Abstract
Purpose
REOLYSIN (Oncolytics Biotech) consists of a wild-type oncolytic reovirus, which has selective cytotoxicity for tumor cells while sparing normal cells. In a phase I study as a single agent, repeated infusions of reovirus were safe with evidence of antitumor activity. Preclinical studies indicate potential for synergy between reovirus and chemotherapeutic agents. A multicenter, phase I dose escalation study was designed to assess the safety of combining reovirus with docetaxel chemotherapy in patients with advanced cancer.
Experimental Design
Patients received 75 mg/m2 docetaxel (day 1) and escalating doses of reovirus up to 3 × 1010 TCID50 (days 1-5) every 3 weeks.
Results
Twenty-five patients were enrolled, and 24 patients were exposed to treatment, with 23 completing at least one cycle and 16 suitable for response assessment. Dose-limiting toxicity of grade 4 neutropenia was seen in one patient, but the maximum tolerated dose was not reached. Antitumor activity was seen with one complete response and three partial responses. A disease control rate (combined complete response, partial response, and stable disease) of 88% was observed. Immunohistochemical analysis of reovirus protein expression was observed in posttreatment tumor biopsies from three patients.
Conclusion
The combination of reovirus and docetaxel is safe, with evidence of objective disease response, and warrants further evaluation in a phase II study at a recommended schedule of docetaxel (75 mg/m2, three times weekly) and reovirus (3 × 1010 TCID50, days 1-5, every 3 weeks).
Reovirus type 3 Dearing (REOLYSIN, Oncolytics Biotech; Reovirus, Oncolytics Biotech), is a wild-type double-stranded RNA virus, which is ubiquitous and non-pathogenic in humans (1). It has been shown to be oncolytic by its ability to replicate selectively in transformed cells, but not in normal cells (2). Despite a significant humoral response, reovirus is capable of oncolysis of tumors after both local injection and systemic administration in murine models and clinical trials (3-7). Activation of the Ras pathway in transformed cells, or the upstream or downstream elements, is an important factor in the permissiveness of a cell to reovirus oncolysis (8). This is in part due to the inability of Ras-activated cells to phosphorylate cellular PKR, but also due to enhancement of virus uncoating, particle infectivity, and apoptosis-dependent release (9). As mutations that activate Ras itself, or elements in its pathway, are present in >60% of cancers (10), these cancers are therefore potential targets for reovirus oncolysis.
Several phase I studies of reovirus as a single agent have been completed in patients with advanced, refractory cancer (reviewed in ref. 11). Three early trials focused on intralesional delivery of reovirus, which was found to be safe with evidence of response in both injected tumor and distant metastases (5). Systemic delivery of reovirus has been shown to be safe and well tolerated, with objective evidence of response in several cases (3, 4, 6, 12). Notably, no maximum tolerated dose (MTD) has been reached in any of the trials. Recent reports by our group and others have established that the antitumor efficacy of reovirus is enhanced by combination with both radiotherapy and chemotherapy (7, 13, 14). More specifically, we and others have found that reovirus in combination with taxanes results in significant synergistic tumor cell kill in vitro and in a murine model (14). Docetaxel acts by disrupting the normal process of microtubule assembly and disassembly. Reoviruses have been shown to associate with microtubules and may require this association for efficient viral replication. We found enhanced microtubule stabilization following combination treatment with reovirus/docetaxel.9 The strong synergy observed led to the selection of docetaxel for this combination study. In addition to the exploitation of oncogene signaling, reovirus activates the host immune response to potentially enhance antitumor responses through the efficient induction of type I interferons (15). Also, the local inflammatory response generated by reovirus-infected tumor cells causes bystander toxicity against reovirus-resistant tumor cells and activation of human myeloid dendritic cells (16). This phase I dose escalation study was designed to determine the MTD and any dose-limiting toxicities (DLT) with the combination of systemic reovirus and docetaxel. Secondary objectives included determining the effect of docetaxel on the expected humoral response to reovirus, pharmacokinetics of docetaxel when administered with reovirus, and assessment of any antitumor activity.
Materials and Methods
Patients
Patients diagnosed with advanced or metastatic solid tumors refractory to standard of care treatment, or for which no curative standard therapy existed, and for whom docetaxel was an appropriate palliative chemotherapy, were considered for enrolment. To be eligible, patients were required to have measurable or evaluable disease; have no continuing residual toxic effects related to any prior anticancer therapy, with any such effect having resolved to grade 1 or lower; be ≥18 years of age; have received no chemotherapy, radiotherapy, biological therapy, and hormone therapy (apart from patients with breast cancer and LHRH analogues in prostate cancer) within 28 days before receiving the study drug; have an Eastern Cooperative Oncology Group (ECOG) performance score of ≤2; and have a life expectancy of at least 3 months.
