Abstract
BACKGROUND
Brain biopsies of superficial cortex are performed for diagnosis of neurological diseases, but preoperative predictors of successful diagnosis and risks are lacking.
OBJECTIVE
We evaluated effectiveness and outcomes of superficial cortical biopsies and determined preoperative predictors of diagnosis, outcomes, morbidities, and mortality.
METHODS
A single-institution retrospective analysis of 170 patients who underwent open brain biopsies of superficial cortex was performed. Clinical predictors of effectiveness and outcomes were determined using univariate/multivariate analyses and a system for risk-benefit stratification was created and tested.
RESULTS
Brain biopsies led to successful diagnosis in 122 of 170 (71.8%) and affected management in 97 of 170 (57.1%) cases. Factors increasing the odds of diagnostic pathology included age older than 45 years (odds ratio [OR]: 2.67, 95% confidence interval [CI]: 1.34-5.27, P < .01), previous cancer diagnosis (OR: 3.64, 95% CI: 1.69-7.85, P < .001), focal (OR: 3.90, 95% CI: 1.91-8.00, P < .001) and enhancing (OR: 5.03, 95% CI: 2.41-10.52, P < .001) lesions on magnetic resonance imaging, biopsy of specific lesions on magnetic resonance imaging (OR: 9.34, 95% CI: 4.29-20.33, P < .001), and use of intraoperative navigation (OR: 6.59, 95% CI: 3.04-14.28, P < .001). Brain biopsies led to symptomatic intracranial hemorrhage, seizures, other significant morbidities, and perioperative mortality in 12.4%, 16.2%, 37.1%, and 8% of cases, respectively. Risk of postoperative intracranial hemorrhage was increased by a history of aspirin use (OR: 2.51, 95% CI: 1.23-5.28, P < .05) and age older than 60 years (OR: 2.66, 95% CI: 1.36-5.18, P < .01).
CONCLUSION
Effectiveness and risk of morbidity/mortality can be estimated preoperatively for patients undergoing open brain biopsies of the superficial cortex. Older age and specific imaging characteristics increase the odds of diagnostic biopsy. Conversely, older age and aspirin use increases the risk of postoperative complications.
Keywords: Brain tumor, Dementia, Efficacy, Hemorrhage, Open brain biopsy, Outcomes, Pathology
Brain biopsy of the superficial cortex is a commonly performed procedure for the diagnosis of neurological illness. However, the decision to pursue an open brain biopsy is not always straightforward. One patient may present with a neurological deficit and a corresponding lesion on brain magnetic resonance image (MRI). Intuitively, biopsy of this lesion would likely produce a successful diagnosis to affect management. Meanwhile, biopsy of a patient with neurological deterioration such as cognitive decline and no specific lesion on imaging may have lower diagnostic yield.
Numerousstudies have evaluated the use of both stereotactic and frameless brain biopsies for malignancy and human immunodeficiency virus infection (HIV)–related diseases.1-4 In patients with discrete intracranial lesions, stereotactic needle biopsies have a reported pathology diagnostic yield of 91.7%.4 Frameless needle biopsy techniques boast similar diagnostic yields.3 Complication rates for stereotactic needle biopsies vary from 0 to 10%.1,5-12 Complications have been associated with hypertension, HIV, biopsy of the pineal region, and thrombocytopenia.5,7,10,13,14
In contrast, the utility of brain biopsies in patients with worsening neurological conditions without focal imaging findings remains unclear. Open brain biopsies for dementia and neurological decline produce nondiagnostic pathology including normal tissue and nonspecific changes in 5% to 71% of cases.15-20 Normal brain tissue is seen in 1% to 45% of biopsy specimens.15-18 Previous reports show that brain biopsies for cognitive decline affects management in 8% to 12% of patients.17,20 Complication rates vary from 2% to 14%.18
This report details the retrospective analysis of our institution's experiences with brain biopsy of the superficial cortex to develop predictors of diagnostic pathology affecting clinical management, outcomes, morbidities, and mortalities. We specifically studied biopsies of the cortical surface (both lesional and nonlesional) rather than needle biopsies. Through this analysis, we developed models that may help estimate the effectiveness and outcomes for patients being evaluated for possible brain biopsy.
