Abstract
Understanding gene regulatory networks controlling properties of pluripotent stem cells will facilitate development of stem cell-based therapies. The transcription factor Foxd3 is critical for maintenance of self-renewal, survival, and pluripotency in murine embryonic stem cells (ESCs). Using a conditional deletion of Foxd3 followed by gene expression analyses, we demonstrate that genes required for several developmental processes including embryonic organ development, epithelium development, and epithelial differentiation were misregulated in the absence of Foxd3. Additionally, we identified 6 novel targets of Foxd3 (Sox4, Safb, Sox15, Fosb, Pmaip1 and Smarcd3). Finally, we present data suggesting that Foxd3 functions upstream of genes required for skeletal muscle development. Together, this work provides further evidence that Foxd3 is a critical regulator of murine development through the regulation of lineage specific differentiation.
Keywords: ES cells, transcription, Foxd3, Sox15, skeletal muscle
1. Introduction
Embryonic stem cells (ESCs) are a unique cell type with the ability to self-renew and differentiate into all embryonic lineages. Due to multiple issues surrounding the feasibility and ethical considerations of using human ESCs (hESCs), one goal of stem cell biologists is to determine the transcriptional networks controlling stem cell properties in other models of pluripotent stem cells including murine ESCs and/or induced pluripotent stem cells (iPSCs). Our lab determined that the Forkhead transcription factor Foxd3 is required for self-renewal and potency of ESCs1. Without Foxd3, several signature stem cell proteins and their corresponding mRNAs (including Oct4, Sox2, and Nanog) are maintained at relatively normal levels suggesting that Foxd3 is not required for their expression. Despite the maintained expression of these genes, ESCs lacking Foxd3 lose key stem cell properties. They are no longer pluripotent; they differentiate into mesendoderm and trophectoderm lineages under conditions that normally maintain pluripotency. Additionally, inducible-mutant ESCs lose self-renewal capacity and undergo aberrant apoptosis1. While Foxd3 is not one of the “core” transcription factors sufficient for reprogramming somatic cells into iPSCs2, it is indispensable for generating iPSCs; mouse embryonic fibroblasts lacking Foxd3 cannot be reprogrammed into pluripotent stem cells (Suflita, Labosky, and Ess, 2013 unpublished data). Together, these data demonstrate that Foxd3 functions downstream of, or in a pathway parallel to, other stem cell factors and is required for self-renewal and pluripotency of ESCs.
Because Foxd3 regulates stem cell properties in multiple lineages1,3–7, Foxd3 target genes must regulate self-renewal, pluripotency, and/or survival of stem cells. Currently, only two direct targets of Foxd3 have been identified (Alb1 and the λ5-preB locus)8,9. Therefore, we sought to identify additional targets of Foxd3. Using microarrays, qRT-PCR, and ChIP assays, we identified 6 novel targets of Foxd3: Sox4, Safb, Sox15, Fosb, Pmaip1 and Smarcd3. Additionally, we present data that Foxd3 functions upstream of genes required for skeletal muscle differentiation.
2. Materials and Methods
2.1 Cell Culture
Foxd3 inducible-mutant ESCs lines were previously characterized1. The cells were maintained using standard procedures10. To generate EBs, ESCs were dissociated into a single cell suspension, preplated to deplete feeder cells, and diluted to a final concentration of 20,000 cells/mL in ESC medium lacking LIF. Tamoxifen (TM, 2μM) was added to mutant cultures, and 400 cells (20 uL) were placed on the underside of a culture dish lid to form hanging drops11. After 3 days in culture, EBs were harvested for RNA analysis.
2.2 Immunocytochemistry
Immunocytochemistry to detect Foxd3 protein was performed following standard techniques1 with the Foxd3 primary antiserum7 diluted in blocking (5% normal donkey serum in PBS) solution (1:1000).
2.3 RNA Isolation and qRT-PCR
ESCs were harvested, RNA extracted as described1, and cDNA generated using the GoScript Reverse Transcription System (Promega). cDNA samples were amplified in an Applied Biosystems 7900HT Real-Time PCR system using GoTaq qPCR Master Mix (Promega). Relative gene expression was calculated as described12. Primer sequences are listed in Table S1. Statistical significance was determined using a two-tailed Student’s t-test.
2.4 Microarray Analysis
Microarray images were scanned with an Affymetrix high resolution GenePix 4000B scanner. Raw .CEL files were uploaded into Partek Genomics Suite version 6.6 (Partek Incorporated), processed using Robust Multi-chip Average (RMA) normalization13, and all three possible individual pairwise comparisons of average group values were analyzed with one-way ANOVA. Probes that showed at least 1.5-fold change with a p-value less than 0.05 were considered significantly altered.