The following baseline laboratory results were required: absolute neutrophil count ≥1,500/μL, platelets ≥100,000/μL, hemoglobin ≥9.0 mg/dL, serum creatinine ≤1.5× institutional upper limit of normal, total bilirubin ≤1.5× institutional upper limit of normal, aspartate transaminase/alanine transaminase ≤2.5× institutional upper limit of normal, and a negative pregnancy test for females of childbearing potential. Exclusion criteria included known brain metastases, concurrent immunosuppressive therapy, known HIV, hepatitis B or C infections, pregnancy or breast-feeding, clinically significant cardiac disease (New York Heart Association class III or IV), and dementia or altered mental state that would prohibit informed consent. The study was approved by the local ethics committees.
Study design
This was an open-label, dose-escalating, nonrandomized, three-center phase I study of reovirus given intravenously combined with docetaxel every 3 weeks. Docetaxel, following oral premedication with dexamethasone (8 mg daily for the 3 days up to treatment), was given as a 60-minute intravenous infusion on day 1, every 21 days. The reovirus used in the study was an isolate of the type 3 replication-competent Dearing strain (REOLYSIN) supplied by Oncolytics Biotech. Reovirus was administered to the patients as an intravenous infusion over 60 minutes from days 1 to 5, every 21 days. On day 1 of each cycle, when both agents were to be given, the docetaxel was given first. Three patients were enrolled in each cohort, at the dose level shown in Table 1, to determine the MTD. At the beginning of each new dose level, only one patient was treated. The second and third patients of the cohort were not treated until at least 2 weeks after the initial patient in that cohort had received the first dose of reovirus.
Table 1.
Dose level by cohort
| Cohort | No. patients | Reovirus dose (TCID50) | No. days | Docetaxel dose (mg/m2) |
|---|---|---|---|---|
| −1* | — | 1 × 109 | ||
| +1 | 3 | 3 × 109 | 5 | 75 |
| +2 | 3 | 1 × 1010 | 5 | 75 |
| +3 | 3 | 3 × 1010 | 5 | 75 |
NOTE: Necessary only if toxicity is encountered at the initial dose level.
Patients continued to receive treatment under this protocol for a maximum of eight cycles, provided there was no evidence of disease progression and the treatment was tolerated.
Dose escalation
Patients were initially enrolled in groups of three and individually assessed for safety and DLTs. Patients were considered evaluable for dose escalation decisions if they had received at least one cycle or withdrew from the study due to drug-related toxicity. If a patient withdrew from the study without meeting these criteria, they were replaced in the cohort.
If one of three patients in a dose group experienced a DLT during the first cycle, three more patients were added to that dose group. If two or more patients in a dose group experience a DLT during any cycle, the previous lower dose would be defined as the MTD. Intrapatient dose escalations were not permitted. Treatment was continued for a maximum of eight cycles, provided it was well tolerated and there was no evidence of disease progression.
Viral administration
Reovirus was supplied by Oncolytics Biotech in single-use 1-mL glass vials containing a frozen viral suspension in PBS. Stock was stored at −70°C and thawed rapidly over 2 minutes, and the appropriate TCID50 dose was diluted to 250 mL in 0.9% sodium chloride and infused over 1 hour through a peripheral line. Treatment was given in a side room, and patients were monitored closely (including blood pressure, temperature, and heart rate measurements) during and for at least 1 hour after infusion.
Dose-limiting toxicity
Toxicities were graded according to Common Terminology Criteria for Adverse Events version 3.0. DLT was defined as any of the following events that were determined to be possibly or probably related to combination therapy during the first cycle of treatment irrespective of whether the toxicity had resolved: absolute neutrophil count <0.5 × 109 lasting for more than 7 days or with sepsis, platelet count <25 × 109/L, grade 2 neurotoxicity or cardiotoxicity, any other drug-related nonhematologic grade 3/4 toxicity, with the exceptions of flu-like symptoms, nausea, and vomiting if appropriate prophylactic or therapeutic measures had not been administered, and the inability to tolerate one course of therapy due to toxicity.
To define DLT, patients were not given prophylactic growth factor support, antidiarrheals, or antipyretics during the first cycle of therapy.
Safety evaluations
Safety was assessed by the evaluation of the type, frequency, and severity of adverse events, changes in clinical laboratory tests (including hematologic, clinical chemistry, and urinalysis), immunogenicity, and physical examination. Electrocardiogram was performed at baseline, after each reovirus infusion for the first cycle, on day 1 of each subsequent cycle and at the end of the study. Patients experiencing any DLT in any cycle had their treatment held until toxicity resolved to baseline or grade 1. On resolution, reovirus and docetaxel therapy was recommenced at a lower dose level.