PATIENTS AND METHODS
Database
After approval by Washington University Institutional Review Board, patients who underwent brain biopsies of the superficial cortex at Washington University School of Medicine between 1998 and 2011 were identified through CPT procedure codes. Inclusion criteria included a patient who underwent a craniotomy/craniectomy for biopsy of the cortical surface. Lesions were on the cortical surface and most amenable to superficial biopsy rather than stereotactic needle biopsy of a deeper lesion. Patients who underwent a tumor resection, endoscopic procedure, stereotactic needle biopsy, or any other procedure more complex than a superficial cortical biopsy were excluded. Over 14 years, 265 patients had procedural codes with burr hole/trephine with biopsy or craniotomy/craniectomy for exploration. Of these, 170 patients met the inclusion/exclusion criteria. A total of 698 patients had procedure codes for stereotactic biopsy, aspiration, or excision with burr hole(s) for intracranial lesion. A database with 170 superficial cortical biopsy patients was created for the retrospective analysis. Database included demographics, pre-operative symptoms and signs, medications, laboratory values, imaging studies, procedural details, postoperative course, postoperative laboratory test results, pathology details, and follow-up hospital and clinic notes. Long-term clinical outcome data were derived from clinic notes. Postoperative complications included seizures, hemorrhages, and deaths occurring within 31 days of the operation. Radiology reports were assessed for focal lesion on head computed tomography (CT) scans and magnetic resonance imaging (MRI), enhancing lesions on MRI, and T2-hyperintensity signal abnormalities. Angiography reports were examined for vasculitis or vasculopathy (determined by artery caliber changes as determined by the neuroradiologist). Postoperative complications were assessed by evaluating medical records for each individual patient. Postoperative intracranial hemorrhages, seizures, deaths, or significant other life-threatening morbidities were recorded and tabulated. Reports of postoperative CT scans or MRI were evaluated for intracranial hemor rhages (ICHs) including intraparenchymal and extra-axial hemorrhages. Radiology reports demonstrating “postoperative blood products” or “postoperative changes” were not included as ICHs. The size and type of hemorrhage were documented and correlated with medical record notes to assess for clinical changes. When available, all CT scans and MRI were reviewed. A positive effect on clinical management was defined by a documented changed in the treatment plan as a result of the biopsy. Long-term clinical outcome data were derived from outpatient and inpatient medical record notes. The outcome data were collected from follow-up documentation of either the surgeon or neurologist who evaluated the patient at the time of biopsy. Based on the assessments, the status was either recorded as worsened, unchanged, improved, or not documented.
Biopsy Procedure
Brain biopsies were performed to diagnose pathology underlying neurological symptoms or signs and/or abnormal imaging findings. The open brain biopsies were performed using standard operative technique. Unless the location of biopsy-targeted radiographic findings, the biopsy was located in the right frontal lobe (occasionally left frontal lobe) anterior to the coronal suture. For some cases, frameless StealthStation (Medtronic Inc, Minneapolis, Minnesota) image-guided navigation was used to pursue a lesion on imaging. After craniotomy or craniectomy was performed at the biopsy site, the dura was opened and a biopsy specimen was taken of a discrete lesion or 10-mm cube of brain parenchyma was removed en bloc. For some procedures, dura was also collected.
Pathology
Specimens were fixed in formalin, processed in paraffin wax, and sectioned for routine histopathological stains such as hematoxylin and eosin and Luxol blue/cresyl violet, and reviewed by a neuropathologist. Additional histopathological and immunohistochemical stains were performed as determined by the neuropathologist. Information for the database was taken from the official pathology report. Nondiagnostic pathology was defined as normal tissue and nonspecific inflammatory changes. Histological vasculopathy was defined as abnormalities found in vasculature without definitive findings of inflammatory cells (which would connote a vasculitis).
Data Analysis
Patient demographic and clinical characteristics as well as the features and outcomes of brain biopsies were summarized as mean ± SD, median, or frequency, as appropriate. Exploratory univariate logistic regression was used to assess the association between each predictor and effectiveness or outcome, and the strength of association was described by odds ratio (OR) and its 95% confidence interval (CI). Multivariate models were created by selecting the most clinically relevant factors among those found during the univariate analysis, and the model development was also assisted by backward stepwise selection procedures. To facilitate an easy clinical interpretation, age at biopsy (the only continuous predictor) was categorized and the selection of optimal cutoff values was assisted by receiver-operating characteristic curves. The overall performance of multivariate logistic analyses were measured by the area under receiver-operating characteristic curves (AUC) and the reliability of the models were also assessed using 10-fold cross-validation.21 AUC measures the ability of a model to correctly classify 2 randomly selected subjects. Its value ranged from 0.5 to 1, with 1 indicating a perfect differentiation. All analyses were 2 sided and significance was set at a P value of .05. Statistical analyses were performed using SAS version 9.0 (SAS Institute, Cary, North Carolina).