Gene functions were determined using NCBI Entrez Gene, Stanford SOURCE, Aceview, and Pubmed databases. Sequences for differential probes not associated with transcripts, based on Affymetrix database annotations, were retrieved from the Affymetrix NetAffx Analysis Center web site. Statistical analyses (including corrections for multiple hypothesis testing) for identification of overrepresented functional categories and pathways were performed using Partek Genomics Suite and DAVID14.
2.5 Chromatin Immunoprecipitation
Chromatin Immunoprecipitation (ChIP) assays were performed using established methods8. DNA was immunoprecipitated, purified, and amplified using qPCR (as above). The ΔΔCt method was used to calculate enrichment of Foxd3 at putative binding sites and Ser5-PolII enrichment at the proximal promoter of target genes. First, the Ct of the immunoprecipitated sample was normalized to input DNA for each amplicon (ΔCt). Next, the ΔCt values obtained from the Foxd3 (Millipore) and Ser5 PolII (Abcam) immunoprecipitated samples were normalized to the ΔCt of non-specific IgG (Santa Cruz) immunoprecipitated sample (ΔΔCt). In the case of PolII ChIP, samples were normalized by dividing the ΔΔCt value of the TM-treated samples by the ΔΔCt value of the untreated samples. Primer sequences are listed in Table S1. Statistical significance was determined using a Student’s t-test comparing the enrichment of not-treated and TM-treated ESCs.
3. Results
3.1 Foxd3 regulates developmental processes
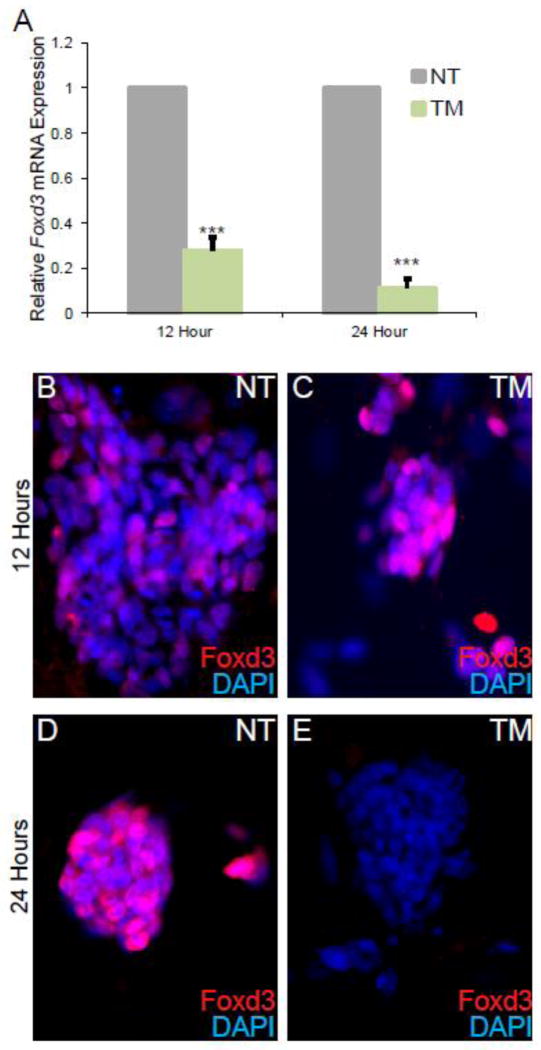
To characterize the function of Foxd3, we used ESCs carrying two conditional alleles of Foxd3 with the entire coding sequence flanked by LoxP sites6. To delete the locus, the ESCs also carried a ubiquitously expressed Cre recombinase transgene1. Upon addition of Tamoxifen (TM), the Foxd3 coding sequence was deleted. Using qRT-PCR, we determined that Foxd3 mRNA levels were not significantly reduced until 12 hours after the addition of TM, and this reduction in Foxd3 mRNA was more pronounced following 24 hours of TM treatment (Fig. 1A). To determine when Foxd3 protein was diminished, we performed fluorescent immunocytochemistry. After 12 hours of culture with TM, Foxd3 protein was reduced but could still be detected (Figs. 1B–C). However, 24 hours after TM addition, Foxd3 protein was only rarely detected (Figs. 1D–E), suggesting that Foxd3 protein persists after loss of Foxd3 mRNA, presumably due to the half-life of the protein. Therefore, to enable us to detect gene regulatory differences due to the loss of Foxd3, we completed our experiments after ESCs were cultured with TM for at least 24 hours.
Figure 1. Foxd3 protein cannot be detected after 24 hours in culture with Tamoxifen (TM).