Response evaluation
Response was assessed by Response Evaluation Criteria in Solid Tumors criteria (17). All patients were clinically evaluated after each course of treatment and radiologically every second course until there was evidence of progressive disease. Tumor markers were also used to assess response in appropriate patients.
Pharmacokinetic and pharmacodynamic endpoints
To assess docetaxel pharmacokinetics, blood samples were taken during the first cycle of treatment at the following time points: baseline, 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 24 hours, and 48 hours. Three patients whose tumors were accessible underwent biopsy to evaluate viral replication. Samples were stored at −80°C until the time of analysis. At this time, the biopsies were thawed and macerated in 1-mL DMEM and centrifuged at 3,600 rpm for 5 minutes. The supernatant was taken, serially diluted (1:10), and placed on to L929 cells in quadruplicates in a 96-well plate. The viral titer was calculated using the Kärber statistical method for a standard TCID50 assay.
Analysis of viral shedding by reverse transcription-PCR
Initial evaluation of the detection limit of reovirus RNA by 35 cycles of reverse transcription-PCR (RT-PCR) was done. Viral RNA was extracted from 140 μL stock using the QIAamp Viral RNA Mini kit (Qiagen) and serially diluted. Five microliters were assayed directly by RT-PCR using the One-Step RT-PCR Enzyme Mix kit (Qiagen). Reovirus s3 cDNA-targeted primers used were 5′-GGGCTGCACATTACCACTGA (forward) and 5′-CTCCTCGCAATACAACTCGT (reverse). PCR conditions were 50°C for 30 minutes, for reverse transcription; 95°C for 15 minutes; and 35 cycles of 95°C for 30 seconds, 62°C for 45 seconds, and 72°C for 45 seconds followed by 72°C for 7 minutes.
All patients had blood samples collected for the detection of reovirus titers during the first two cycles. Samples were taken at baseline, 4 hours after the last dose of reovirus on days 5 and 15. Blood from the contralateral arm was collected into EDTA tubes, centrifuged at 1,200 × g for 10 minutes at 4°C, and stored at −70°C. Urine, sputum, and fecal swab (after PBS elution) samples were also stored at −70°C. Samples were analyzed after the last treatment dose in each cycle and weekly using the 35-cycle RT-PCR. Reovirus RNA (300-bp PCR product) and water were included in all experiments as positive and negative controls, respectively.
Detection of neutralizing antireoviral antibodies
A modified neutralizing antibody assay was used to detect antibody titers at baseline and weekly during the first two cycles of treatment by measuring the effect of patient serum samples on the ability of reovirus to kill a monolayer of target mouse L929 cells. The neutralizing antireoviral antibody (NARA) titer of serum samples was expressed as the last dilution causing <80% cell killing as described previously (18). Assays were performed in duplicate by two different technicians to verify the results.
Immunohistochemistry of biopsies for reoviral protein
Where possible, fine-needle core biopsies were taken from accessible metastatic sites after reovirus and docetaxel treatment, on day 5 of cycle 2. Biopsies were fixed in formalin and paraffin embedded. Immunohistochemical analysis of reovirus protein expression in 5-μm sections followed a published protocol. The Benchmark LT automated system (Ventana Medical Systems) was used. In brief, optimal conditions were determined from blinded analysis of cells either infected with reovirus or not infected using 1:3,000 diluted primary antibody following pretreatment in Ventana’s cell conditioning 1 for 30 minutes (antigen retrieval). The primary antibody was provided by co-author M. Coffey and was derived in goat; a rabbit anti-goat (Abcam) secondary antibody was used at a dilution of 1:3,000. The antigen was detected with the Ultraview Universal DAB (3,3′-diaminobenzidine) or Fast Red system from Ventana with a counterstain of hematoxylin. The negative controls included omission of the primary antibody and carcinomas from patients who had not been treated with the reovirus.
Analysis of the colocalization of microtubular protein and reovirus protein was done using the nuance system from Cambridge Research Institute. Optimal conditions for microtubular protein involved protease digestion (Ventana protease 1, 4 minutes) and a dilution of 1:5. After immunohistochemical analysis of microtubular protein using DAB as the chromogen, the same slide was then tested for reovirus protein using immunohistochemistry and the Fast Red chromogen.
Results
Patients
A total of 25 patients were enrolled into the study across three centers between June 2007 and January 2009. Their demographics are summarized in Table 2. One patient failed to start treatment due to worsening liver function tests after enrolment. Another patient developed leucopenia on day 2, cycle 1 and was taken off study and replaced in the cohort. All others received at least one cycle. They were treated over three dose levels and received a total of 98 cycles (median, 3; range, 1-8), summarized in Table 3A. Sixteen patients completed at least two cycles of treatment and were therefore eligible for response assessment.
Table 2.
