this does not lessen the overall importance of the findings of the present study. The data demonstrate that mifepristone modifies central stress responsiveness in distinct neural regions that are associated with stress-responding and mood.
HPA axis dysregulation (i.e., hypercortisolemia) is often observed in depressed individuals but clinical depression is a complex psychopathology, likely due to several factors. Clearly, not all depressed individuals have alterations in HPA axis function; likewise, disturbances in HPA axis function do not necessarily lead to psychopathology. In fact, only a specific subpopulation of depressed individuals (psychotic depression) consistently demonstrate hypercortisolemia (DeBattista and Belanoff, 2006). Based on this knowledge, there are some limitations to the translation of the findings in the present study. For example, we examined the effect of mifepristone on HPA axis activation and mood in otherwise “normal” animals. However the present study was designed to determine if the reported antidepressant effects of mifepristone were due to decreased neuroendocrine and central responses to stress. In this manner, the FST served as both the stressor as well as a tool to assess depression-like behavior. The modest effects of mifepristone on depression-like behavior in the modified FST may be due to the fact that the drug appears to be particularly effective in alleviating depression-like behavior in individuals with elevated cortisol concentrations as seen in psychotic depressives (Belanoff et al., 2001; DeBattista and Belanoff, 2006; Flores et al., 2006). Consistent with this fact, the modulating effects of mifepristone on neuroendocrine and central stress responses were evident only during periods of elevated glucocorticoid secretion as seen with FST exposure. Therefore, studying the effects of the compound on the behavioral, neuroendocrine and central stress responses under regimens which induce both HPA axis hyperactivity and/or psychopathology are clearly warranted.
Acknowledgments
The authors would like to thank Benjamin Packard for assistance with radioimmunoassays and Kenneth Jones and Nathan Evanson PhD. for assistance with data imaging analysis. We would like to thank Ryan Jankord PhD, Rong Zhang PhD, Yve Ulrich-Lai, PhD, Jonathan Flak, Anne Christiansen and Annette Dekloetfor helping with blood, organ and brain collectssion for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann CG, Linthorst AC, Holsboer F, Reul JM. Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology. 2003;28:1056–1067. doi: 10.1038/sj.npp.1300158. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Bilang-Bleuel A, De Carli S, Linthorst AC, Reul JM. The selective glucocorticoid receptor antagonist ORG 34116 decreases immobility time in the forced swim test and affects cAMP-responsive element-binding protein phosphorylation in rat brain. Neuroendocrinology. 2005;81:129–136. doi: 10.1159/000086413. [DOI] [PubMed] [Google Scholar]
- Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–286. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–254. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. 2001;21:516–521. doi: 10.1097/00004714-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005a;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005b;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, De Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Delbende C, Contesse V, Mocaer E, Kamoun A, Vaudry H. The novel antidepressant, tianeptine, reduces stress-evoked stimulation of the hypothalamo-pituitary-adrenal axis. Eur J Pharmacol. 1991;202:391–396. doi: 10.1016/0014-2999(91)90284-w. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658:21–26. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology. 2006;31:628–636. doi: 10.1038/sj.npp.1300884. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Yu WK, Weinberg J. Effects of mineralocorticoid and glucocorticoid receptor blockade on hypothalamic-pituitary-adrenal function in female rats prenatally exposed to ethanol. Alcohol Clin Exp Res. 2006;30:1916–1924. doi: 10.1111/j.1530-0277.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Ishizaki J, Yamamoto H, Takahashi T, Takeda M, Yano M, Mimura M. Changes in regional cerebral blood flow following antidepressant treatment in late-life depression. Int J Geriatr Psychiatry. 2008;23:805–811. doi: 10.1002/gps.1980. [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol. 1991;261:R1257–1268. doi: 10.1152/ajpregu.1991.261.5.R1257. [DOI] [PubMed] [Google Scholar]
- Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Schlapfer TE, Keysers C, Klingmuller D, Maier W, Fink GR, Hurlemann R. Modeling a negative response bias in the human amygdala by noradrenergic-glucocorticoid interactions. J Neurosci. 2008;28:12868–12876. doi: 10.1523/JNEUROSCI.3592-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailliet F, Qi H, Rocher C, Spedding M, Svenningsson P, Jay TM. Protection of stress-induced impairment of hippocampal/prefrontal LTP through blockade of glucocorticoid receptors: implication of MEK signaling. Exp Neurol. 2008;211:593–596. doi: 10.1016/j.expneurol.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Mayer JL, Klumpers L, Maslam S, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18:629–631. doi: 10.1111/j.1365-2826.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudery M, Mocaer E, Froger N, Lanfumey L, Laviola G, Casolini P, Zuena AR, Marzano L, Hamon M, Maccari S. Chronic treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT(1A) receptor mRNA in prenatally stressed rats. Neuropharmacology. 2004;47:841–847. doi: 10.1016/j.neuropharm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145:3763–3768. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- Pace TW, Spencer RL. Disruption of mineralocorticoid receptor function increases corticosterone responding to a mild, but not moderate, psychological stressor. Am J Physiol Endocrinol Metab. 2005;288:E1082–1088. doi: 10.1152/ajpendo.00521.2004. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Depression, stress and the adrenal axis. J Neuroendocrinol. 2003;15:811–812. doi: 10.1046/j.1365-2826.2003.01058.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Ratka A, Sutanto W, Bloemers M, de Kloet ER. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989;50:117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- Reul JM, Labeur MS, Grigoriadis DE, De Souza EB, Holsboer F. Hypothalamic-pituitary-adrenocortical axis changes in the rat after long-term treatment with the reversible monoamine oxidase-A inhibitor moclobemide. Neuroendocrinology. 1994;60:509–519. doi: 10.1159/000126788. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Kim PJ, Kalman BA, Cole MA. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology. 1998;139:2718–2726. doi: 10.1210/endo.139.6.6029. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson F, Craighead M. Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochem Res. 2008;33:691–707. doi: 10.1007/s11064-007-9518-3. [DOI] [PubMed] [Google Scholar]
- van Haarst AD, Oitzl MS, Workel JO, de Kloet ER. Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology. 1996;137:4935–4943. doi: 10.1210/endo.137.11.8895366. [DOI] [PubMed] [Google Scholar]
- Xu Y, Day TA, Buller KM. The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1beta administration. Neuroscience. 1999;94:175–183. doi: 10.1016/s0306-4522(99)00311-5. [DOI] [PubMed] [Google Scholar]