RESULTS
Demographics
Over 14 years, 170 cases met the inclusion criteria. Demographics and case characteristics are detailed in Table 1. The mean age of the patients at the time of biopsy was 48.6 years (SD = 22.2; 1 day-85 years). Fourteen percent of patients were younger than 21 years of age; 17% were 21 to 40 years of age; 34% were 41 to 60 years of age; and 35% were older than 60 years of age. Ninety-nine patients 58.2%) were male. Forty-two patients (25.9%) smoked tobacco, and 21 patients (12.7%) had a history of coronary artery disease. Fifty-three patients (31.7%) had vascular disease, including hypertension. Three patients had a diagnosis of HIV (1.8%). Seventy-seven patients (46.1%) had a previous cancer diagnosis. Patients presented with headaches, cognitive decline, behavioral changes, hallucinations, focal weakness, myoclonus, and seizures, respectively. Forty-one patients (24.6%) were taking aspirin on initial neurosurgical consultation. Fifty-eight percent were taking 81 mg/d, and aspirin was held 1 to 30 days before surgery. Seven (4.2%), 7 (4.2%), 8 (4.9%), and 60 (36.4%) were taking clopidogrel, warfarin, nonsteroidal anti-inflammatory drugs, or steroids, respectively.
TABLE 1.
Patient Demographics and Characteristicsa
| Variable | No. (%) of Total |
|---|---|
| Age, mean ± SD, y | 48.6 ± 22.1 |
| Male | 99 (58.2) |
| Comorbidities | |
| Smoker | 42 (25.9) |
| Coronary artery disease | 21 (12.7) |
| Vascular disease | 53 (31.7) |
| HIV | 3 (1.8) |
| History of cancer | 77 (46.1) |
| Presenting symptoms and signs | |
| Headaches | 68 (40.7) |
| Cognitive decline | 85 (50.3) |
| Behavioral change | 63 (37.5) |
| Hallucinations | 10 (6.0) |
| Focal weakness | 68 (40.5) |
| Myoclonus | 6 (3.6) |
| Seizures | 57 (33.7) |
| Medications on consultation | |
| Aspirin | 41 (24.6) |
| Clopidogrel | 7 (4.2) |
| Coumadin | 7 (4.2) |
| NSAIDs | 8 (4.9) |
| Steroids | 60 (36.4) |
HIV, human immunodeficiency virus; NSAIDs, nonsteroidal anti-inflammatory drugs.
Preoperative Evaluation
Before open brain biopsy, each patient underwent an extensive preoperative evaluation (Table 2). Radiological evaluations included a head CT scan, brain MRI, magnetic resonance angiography, CT angiography, catheter angiography, and additional studies such as positron emission tomography and spectroscopy. Fifty-nine of 102 head CT scans (57.8%) revealed a focal lesion. Focal abnormalities on brain MRI were noted in 115 of 167 patients (68.9%). An enhancing lesion on MRI was noted in 93 of 163 patients (57.1%). MRI revealed hyperintense T2 signal changes in 139 of 163 patients (85.3%). Catheter angiography, CT angiography, and MR angiography were performed in select patients and showed signs of vasculitis/ vasculopathy in 11 of 169 patients (6.5%). Spectroscopy, positron emission tomography, electroencephalography, basic laboratory studies, bleeding studies, and cerebral spinal fluid (CSF) studies were performed on select patients.
TABLE 2.