Patient characteristics
| Patient characteristics | No. patients (n = 25) |
|---|---|
| Gender | |
| Male | 21 |
| Female | 4 |
| Age (y) | |
| Median | 60 |
| Range | 32-77 |
| Previous chemo lines | |
| 0 | 2 |
| 1 | 17 |
| 2 | 4 |
| >2 | 2 |
| Cancer diagnosis | |
| Esophagus | 6 |
| Prostate | 4 |
| Melanoma | 4 |
| Pancreas | 4 |
| Unknown 1° | 2 |
| Breast | 1 |
| Stomach | 1 |
| Mesothelioma | 1 |
| Hapatocellular | 1 |
| Bronchoalveolar | 1 |
| ECOG status | |
| 0 | 5 |
| 1 | 20 |
| 2 | 0 |
Table 3.
Grade ≥3 toxicity by dose level
| A. Patients treated at each dose level and grade 4 events observed
| ||||
|---|---|---|---|---|
| Cohort | No. patients | Reovirus dose (TCID50) | Total no. cycles (range) | Grade 4 event (n) |
| 1 | 4 | 3 × 109 | 16 (1-6) | 0 |
| 2 | 4 | 1 × 1010 | 20 (2-8) | 0 |
| 3 | 16 | 3 × 1010 | 62 (1-8) | 4* |
|
B. Grade ≥3 toxicity observed for each reovirus dose level (n = 24)
| ||||
| Toxicity |
Dose level
|
Total, n = 24 (%) | ||
| 1 | 2 | 3 | ||
|
| ||||
| Neutropenia | 1 | 2 | 3 | 6 (25) |
| Neutropenia with fever | 1 | 1 (4) | ||
| Thrombocytopenia | 1 | 1 (4) | ||
| Pancytopenia | 1 | 1 (4) | ||
| Fatigue | 1 | 1 (4) | ||
| Vomiting | 1 | 1 (4) | ||
| Diarrhea | 1 | 1 | 2 (8) | |
| Abdominal pain | 1 | 1 (4) | ||
| Bowel obstruction | 1 | 1 (4) | ||
| Nausea | 1 | 1 (4) | ||
| Dehydration | 2 | 2 (8) | ||
| Gastric bleed | 1 | 1 (4) | ||
| Tachycardia | 1 | 1 (4) | ||
| Increased alanine aminotransferase | 1 | 1 (4) | ||
| Perianal abscess | 1 | 1 (4) | ||
| Pyrexia | 1 | 1 (4) | ||
| Hypotension | 1 | 1 (4) | ||
| Hypokalemia | 1 | 1 (4) | ||
Grade 4 neutropenia (one of them developed grade 4 lymphopenia).
Safety and toxicities
The treatment was well tolerated, with the most common side effects being flu-like symptoms (fever, chills, and headache), diarrhea, fatigue, and neutropenia. The grade 3 to 4 toxicities are given in Table 3. There were a total of six grade 4 toxicities. This included four episodes of grade 4 neutropenia in cohort 3, all of which were thought to be due to the docetaxel therapy alone. One man with prostate carcinoma and a retrovesical fistula had grade 4 neutropenia after his first cycle of treatment. He continued on the study on the cohort 2 dose of reovirus and a 20% reduction in docetaxel for a further four cycles without any further DLTs. One episode of grade 4 neutropenia was complicated by sepsis, although the patient fully recovered and completed eight cycles of treatment and the same patient developed grade 4 lymphopenia during treatment. All other toxicities were grade 3 or less. One patient with hepatocellular carcinoma developed a grade 3 rise in aspartate aminotransferase on day 5 of cycle 1, and treatment was withheld. Consequently, this patient was replaced in the cohort.
Flu-like symptoms typically occurred 2 to 4 days after reovirus administration and were easily controlled with paracetamol and nonsteroidal anti-inflammatory medication. Symptoms seemed to be more common in the first cycle of treatment and milder in subsequent cycles. The incidence of neutropenia was not affected by the dose level of reovirus; however, there was a relationship between neutropenia and the amount of previous chemotherapy received, with all the patients who had grade 4 neutropenia having received at least six cycles of chemotherapy before enrolment.
Viral biodistribution
Pretreatment and posttreatment serum, urine, saliva, and anal swabs were negative in all but two patients using RT-PCR screening based on 35-cycle amplification. In patient 0308, positive signal was detected in the serum on day 5 of cycle 1. Patient 0317 had positive signal in the urine and saliva on day 15 of cycle 1, and also in the serum on day 5, and the anal swab on day 15 of cycle 2. This patient also had a positive reading in the pretreatment serum sample, which may indicate contamination. The analysis of viral shedding including representative gels is illustrated in the Supplementary Data.