A. qRT-PCR analysis of Foxd3 mRNA levels after 12 and 24 hours of culture with TM. Relative Foxd3 expression is decreased in TM-treated ESCs (green) at both time points compared to untreated controls (grey). Error bars indicate SEM. *** p<0.001. N=3 experiments. The expression of Foxd3 in NT cells is set to 1. B–E. Immunocytochemistry analysis of Foxd3 protein expression (red) after 12 (B–C) and 24 (D–E) hours in culture in NT (B,D) and TM-treated (C,E) ESCs. Nuclei are indicated by DAPI (blue).
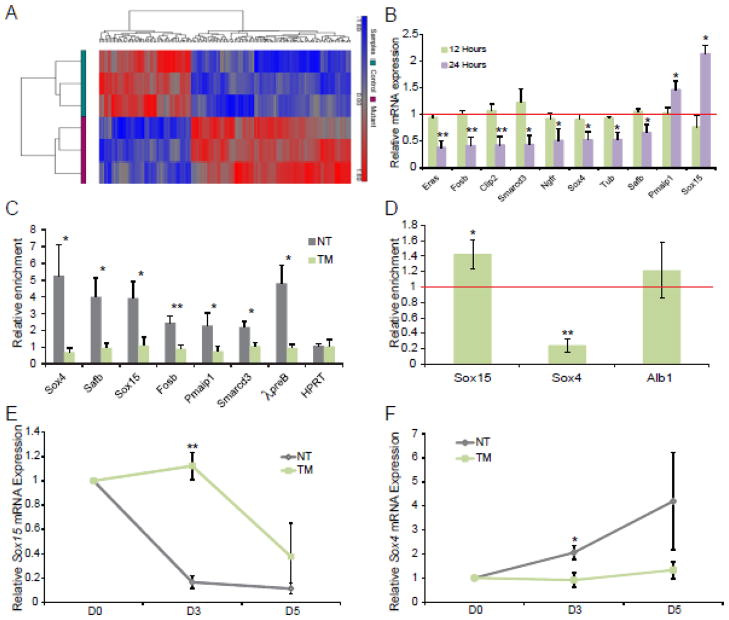
To characterize genes misregulated in the absence of Foxd3, we used Affymetrix Gene/Exon microarrays to determine which genes were misregulated in the absence of Foxd3 after 24 hours of TM treatment (n=3 hybridizations of each group). Statistical analysis of the TM treated versus not treated cells yielded 423 significantly differentially expressed probes (Table S2). Hierarchical clustering of normalized hybridization signals for these 423 probes successfully separated the TM-treated from untreated cells based on gene expression patterns (Fig. 2A), suggesting that the findings from each experiment were highly reproducible and gene expression patterns between control cells and TM-treated cells were distinct.
Figure 2. Identification of direct targets of Foxd3.
A. Hierarchical clustering of 423 probes detected as significantly differential (at least 1.5-fold, p-value < 0.05) between NT and TM treated ESCs. Values shown are log2, and bright red, bright blue, and gray indicate the highest, lowest, and median normalized signal values, respectively. Vertical dendrograms represent the individual samples, of which there are three replicates for each sample type. B. qRT-PCR validation of misregulated genes identified using microarrays at 12 (green) and 24 (purple) hours post TM treatment. The red line indicates the relative gene expression levels in NT control ESCs. N=4 experiments. C. qPCR following ChIP experiments was used to identify novel targets of Foxd3. The data are portrayed as enrichment over a non-specific rabbit IgG antibody in untreated (NT, grey) and TM treated (green) ESCs after 24 hours in culture. The λ5preB locus serves as a positive control while the HPRT coding sequence serves as a negative control. N=5 experiments. D. qPCR following ChIP experiments to determine if Ser5-PolII occupancy is altered at the proximal promoters of Sox15 and Sox4 in the absence of Foxd3. The data depict the enrichment of Ser5-PolII in induced mutant (TM) ESCs after 48 hours in culture normalized to untreated cells (red line). The enrichment at a sequence −7kb from the Alb1 gene serves as a negative control; enrichment in both TM and untreated cells are at least 3-fold less at this sequence than at Sox4 and Sox15 proximal promoters. E–F. qRT-PCR analysis of Sox15 (E) and Sox4 (F) expression in untreated (control, grey) and TM treated (induced mutant, green) EBs at Day 0 (D0) and Day 3 (D3) normalized to expression in untreated ESCs at D0. N=3 experiments. In all panels, error bars indicate SEM. *p<0.05 **p<0.01, ***p<0.001.