Preoperative Evaluationa
| Imaging and Clinical Studies | No. (%) of Total |
|---|---|
| Focal lesion on CT scan | 59/102 (57.8) |
| Focal lesion on MRI | 115/167 (68.9) |
| Enhancing lesion on MRI | 93/163 (57.1) |
| Catheter angiogram performed | 44/169 (26.0) |
| CT angiogram performed | 2/169 (1.2) |
| MR angiogram performed | 26/169 (15.4) |
| Vasculitis/vasculopathy on angiogram | 11/169 (6.5) |
| PET | 21/166 (12.7) |
| Spectroscopy | 18/165 (10.9) |
| EEG | 53/165 (32.1) |
| Normal | 12 (7.2) |
| Slowing | 30 (18.2) |
| Seizure | 11 (6.7) |
| Laboratory Studies | Mean (SD) | No. of Patients Tested |
|---|---|---|
| INR | 1.0 (0.1) | 152 |
| Platelet count, ×1000 cells/mm3 | 270 (105) | 160 |
| ESR, mm/h | 28.5 (31.0) | 73 |
| CRP, mg/L | 19.5 (33.3) | 40 |
| WBCs, ×100 cells/mm3 | 9.8 (8.8) | 160 |
| Cerebrospinal fluid studies | ||
| Nucleated cells, cells/μL | 60 (224) | 92 |
| Protein, mg/dL | 132.2 (214) | 91 |
| Glucose, mg/dL | 68.4 (25.1) | 91 |
| IgG index | 0.96 (1.7) | 30 |
| Platelet function screen | 28 | |
| Epinephrine, s | 147 (76) | |
| ADP, s | 76 (47) |
CT, computed tomography; MRI, magnetic resonance imaging; MR, magnetic resonance; PET, positron emission tomography; EEG, electroencephalography; INR, international normalized ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; WBCs, white blood cells; IgG, immunoglobulin G, ADP, adenosine diphosphate. Platelet function screen normal values: epinephrine (60-160), ADP (50-110).
Procedure
Surgeons pursued a discrete lesion on MRI and used image-guided navigation in 67.3% and 65.2% of biopsies, respectively. Biopsies of the right and left hemispheres were performed in 55.9% and 36.1% of cases, respectively (see Table 1, Supplemental Content 1, http://links.lww.com/NEU/A538). An intraoperative complication, a seizure, was noted in 1 of the 170 biopsies.
Pathology
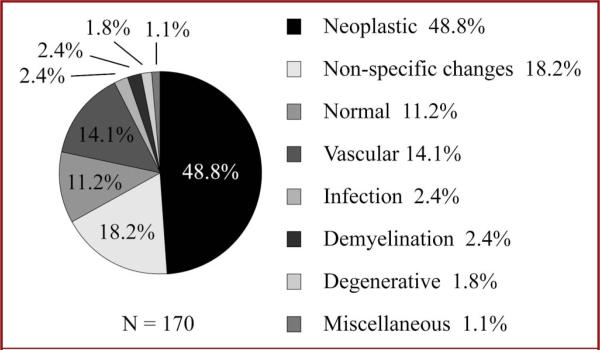
Overall pathology findings are shown in Figure 1 and revealed diagnostic pathology in 70.6% of patients. Forty-nine percent (83/170) of samples were neoplastic. Neoplasms included glioblastoma (32.5%), astrocytoma (22.9%), meningioma (9.6%), oligoastrocytoma (7.2%), oligodendroglioma (7.2%), CNS lymphoma (10.8%), other primary nervous system neoplasms (6.0%), and metastases (3.6%). Open brain biopsy pathology showed non-diagnostic pathology in 29.4% of open brain biopsies: nonspecific inflammatory changes in 18.2% (31/170) and normal tissue in 11.2% (19/170) of cases. Fourteen percent of brain biopsies (24/170) were found to have vascular anomalies including vasculitis (2.9%), vasculopathy (3.5%), vascular malformations (1.2%, a capillary telangiectasia and an arteriovenous malformation), and amyloid angiopathy (6.5%). Infectious etiologies and demyelination were found in 2.4% of open brain biopsies (4/170 and 4/170) each. Degenerative pathology was found in 1.8% (3/170) of open brain biopsies including spongiform changes (1.2%) and Alzheimer plaques (0.6%). Lymphohistiocytic infiltrate and neuronal ceroid lipofuscinosis were found in 2 patients.
FIGURE 1.
Distribution of pathology results for 170 brain biopsies of the superficial cortex.