NARA response
Twenty-two patients had data available for analysis of the increase in NARA titers. This was expressed as the fold increase in antibody titer compared with pretreatment. All patients showed an increase in NARA titers with a range of 27 to 2,187 (median, 243) at day 15, cycle 1, and 27 to 6,561 (median, 729) at peak (Fig. 1). From previous studies, evidence of effective immune modulation was taken if the day 15, cycle 1 NARA showed only a 10- to 50-fold increase over the pretreatment control. In this study, only one patient, 101, had evidence of immune modulation. However, this patient had a dramatic fall in his neutrophil and lymphocyte count after the second day of treatment and was consequently taken off study. All the other patients had increases in their NARA of 80-fold or more, indicating that docetaxel had no effect on the production of neutralizing antibodies to reovirus.
Fig. 1.
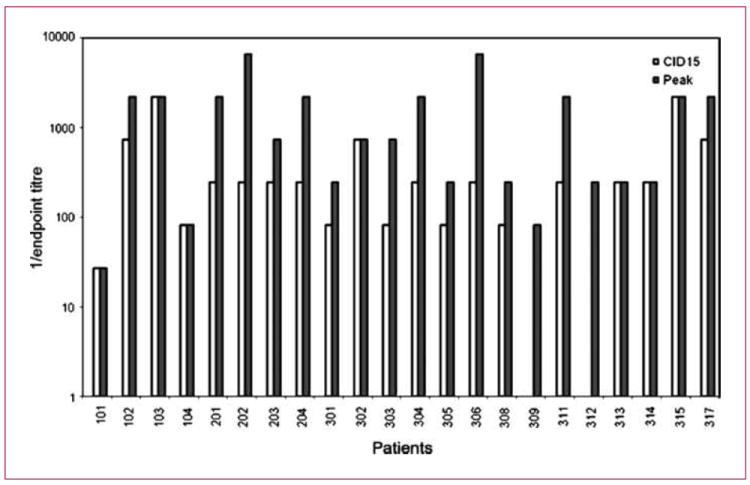
Fold increase in reoviral antibody titer. Graph shows the fold increase in reoviral antibody titer over pretreatment in the 22 patients for whom data were available. Both the cycle 1, day 15 (white) and peak (gray) reoviral antibody titer are shown.
Response assessment
Sixteen patients were eligible for response assessment having completed two cycles of treatment (Table 4). Objective radiological responses were seen in four patients. There was one complete response in the liver of a patient with metastatic breast carcinoma. This patient completed eight cycles of treatment with no evidence of disease recurrence in the liver at the end of study. Three patients had objective evidence of a partial response: a patient with ocular melanoma had a 30% reduction in the size of liver metastases, a patient with gastric carcinoma had a 32% reduction in the size of target lymph node metastases, and a patient with gastroesophageal carcinoma had a 32% reduction in lung metastases. Ten patients had evidence of stable disease for at least two cycles, six of whom completed at least six cycles of treatment. Three patients with radiologically stable disease had evidence of a minor response: a patient with mesothelioma, who received six cycles, had a 23% decrease in the size of a target mediastinal lymph node, a patient with a pancreatic carcinoma had a 48% decrease in Ca19.9 tumor marker, and there was a 30% fall in prostate-specific antigen (PSA) in a patient with prostate carcinoma.
Table 4.
Antitumor activity in evaluable patients (n = 16)
| Best response | No. patients | Tumor types | Reduction |
|---|---|---|---|
| Partial response | 4 | Breast | Complete response in liver; stable disease in bone |
| Stomach | ↓32% in lymph nodes | ||
| Gastroesophageal | |||
| Ocular melanoma | |||
| Minor response | 3 | Mesothelioma | ↓23% in lymph nodes |
| Prostate | ↓30% in PSA | ||
| SCC H + N | ↓26% in lymph node | ||
| Stable disease | 7 | Prostate | |
| Unknown 1° | |||
| Melanoma | |||
| Esophagus | |||
| Pancreas |
Posttreatment biopsy evaluation
Where possible, fine-needle core biopsies of accessible metastases were taken on day 5, cycle 2 of treatment. Evaluable tissue was obtained from three patients with prostate cancer (iliac lymph node biopsy), unknown primary tumor (liver biopsy), and pleural mesothelioma (pleural biopsy), and immunohistochemistry for reovirus protein was completed (Fig. 2A-E). Compared with control sections of normal human liver, reoviral protein expression was observed in tumor cells in all three biopsies. The staining was mainly cytoplasmic, and the strongest expression was seen in metastatic mesothelioma.
Fig. 2.