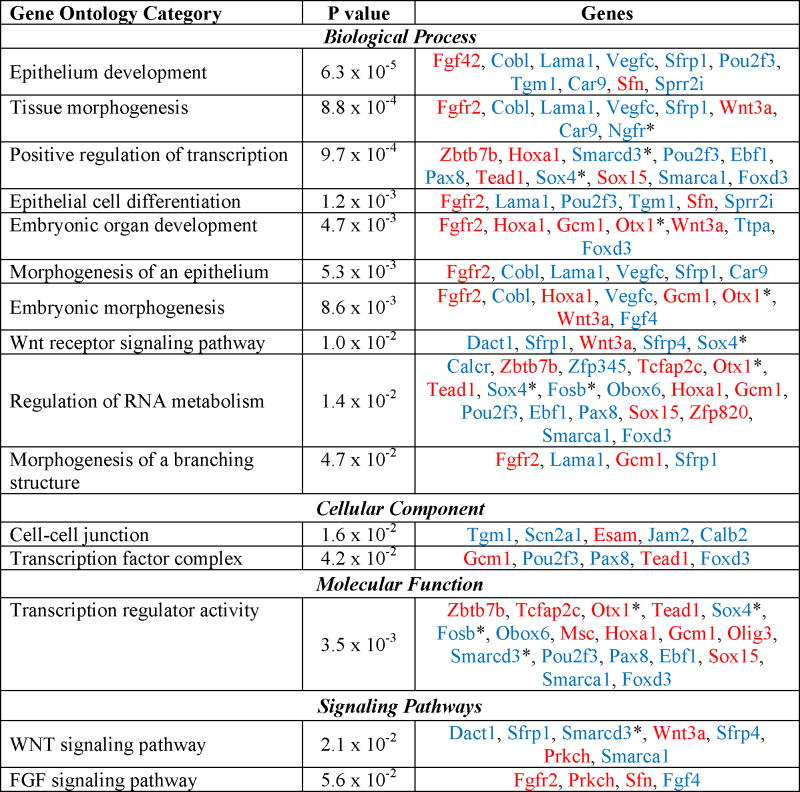
To further analyze the function of genes misregulated in the absence of Foxd3, we used functional analysis program, DAVID, to identify significantly enriched gene ontologies (Table 1). These data suggested that Foxd3 regulates genes controlling several developmental processes including embryonic organ development, epithelium development, and epithelial differentiation. On a pathway level, Foxd3 regulates components of the Wnt and FGF signaling pathways (Table 1), specifically Fgf4 and its receptor Fgf2r15. Strikingly, mice lacking β-catenin, a downstream mediator of canonical Wnt signaling, die at approximately 6.5 days post coitum (dpc) with disrupted embryonic tissues and morphologically normal extraembryonic tissue16, and Fgf4 null embryos die around implantation due to impaired expansion of the epiblast17. The timing of lethality and phenotype of both the Wnt and FGF4 signaling mutants is similar to the lethality of Foxd3 null embryos, consistent with the possibility that Foxd3 regulates these pathways in vivo3. Finally, loss of Foxd3 in ESCs also impacts expression of transcription factors as indicated by enrichment of three functional categories: transcription regulator activity, transcription factor complex, and positive regulation of transcription. Interestingly, as summarized in Table 1, expression of some misregulated genes is increased in the absence of Foxd3 (red) while expression of others is decreased (blue) suggesting that Foxd3 both activates and represses these biological processes. Our findings also suggest that Foxd3 functions in stem cells by primarily regulating development and differentiation.
Table 1. Enriched Functional Categories in Mutant Cells.
Functional classification of genes misregulated after 24 hours of TM treatment. The p-value was calculated by the online database and functional analysis program, DAVID using Fisher’s Exact Test. Upregulated genes are indicated by red text while downregulated genes are indicated by blue text.
|
These genes fell below the cut-off criteria chosen for analysis of microarray data but were validated by qRT-PCR.
Analysis of the results obtained from the microarray allowed us to prioritize the verification of changes in expression of genes encoding proteins known to control stem cell properties in addition to those with unknown function. Using qRT-PCR to assay gene expression, we confirmed 10 genes of interest that were significantly misregulated (p-value < 0.05) after 24 hours of TM treatment (Fig. 2B, purple). We also assayed gene expression after 12 hours of TM treatment, and unsurprisingly, found that no genes of interest were misregulated (Fig. 2B, green). Eight of the misregulated genes (Eras, Fosb, Clip2, Smarcd3, Ngfr, Sox4, Tub, and Safb) were downregulated while 2 (Pmaip1 and Sox15) were upregulated, suggesting that Foxd3 both positively and negatively regulates expression of putative target genes.