Predictors of Diagnostic Pathology and Outcome
We evaluated for preoperative predictors of successful diagnostic pathology using a hypothesis-generated and exploratory univariate analysis (Table 3). Patients older than 45 years of age had 2.67 times the odds of diagnostic pathology than younger patients (95% confidence interval [CI]: 1.34-5.29; P < .01). Patients older than 45 years of age at the time of biopsy had diagnostic pathology in 80% of biopsies. Conversely, patients 45 years of age and younger had diagnostic pathology in 60%. History of a cancer diagnosis increased the odds of diagnostic pathology by 3.64 (95% CI: 1.69-7.85; P < .001). A focal lesion on CT (OR: 5.54, 95% CI: 2.13-14.43,P < .001), a focal lesion on MRI (OR: 3.90, 95% CI: 1.91-8.00, P < .001), and an enhancing lesion on MRI (OR: 5.03, 95% CI: 2.41-10.52, P < .001) were associated with diagnostic pathology. Biopsy of a discrete lesion on MRI (OR: 9.34, 95% CI: 4.39-20.33, P < .001), and use of a frameless image-guided surgical technique (OR: 6.59, 95% CI: 3.04-14.28, P < .001) increased the odds of diagnostic pathology. No statistically significant associations were identified with sex, smoking, vascular disease, HIV, headaches, behavioral changes, cognitive decline, hallucinations, myoclonus, seizures, steroid use, T2 signal changes on MRI, findings of vasculitis or vasculopathy on angiograms, white blood cell count, inflammatory markers, CSF studies, or use of positron emission tomography, spectroscopy, or electroencephalography.
TABLE 3.
Predictors of Diagnostic Tissue Pathologya
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Age >45 y | 2.67 | 1.34-5.29 | <.01 |
| Male sex | 0.78 | 0.39-1.56 | |
| Comorbidities | |||
| Smoker | 0.85 | 0.39-1.82 | |
| Coronary artery disease | 4.28 | 0.95-19.14 | |
| Vascular disease | 0.86 | 0.42-1.77 | |
| HIV | 0.78 | 0.07-8.81 | |
| Previous cancer diagnosis | 3.64 | 1.69-7.85 | <.001 |
| Presenting symptoms and signs | |||
| Headaches | 0.66 | 0.34-1.30 | |
| Cognitive decline | 0.72 | 0.45-1.15 | |
| Behavioral change | 0.61 | 0.31-1.21 | |
| Hallucinations | 1.63 | 0.33-8.00 | |
| Focal weakness | 1.00 | 0.50-1.99 | |
| Myoclonus | 0.18 | 0.03-1.04 | |
| Seizures | 2.06 | 0.95-4.44 | |
| Medications on consultation | |||
| Steroids | 0.93 | 0.47-1.87 | |
| Imaging and clinical studies | |||
| Focal lesion on CT scan | 5.54 | 2.13-14.43 | <.001 |
| Focal lesion on MRI | 3.90 | 1.91-8.00 | <.001 |
| Enhancing lesion on MRI | 5.03 | 2.41-10.52 | <.001 |
| Catheter angiography performed | 0.35 | 0.17-0.72 | <.01 |
| CT angiography performed | 0.39 | 0.02-6.39 | |
| MR angiography performed | 0.39 | 0.17-0.93 | <.05 |
| Vasculitis/vasculopathy on angiography | 0.30 | 0.09-1.04 | |
| Surgical technique | |||
| Biopsy of specific lesion on MRI | 9.34 | 4.29-20.33 | <.001 |
| Image-guided surgery | 6.59 | 3.04-14.28 | <.001 |
OR, odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; CT, computed tomography; MRI, magnetic resonance imaging.
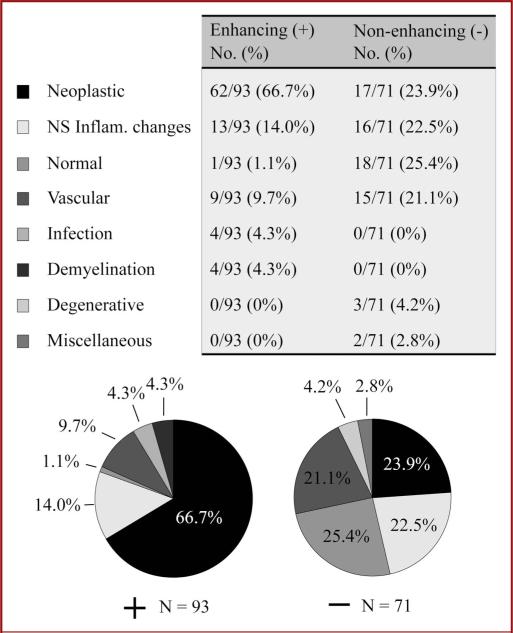
An enhancing lesion on MRI altered the diagnoses attained with open brain biopsy (Figure 2). Enhancing lesions on MRI had a significantly different distribution of diagnoses compared with nonenhancing lesions (P < .0001, Fisher exact test). If MRI showed an enhancing lesion, pathology revealed a neoplasm in 66.7% of biopsies. Normal pathology and nonspecific inflammatory changes accounted for 1.1% and 14.0% of biopsies, respectively. The remainder of biopsies showed vascular disease (9.7%), infections (4.3%), and demyelinating disorders (4.3%). Without an enhancing lesion on MRI, pathology showed normal tissue in 25.4% and nonspecific inflammatory changes in 22.5% of biopsies: 47.9% nondiagnostic. Neoplasms (23.9%) and vascular diseases (21.1%) accounted for the majority of other diagnoses.