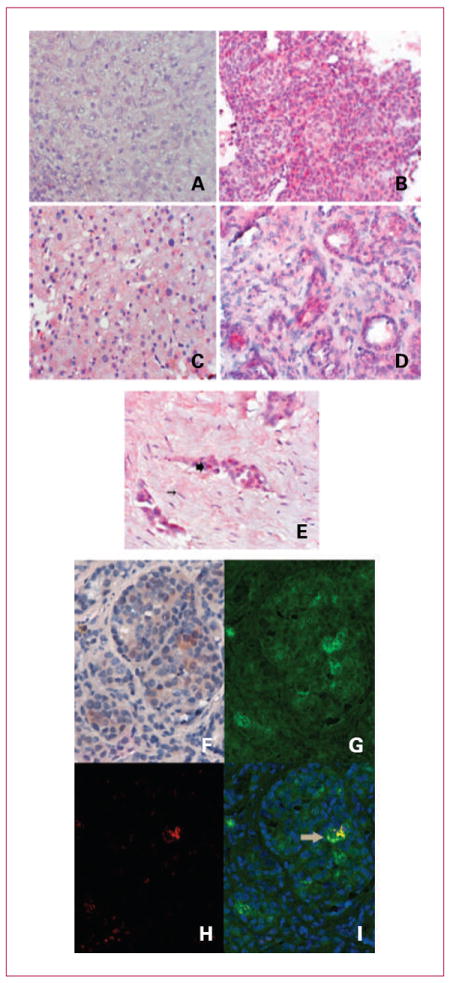
A to E, expression of reoviral protein (red stain) in posttreatment biopsies. A, normal liver (magnification, ×400). B, liver biopsy from metastasis from carcinoma unknown primary (magnification, ×400). C, normal liver adjacent to liver metastasis from carcinoma. D, metastatic prostate cancer in an iliac lymph node (magnification, ×200). E, pleural biopsy malignant mesothelioma (magnification, ×400). Large arrow indicates reoviral protein in tumor cells (red) with no staining in supporting fibroblasts (thin arrow). F to I, RGB image analysis of tissue with reovirus (red) and microtubular protein (brown) using the nuance system. This system converts the RGB image of the DAB signal to fluorescent green (microtubular protein; G) and the RGB image of the Fast Red system to fluorescent red (reovirus; H), and then mixes them with the fluorescent green representing cells that express the reovirus in the microtubular complex (I, arrow). Magnification, ×400.
In one patient, we were able to show colocalization of reovirus to microtubule protein, consistent with virus replication in the tumor cells (Fig. 2F-I).
Pharmacokinetics
The effect of reovirus on docetaxel pharmacokinetics was assessed by the measurement of serial serum samples after intravenous delivery of docetaxel. All patients had similar results with no difference in docetaxel clearance than would be expected in patients receiving docetaxel alone. Peak concentration of docetaxel ranged from 1,510 to 4,080 ng/mL between 15 and 30 minutes after docetaxel administration. The concentration of docetaxel fell below 100 ng/mL within 2 hours in all patients. There was no correlation between docetaxel clearance and dose escalation of reovirus.
Discussion
The purpose of this study was to determine the safety, DLT, and MTD of intravenous reovirus in combination with docetaxel chemotherapy in patients with advanced solid malignancies. In keeping with experience with other viral therapies, it was always anticipated that the therapeutic potential of reovirus would be realized through combination with other anticancer agents. Supporting preclinical data suggested strong potential synergy between the two agents through enhanced tumor apoptosis. This study also allowed an evaluation of any immunomodulatory effects of docetaxel on the humoral response to reovirus and also whether concurrent treatment with reovirus affected the pharmacokinetics and clearance of docetaxel.
Several chemotherapy/oncolytic virus combinations have been evaluated to date and have been shown to result in marked antitumor effects without compromising safety. Onyx-015, an oncolytic adenovirus engineered to replicate in p53 mutant tumor cells, showed enhanced clinical efficacy when combined intratumorally with systemic cisplatin and 5-fluorouracil compared with chemotherapy alone (19). A large number of preclinical reports indicate marked synergy between oncolytic viruses, with varied mechanisms of action, and a range of chemotherapeutic agents (20-25). Not surprisingly, the mechanisms underlying the observed synergies are incompletely understood.
As a single agent, reovirus has been shown to be safe in human use and has been associated with minimal toxicity when given intravenously or intratumorally in patients with a wide range of solid cancers (4, 6). Antitumor effects have been regularly observed even in phase I studies (reviewed in ref. 26).
The toxicities seen in this study included fever, flu-like symptoms, fatigue, and nausea, and were similar to what has been reported with either agent alone. Grade 3 or greater neutropenia was observed in 46%, which seems consistent to the incidence of that toxicity for docetaxel monotherapy (65%) at the same dose and schedule according to prescribing information. As only one DLT, of grade 4 neutropenia, was encountered, the MTD was not technically reached. However, the highest dose of reovirus available for administration is 3 × 1010 TCID50, and this, combined with docetaxel (75 mg/m2), is the recommended dose for ongoing studies.