3.2 Identification of direct targets of Foxd3
To determine whether the misregulated genes are direct targets of Foxd3, we used rVista to identify putative Foxd3 binding sites less than 20 kb away from the misregulated genes, and verified Foxd3 occupancy using chromatin immunoprecipitation (ChIP) assays followed by quantitative PCR (qPCR). Using this assay, we determined that 6 of the 10 misregulated genes (Sox4, Safb, Sox15, Fosb, Pmaip1 and Smarcd3) were direct targets of Foxd3 (Fig. 2C, grey bars). Foxd3 occupancy at the λpreB locus served as a positive control8, while the Hprt coding sequence served as a negative control. To validate specificity of the antibody, we analyzed Foxd3 occupancy in TM treated ESCs and, as expected, did not detect Foxd3 at any loci (Fig. 2C, green bars). Together, these results indicate that the identified binding sites are novel targets of Foxd3. The Foxd3 binding sites near Fosb, Safb, Smarcd3, and Sox4 are conserved among mice, rats, and humans; however, the antibody used for ChIP assays did not provide reproducible results in hESCs (data not shown).
To further characterize the role of Foxd3 in regulating ES cell properties, we chose to analyze developmental processes misregulated in the absence of Foxd3. Furthermore, published data demonstrates that Foxd3 and Sox2 interact in ESCs and are replaced by Foxp1 and Sox4 in differentiating B cells8. Therefore, we focused our analyses on the misregulated Sox family members, Sox15 and Sox4. We detected Foxd3 bound at regions 4.8 kb upstream of the Sox15 transcriptional start site and 9.5 kb downstream of the Sox4 gene (Fig. 2C). To determine if Sox15 and Sox4 transcription is altered in the absence of Foxd3, we used ChIP assays to analyze the occupancy of RNA Polymerase II phosphorylated at Serine5 (Ser5-PolII). There was a significant increase in Ser5-PolII occupancy at the Sox15 proximal promoter in TM-treated cells compared to controls. Alternatively, Ser5-PolII occupancy was drastically decreased at the Sox4 promoter in ESCs lacking Foxd3 (Fig. 2D). These data suggest that Foxd3 directly regulates the transcription of these two target genes.
Additionally, using an embryoid body (EB) assay to analyze gene expression in differentiating cells, we determined that Sox15 mRNA levels quickly decreased while Sox4 mRNA levels gradually increased upon differentiation of untreated ESCs (Fig. 2E–F, grey). Consistent with increased Sox15 mRNA levels in ESCs lacking Foxd3, Sox15 mRNA is maintained in EBs lacking Foxd3 (Figure 2E, green), while Sox4 mRNA was decreased in TM-treated cells (Figure 2F, green). Together, these data indicate that Sox4 and Sox15 expression is altered in EBs lacking Foxd3.
3.3 Foxd3 functions upstream of genes required for skeletal muscle development
While some data suggest that Foxd3 functions as a transcriptional activator18–20, compelling evidence indicates that Foxd3 functions as a transcriptional repressor in mesoderm induction in Xenopus21,22. Therefore, we focused our analyses on Sox15, which is repressed by Foxd3. Because Sox15 is regulated by Foxd3, and Sox15 is a critical regulator of skeletal muscle differentiation in vitro23–25, we sought to first characterize the effects of loss of Foxd3 on genes functioning downstream of Sox15 required to regulate skeletal muscle development. Skeletal muscle is derived from paraxial mesoderm and requires the myogenic bHLH transcription factors MyoD and Myf5 for differentiation26. Following determination to the skeletal muscle lineage, myoblast progenitor cells divide, align, and fuse to generate multinucleated myotubes, resulting in mature muscle fibers that also contain muscle stem cells. A putative stem cell population, the satellite cells, is capable of proliferating to generate new myoblasts that fuse with mature muscle fibers27.