FIGURE 2.
Pathology results for open brain biopsy patients who underwent a preoperative magnetic resonance imaging study including 93 who showed enhancing lesions (+, left) and 71 with no enhancing lesions (–, right). Quantitation and pie charts are shown each subgroup of patients. NS Inflam., nonspecific inflammatory changes.
Biopsy affected the clinical management plan in 97 of 170 patients (57.1%). Biopsy affected management in 75.0% of patients with a cancer history vs 55.4% in patients without a previous cancer diagnosis (P < .001). Myoclonus decreased the likelihood that biopsy affected management (P < .05). A focal CT lesion (P < .01), a focal MRI lesion (P < .001), and an enhancing MRI lesion (P < .001) increased the probability that biopsy affected management. Biopsy of a discrete MRI lesion and use of intraoperative navigation increased the odds that biopsy affected management (P < .001). Patients who had catheter angiography performed were less likely to have a biopsy finding that affected management (P < .01). Brain biopsy affected management in 9.1% of patients who had angiographic signs of vasculitis vs 63.7% in other patients (P < .001).
Several factors correlated with long-term outcomes after open brain biopsies. Overall, 72 of 149 patients (48.3%) were improved on long-term follow-up after open brain biopsies. Twenty-one patients were lost to follow-up. Median length of follow-up time was 28.0 months (interquartile range, 5.7-57.0 months). Thirty-seven percent of previous cancer patients were improved on follow-up vs 43.6% of patients without a previous cancer diagnosis (P < .05). Patients who improved on follow-up were younger (42.9 ± 20.6 years old) than those who did not improve (51.5 ± 22.7 years; P < .01). Thirty-seven percent of patients who presented with cognitive decline improved on follow-up compared with 59.5% patients without cognitive decline (P < .01). In contrast, presenting with headaches increased the chance of improvement on follow-up from 38.8% to 62.3% (P < .01).
Morbidities and Mortalities
Postoperative morbidities and mortalities were recorded for each patient (Table 4). Fifty-five patients (32.5%) had reports of postoperative ICH. Of these, 21 (12.4%) were symptomatic and 34 (20.1%) were asymptomatic. The mean ICH size was 1.5 ± 1.8 cm. A symptomatic ICH was 3.9 times larger than an asymptomatic ICH (3.1 ± 2.3 vs 0.8 ± 0.6 cm, respectively; P < .01). Five biopsies (3%) were complicated by ICH requiring surgical intervention. Twenty-seven patients (16.2%) experienced postoperative seizures. Of these, 10 of 27 experienced de novo seizures. Sixty-two (37.1%) experienced significant postoperative morbidities, including major systemic complications, infections, neurological deficits, strokes, and hemorrhages. Thirteen biopsies (8%) were associated with perioperative mortality (within 31 days postoperatively; range, 1-31 days). Three (1.8%) died of postoperative ICH and 3 (1.8%) died of disease progression.
TABLE 4.
Postoperative Morbidity and Mortalitya
| Morbidity and Mortality | No. (%) of Patients | Size, cm (SD) |
|---|---|---|
| ICH | 55 (32.5) | 1.5 (1.8) |
| Symptomatic ICH | 21 (12.4) | 3.1 (2.3) |
| Asymptomatic ICH | 34 (20.1) | 0.8 (0.6)b |
| ICH requiring surgical intervention | 5 (3.0) | |
| Seizure | 27 (16.2) | |
| Significant morbidities | 62 (37.1) | |
| Perioperative mortality | 13 (8.0) | |
| Mortality 2°/2 disease progression | 3 (1.8) | |
| Mortality 2°/2 ICH | 3 (1.8) |
ICH, intracranial hemorrhage.