As in previous trials of single-agent reovirus, viral shedding was observed infrequently (two patients). One had positive samples from the serum, saliva, urine, and anal swab, whereas the other patient just had a positive serum sample. The results suggest that there is rapid clearance of the virus from the circulation, which is unaffected by the administration of docetaxel. There was a rapid induction of a humoral response to reovirus, with all patients showing an increase in the NARA titers after the first cycle. Despite its reported immunomodulatory effects (27), docetaxel seemed to have no effect on the NARA response. From the single-agent reovirus study by Vidal et al., the median increase in NARA titers above baseline was 250-fold. They proposed that successful immune modulation could be defined as a rise in NARA titer that is at least 10-fold lower than observed in the single-agent reovirus studies. Only one patient in this study met that criteria, and he had an unusual and dramatic fall in his neutrophil and lymphocyte count on cycle 1, day 2 and was taken off study. It is possible that the reduced exposure to reovirus and myelosuppression may have limited the extent of the NARA response. The potential reduction/elimination of reovirus oncolysis due to the NARA response has been extensively debated. In our view, it is most likely that NARA has a role in preventing the efficient spread of progeny but not viremia. The virus may gain access to immune-privileged areas of tumors and escape detection. There is additional evidence that reovirus persists for several months after treatment despite NARA and late tumor marker and radiological antitumor responses have been observed (4-6).
The clearance of docetaxel was similar in all patients and across all cohorts with no obvious relationship to the reoviral dosage.
Although not a primary endpoint of the study, objective radiological tumor responses were seen. Given that all patients were docetaxel naïve, it is impossible to determine whether responses were a result of either agent alone or their combination. Only two patients had not received prior chemotherapy, both had hormone-refractory prostate cancer. The majority of patients had received one previous line of chemotherapy, although six patients had two or more lines of chemotherapy. Of note, two patients had received prior paclitaxel chemotherapy. One of these with breast cancer and liver metastases completed eight cycles of treatment on the study with a complete response in her liver. The other patient had carcinoma of unknown primary site and completed six cycles of treatment with stable disease.
Partial responses were seen in three further patients with ocular melanoma, gastric carcinoma, and esophageal cancer. All three completed at least six cycles of treatment. A further 10 patients had stable disease as a best response, and only two of the evaluable patients had disease progression after the first two cycles. Taken together, this translates into a disease control rate of 88% and an objective response rate of 25%. Although this response rate fits in with reported response rates for second-line single-agent docetaxel [melanoma, 14% (28); gastric, 20% (29); breast, 30-50% (30)], the high disease control rate and response in two patients previously treated with a taxane is encouraging. In our previous study (REO-005) where reovirus was administered as a single agent, we were able to isolate and propagate reovirus from posttreatment tumor biopsy tissue. We were unable to show this in the current studies. However, in all three patients where posttreatment tumor biopsies were taken, we found reoviral protein expression in tumor cells by immunohistochemistry, which is clear evidence of virus tracking to sites of metastases. The reovirus staining was mainly cytoplasmic and largely restricted to tumor cells with much lower expression in normal adjacent tissue (liver and fibroblasts). Furthermore, in one biopsy, we showed colocalization of replicating virus in microtubular protein consistent with proliferating virus in the tumor.
Reovirus as a single agent is safe and shows modest efficacy. However, the future place of reovirus as an anticancer agent is likely to depend on strategies that improve systemic delivery to the tumor, avoid rapid viral clearance by the immune system, and combine reovirus with other anticancer agents. Here, we present the first clinical trial of reovirus combined with a chemotherapeutic agent. The combination is safe, and the recommended dose for future studies is 3 × 1010 TCID50 of reovirus and 75 mg/m2 of docetaxel. Disease stabilization rates for this combination are promising, and further studies are warranted.
Supplementary Material
Translational Relevance.
Oncolytic viruses are currently undergoing rapid evaluation in the metastatic cancer setting. Their true potential will be realized in combination with conventional chemotherapy, radiotherapy, or targeted therapy. In particular, oncolytic reovirus has been shown in numerous single-agent studies to be safe and able to target tumor sites. This study is the evolution of the original single-agent systemic study (REO-005), with the addition of docetaxel to systemic reovirus. It is the first study to examine a reovirus/full-dose chemotherapy combination in humans. Preclinical work has shown marked synergy in terms of tumor kill but also mechanistically through enhancement of apoptosis and stabilization of microtubules. In combination, reovirus was safe and no dose-limiting toxicity was observed. Although the antitumor effects of the individual agents were not measurable, reovirus was able to track to remote tumor sites, with reoviral protein expression in metastases. This study also reiterates the feasibility of virus/chemotherapy combinations in the outpatient setting and provides the framework for phase II and III studies.
Acknowledgments
We thank all our participating patients, our research nurses and coordinators in our respective treatment centers, Chris Lyne, Ventana Medical Systems (Kathleen Sergott) and Phylogeny for assistance with the immunohistochemistry work.