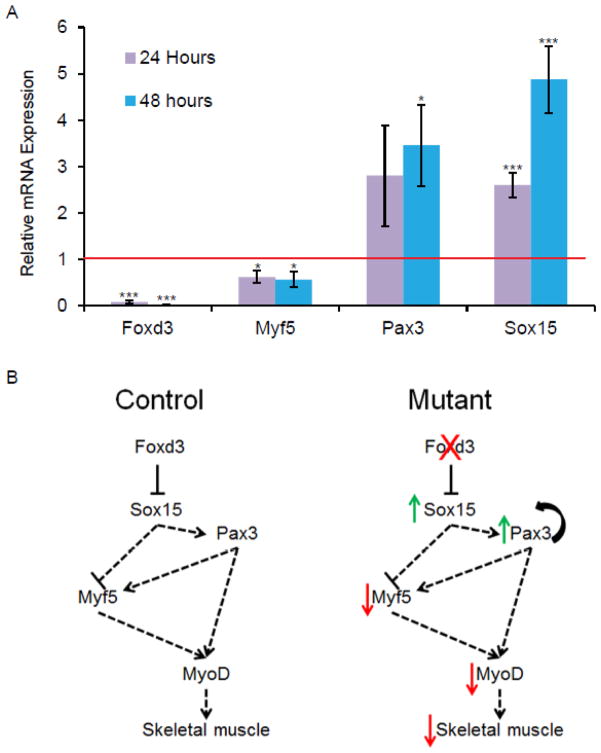
The transcription factors Pax3 and Sox15 function upstream of Myf5 and MyoD23–25. Sox15 null animals cannot regenerate skeletal muscle following injury, while overexpression of Sox15 results in increased Pax3 expression, decreased Myf5 expression, and an expansion of immature myoblasts25,28. Because Sox15 is upregulated in Foxd3 induced mutant ESCs and Sox15 inhibits myogenesis, we hypothesized that ESCs lacking Foxd3 cannot be directed to produce mature skeletal muscle. Additionally, Foxd3 is expressed in the paraxial mesoderm of mouse embryos, further suggesting that Foxd3 may be an important regulator of skeletal muscle development29. To determine if genes functioning downstream of Sox15 are misregulated in the absence of Foxd3, we assayed mRNA levels of these myogenic genes in differentiating ESCs using qRT-PCR. Consistent with our hypothesis, Myf5 expression decreased while Pax3 expression increased (Fig. 3A), suggesting that Foxd3 functions upstream of Sox15 to regulate myogenesis.
Figure 3. Foxd3 functions upstream of genes required for skeletal muscle development.
A. qRT-PCR data demonstrating the relative expression of Foxd3, Myf5, and Pax3 mRNA in Foxd3 induced mutant ESCs cultured for 24 (purple) and 48 (blue) hours. Red line indicates expression of these mRNAs in untreated ESCs. Error bars indicate SEM. *p< 0.05, ***p< 0.001 B. Model of Foxd3 and Sox15 in mESCs in the process of skeletal muscle differentiation. In a control progenitor cell (left), Foxd3 represses Sox15 allowing precise regulation of Pax3 and Myf5 and proper skeletal muscle development. In the absence of Foxd3 (right), Sox15 is upregulated, resulting in an increase in Pax3 and a decrease in Myf5 expression.
4. Discussion
We identified several genes, pathways, and biological functions that are misregulated in ESCs lacking Foxd3. Additionally, we identified 6 novel targets of Foxd3: Sox4, Safb, Sox15, Fosb, Pmaip1, and Smarcd3. We further characterized the expression of genes that function downstream of Sox15, and we showed that Foxd3 directly or indirectly regulates genes required for skeletal muscle development and regeneration, uncovering a novel role for Foxd3.
The data presented in Figure 3, together with previous work in the lab1, suggest that Foxd3 induced mutant ESCs precociously express genes required for mesoderm induction, but they are likely unable to differentiate into skeletal muscle. These data are consistent with the model shown in Fig. 3B in which Foxd3 represses Sox15 transcription resulting in increased Pax3 and decreased Myf5 expression in ESCs undergoing differentiation. An increase in Pax3 in skeletal muscle progenitors may result in increased self-renewal and decreased differentiation, limiting the number of mature skeletal muscle fibers30,31. Additionally, decreased Myf5 may result in decreased generation of skeletal muscle. The data presented here are consistent with a recent publication demonstrating the function of FOXD3 in hESCs; overexpression of FOXD3 in hESCs induces differentiation to paraxial mesoderm, including differentiation into skeletal myoblasts29. Together, these data suggest a conserved function for Foxd3 in regulating skeletal muscle development in mammals.
We hypothesize that the other targets of Foxd3 (Sox4, Safb, Fosb, Pmaip1 and Smarcd3) also regulate ES cell properties, and based on published accounts, several of these targets are of future interest. The transcription factor Sox4 is required for cardiac outflow tract development32–34, a process regulated by the cardiac neural crest, another multipotent progenitor population in which Foxd3 function is critical5,6,35. The transcription factor FBJ osteosarcoma oncogene B (Fosb) promotes osteoblast differentiation while inhibiting adipogenesis36 suggesting that inhibition of Fosb by Foxd3 regulates differentiation of these lineages. Smarcd3 (also called Baf60c), a member of the Swi/Snf chromatin remodeling complex, associates with MyoD to promote transcription of genes required for myogenesis37,38. While Sox15, Sox4, Fosb, and Smarcd3 have been implicated in regulating differentiation of disparate lineages, no one has carefully investigated the role of these proteins in maintaining ESC properties, and it is possible that Sox4, Fosb, and Sox15 are involved in maintaining pluripotency in ESCs.