Grant Support
Oncolytics Biotech, Inc. (C. Comins, M. Coffey, and K. Mettinger); Guys and St. Thomas’ NHS Trust (J. Spicer); Churchill Hospital NHS Trust (A. Protheroe); Cancer Research UK (V. Roulstone, K. Twigger, C. White, A. Melcher, and K. Harrington); Mayo Clinic National Institutes of Health (grant R01 CA107082)(R. Vile); Heart and Lung Research Institute (G. Nuovo, D. Cohn, and M. Phelps); and Prostate Project (H.S. Pandha).
Footnotes
Submitted for publication.
Disclosure of Potential Conflicts of Interest
H.S. Pandha: commercial research grant, Oncolytics Biotech.
References
- 1.Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 2.Hashiro G, Loh PC, Yau JT. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch Virol. 1977;54:307–15. doi: 10.1007/BF01314776. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–32. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 4.Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of Reovirus®, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–9. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal LKV, Beirne D, Twigger K, et al. Phase I trial of intratumoral administration of reovirus type 3 with radiation in patients with advanced malignancies. J Clin Oncol. 2007;25:14009. [Google Scholar]
- 6.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 7.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15:1522–30. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 10.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 11.Comins C, Heinemann L, Harrington K, Melcher A, De Bono J, Pandha H. Reovirus: viral therapy for cancer ‘as nature intended’. Clin Oncol (R Coll Radiol) 2008;20:548–54. doi: 10.1016/j.clon.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Thirukkumaran CM, Nodwell MJ, Hirasawa K, et al. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res. 2010;70:2435–44. doi: 10.1158/0008-5472.CAN-09-2408. [DOI] [PubMed] [Google Scholar]
- 13.Pandha HS, Heinemann L, Simpson GR, et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res. 2009;15:6158–66. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- 14.Sei S, Mussio JK, Yang QE, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steele TA, Cox DC. Reovirus type 3 chemoimmunotherapy of murine lymphoma is abrogated by cyclosporine. Cancer Biother. 1995;10:307–15. doi: 10.1089/cbr.1995.10.307. [DOI] [PubMed] [Google Scholar]
- 16.Errington F, Steele L, Prestwich R, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–26. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–20. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 19.Khuri FR, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 20.Angelova AL, Aprahamian M, Grekova SP, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009;15:511–9. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 21.Petrowsky H, Roberts GD, Kooby DA, et al. Functional interaction between fluorodeoxyuridine-induced cellular alterations and replication of a ribonucleotide reductase-negative herpes simplex virus. J Virol. 2001;75:7050–8. doi: 10.1128/JVI.75.15.7050-7058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutermann A, Mayer E, von Dehn-Rothfelser K, et al. Efficacy of oncolytic herpesvirus NV1020 can be enhanced by combination with chemotherapeutics in colon carcinoma cells. Hum Gene Ther. 2006;17:1241–53. doi: 10.1089/hum.2006.17.1241. [DOI] [PubMed] [Google Scholar]
- 23.Pawlik TM, Nakamura H, Mullen JT, et al. Prodrug bioactivation and oncolysis of diffuse liver metastases by a herpes simplex virus 1 mutant that expresses the CYP2B1 transgene. Cancer. 2002;95:1171–81. doi: 10.1002/cncr.10776. [DOI] [PubMed] [Google Scholar]
- 24.Raki M, Kanerva A, Ristimaki A, et al. Combination of gemcitabine and Ad5/3-Δ24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther. 2005;12:1198–205. doi: 10.1038/sj.gt.3302517. [DOI] [PubMed] [Google Scholar]
- 25.Tyminski E, Leroy S, Terada K, et al. Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan. Cancer Res. 2005;65:6850–7. doi: 10.1158/0008-5472.CAN-05-0154. [DOI] [PubMed] [Google Scholar]
- 26.Harrington KJ, Vile RG, Melcher A, Chester J, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–8. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–44. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verweij J, Catimel G, Sulkes A, et al. Phase II studies of docetaxel in the treatment of various solid tumours. EORTC Early Clinical Trials Group and the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 1995;31A(Suppl 4):S21–4. doi: 10.1016/0959-8049(95)00362-m. [DOI] [PubMed] [Google Scholar]
- 29.Roth AD, Ajani J. Docetaxel-based chemotherapy in the treatment of gastric cancer. Ann Oncol. 2003;14(Suppl 2):ii41–4. doi: 10.1093/annonc/mdg728. [DOI] [PubMed] [Google Scholar]
- 30.Pivot X, Asmar L, Hortobagyi GN. The efficacy of chemotherapy with docetaxel and paclitaxel in anthracycline-resistant breast cancer (review) Int J Oncol. 1999;15:381–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