In addition to genes regulating pluripotency, two novel Foxd3 targets have the potential to regulate self-renewal of ESCs. Smarcd3 is a component of a Swi/Snf complex and is required to regulate self-renewal of neural stem cells39. While Smarcd3 mRNA can be detected in ESCs (Figure 2B), to date, no one has analyzed the requirement for this protein in regulating self-renewal of ESCs. In addition, the function of the ubiquitously expressed nuclear scaffolding protein, Safb, has yet to be determined. It has been suggested that Safb may regulate the cell cycle, consistent with the possibility that Safb is required for ES cell proliferation and/or self-renewal40–42. Together, this evidence from the literature is consistent with the hypothesis that these new targets of Foxd3 may regulate self-renewal in ESCs.
Lastly, some targets of Foxd3 are also required to prevent aberrant apoptosis. Safb indirectly represses apoptotic genes in breast cancer cells43,44. Therefore, decreased Safb expression in Foxd3 mutant ESCs may lead to an increase in apoptosis. Finally, Pmaip1 (also called Noxa) is a direct target of Foxd3 and is a critical regulator of cell death. Pmaip1 is required for the activation of caspases and contributes to p53-dependent apoptosis45–47.
Altogether, these data from our laboratory and others suggest that Foxd3 functions upstream of critical regulators of stem cell properties. Prior to this manuscript, only two direct targets of Foxd3 were identified, and the work reported here has uncovered several factors that function downstream of Foxd3. Additional characterization of the function of these factors in ESCs will further elucidate gene regulatory networks controlling stem cell properties.
Supplementary Material
Highlights.
Foxd3 regulates expression of genes controlling developmental processes
Foxd3 both positively and negatively regulates expression of downstream genes
Newly identified direct targets of Foxd3 include: Sox4, Safb, Sox15, Fosb, Pmaip1, and Smarcd3
Foxd3 functions upstream of genes required for skeletal muscle development
Acknowledgments
We thank Adam Bazinet and Drs. Steve Dalton and Ali Brivanlou for their careful reading of this manuscript. P.A.L. was supported by NIH R01HD036720, a pilot grant from NIH 5P01GM085354, and the Vanderbilt University Medical Center Academic Support program. J.L.P. was supported by AHA 10PRE4500024. M.T.S was supported by NIH T32HD007043. C.L.G. was supported by NIH U01HL100398.
Footnotes
All experiments were carried out at Vanderbilt University Medical Center.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu Y, Labosky PA. Regulation of embryonic stem cell self-renewal and pluripotency by Foxd3. Stem Cells. 2008;26:2475–84. doi: 10.1634/stemcells.2008-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–61. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundell NA, et al. Enteric nervous system specific deletion of Foxd3 disrupts glial cell differentiation and activates compensatory enteric progenitors. Dev Biol. 2012;363:373–87. doi: 10.1016/j.ydbio.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mundell NA, Labosky PA. Neural crest stem cell multipotency requires Foxd3 to maintain neural potential and repress mesenchymal fates. Development. 2011;138:641–52. doi: 10.1242/dev.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–24. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tompers DM, Foreman RK, Wang Q, Kumanova M, Labosky PA. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Dev Biol. 2005;285:126–37. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Liber D, et al. Epigenetic priming of a pre-B cell-specific enhancer through binding of Sox2 and Foxd3 at the ESC stage. Cell Stem Cell. 2010;7:114–26. doi: 10.1016/j.stem.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, et al. Pioneer factor interactions and unmethylated CpG dinucleotides mark silent tissue-specific enhancers in embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:12377–82. doi: 10.1073/pnas.0704579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy A. Manipulating the mouse embryo : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2003. p. viii.p. 764. [Google Scholar]
- 11.Samuelson LC, Metzger JM. Differentiation of Embryonic Stem (ES) Cells Using the Hanging Drop Method. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot4485. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 14.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 15.Bellosta P, et al. Identification of receptor and heparin binding sites in fibroblast growth factor 4 by structure-based mutagenesis. Mol Cell Biol. 2001;21:5946–57. doi: 10.1128/MCB.21.17.5946-5957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haegel H, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 17.Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267:246–9. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- 18.Lee HC, Huang HY, Lin CY, Chen YH, Tsai HJ. Foxd3 mediates zebrafish myf5 expression during early somitogenesis. Dev Biol. 2006;290:359–72. doi: 10.1016/j.ydbio.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Thomas AJ, Erickson CA. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development. 2009;136:1849–58. doi: 10.1242/dev.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan G, Li J, Zhou Y, Zheng H, Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J. 2006;20:1730–2. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- 21.Steiner AB, et al. FoxD3 regulation of Nodal in the Spemann organizer is essential for Xenopus dorsal mesoderm development. Development. 2006;133:4827–38. doi: 10.1242/dev.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaklichkin S, Steiner AB, Lu Q, Kessler DS. FoxD3 and Grg4 physically interact to repress transcription and induce mesoderm in Xenopus. J Biol Chem. 2007;282:2548–57. doi: 10.1074/jbc.M607412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beranger F, Mejean C, Moniot B, Berta P, Vandromme M. Muscle differentiation is antagonized by SOX15, a new member of the SOX protein family. J Biol Chem. 2000;275:16103–9. doi: 10.1074/jbc.275.21.16103. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, et al. Sox15 is required for skeletal muscle regeneration. Mol Cell Biol. 2004;24:8428–36. doi: 10.1128/MCB.24.19.8428-8436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage J, Conley AJ, Blais A, Skerjanc IS. SOX15 and SOX7 differentially regulate the myogenic program in P19 cells. Stem Cells. 2009;27:1231–43. doi: 10.1002/stem.57. [DOI] [PubMed] [Google Scholar]
- 26.Rudnicki MA, et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–9. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 27.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama M, Ichisaka T, Nakagawa M, Yamanaka S. Differential roles for Sox15 and Sox2 in transcriptional control in mouse embryonic stem cells. J Biol Chem. 2005;280:24371–9. doi: 10.1074/jbc.M501423200. [DOI] [PubMed] [Google Scholar]
- 29.Arduini BL, Brivanlou AH. Modulation of FOXD3 activity in human embryonic stem cells directs pluripotency and paraxial mesoderm fates. Stem Cells. 2012;30:2188–98. doi: 10.1002/stem.1200. [DOI] [PubMed] [Google Scholar]
- 30.Epstein JA, Lam P, Jepeal L, Maas RL, Shapiro DN. Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem. 1995;270:11719–22. doi: 10.1074/jbc.270.20.11719. [DOI] [PubMed] [Google Scholar]
- 31.Young AP, Wagers AJ. Pax3 induces differentiation of juvenile skeletal muscle stem cells without transcriptional upregulation of canonical myogenic regulatory factors. J Cell Sci. 2010;123:2632–9. doi: 10.1242/jcs.061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schilham MW, et al. Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature. 1996;380:711–4. doi: 10.1038/380711a0. [DOI] [PubMed] [Google Scholar]
- 33.Ya J, et al. Sox4-deficiency syndrome in mice is an animal model for common trunk. Circ Res. 1998;83:986–94. doi: 10.1161/01.res.83.10.986. [DOI] [PubMed] [Google Scholar]
- 34.Maschhoff KL, Anziano PQ, Ward P, Baldwin HS. Conservation of Sox4 gene structure and expression during chicken embryogenesis. Gene. 2003;320:23–30. doi: 10.1016/j.gene.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Nelms BL, Pfaltzgraff ER, Labosky PA. Functional interaction between Foxd3 and Pax3 in cardiac neural crest development. Genesis. 2011;49:10–23. doi: 10.1002/dvg.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabatakos G, et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–90. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 37.Forcales SV, et al. Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J. 2012;31:301–16. doi: 10.1038/emboj.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochi H, Hans S, Westerfield M. Smarcd3 regulates the timing of zebrafish myogenesis onset. J Biol Chem. 2008;283:3529–36. doi: 10.1074/jbc.M708594200. [DOI] [PubMed] [Google Scholar]
- 39.Lamba DA, Hayes S, Karl MO, Reh T. Baf60c is a component of the neural progenitor-specific BAF complex in developing retina. Dev Dyn. 2008;237:3016–23. doi: 10.1002/dvdy.21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huerta M, et al. Cyclin D1 is transcriptionally down-regulated by ZO-2 via an E box and the transcription factor c-Myc. Mol Biol Cell. 2007;18:4826–36. doi: 10.1091/mbc.E07-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapia R, et al. Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle. Mol Biol Cell. 2009;20:1102–17. doi: 10.1091/mbc.E08-03-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debril MB, et al. Scaffold attachment factor B1 directly interacts with nuclear receptors in living cells and represses transcriptional activity. J Mol Endocrinol. 2005;35:503–17. doi: 10.1677/jme.1.01856. [DOI] [PubMed] [Google Scholar]
- 43.Lee YB, Colley S, Norman M, Biamonti G, Uney JB. SAFB re-distribution marks steps of the apoptotic process. Exp Cell Res. 2007;313:3914–23. doi: 10.1016/j.yexcr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Chan CW, et al. A novel member of the SAF (scaffold attachment factor)-box protein family inhibits gene expression and induces apoptosis. Biochem J. 2007;407:355–62. doi: 10.1042/BJ20070170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–70. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 46.Oda E, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 47.Yakovlev AG, et al. BOK and NOXA are essential mediators of p53-dependent apoptosis. J Biol Chem. 2004;279:28367–74. doi: 10.1074/jbc.M313526200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.