Different from symptomatic ICH, P < .01; size = mean diameter.
Predictors of Morbidities
Several preoperative variables were associated with postoperative ICH (Table 5). Patients without a postoperative ICH had a mean age of 46.5 ± 24.2 years, whereas postoperative patients with ICH had a mean age of 53.2 ± 21.8 years (P < .05). Age older than 60 years at the time of surgery increased the odds of postoperative ICH by 2.66 (95%: CI 1.36-5.18, P < .01). Forty-nine percent of patients who took aspirin on initial consultation had a postoperative ICH compared with 27.2% of those who did not take aspirin (OR: 2.51, 95% CI: 1.23-5.28, P < .05). Exploratory univariate analysis of risk factors found no associations with postoperative ICH and coagulation studies, platelet count or function tests, surgical technique or diagnosis. Pre-operative seizures increased the incidence of postoperative seizures from 9.0% to 39.9% (P < .001). Forty-nine percent of patients with a previous cancer diagnosis had a postoperative morbidity compared with 28.4% of patients without previous cancer (P < .01).
TABLE 5.
Predictors of Postoperative ICHa
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Age >60 y | 2.66 | 1.36-5.18 | <.01 |
| Male sex | 1.22 | 0.63-2.36 | |
| Comorbidities | |||
| Smoker | 0.93 | 0.43-1.98 | |
| Coronary artery disease | 0.83 | 0.30-2.26 | |
| Vascular disease | 1.30 | 0.65-2.59 | |
| HIV | 1.07 | 0.09-12.04 | |
| Previous cancer diagnosis | 1.31 | 0.69-2.51 | |
| Medications on consultation | |||
| Aspirin | 2.51 | 1.23-5.28 | <.05 |
| Clopidogrel | 0.83 | 0.16-4.43 | |
| Warfarin | 0.84 | 0.16-4.47 | |
| NSAIDs | 0.29 | 0.03-2.41 | |
| Steroids | 1.26 | 0.64-2.48 | |
| Imaging and clinical studies | |||
| Focal lesion on CT scan | 2.19 | 0.91-5.29 | |
| Focal lesion on MRI | 1.33 | 0.65-2.72 | |
| Enhancing lesion on MRI | 2.00 | 1.01-3.99 | |
| Catheter angiography performed | 0.52 | 0.23-1.14 | |
| CT angiography performed | 2.07 | 0.13-33.8 | |
| MR angiography performed | 0.32 | 0.11-0.99 | <.05 |
| Vasculitis/vasculopathy on angiography | 0.44 | 0.09-2.09 | |
| Surgical technique | |||
| Biopsy of specific lesion on MRI | 1.87 | 0.90-3.90 | |
| Image-guided surgery | 1.58 | 0.77-3.24 |
ICH, intracranial hemorrhage; OR, odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; NSAIDs, nonsteroidal anti-inflammatory drugs; CT, computed tomography; MRI, magnetic resonance imaging.
Models for Predicting Effectiveness and Outcome
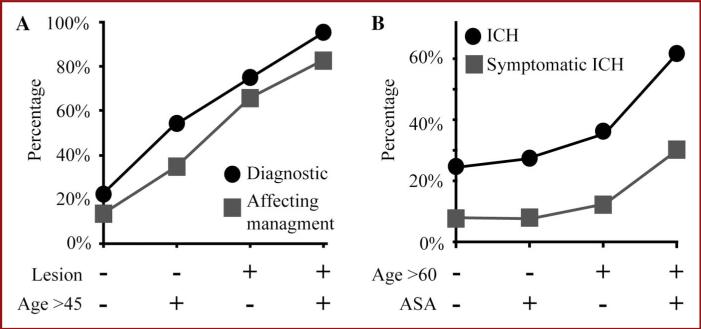
Multivariate analyses identified age older than 45 years and biopsy of a discrete lesion on MRI as the 2 independent predictors, and the 10-fold cross-validation21 demonstrated a reliable prediction of both diagnostic pathology (AUC = 0.82) and surgery (AUC = 0.76) affecting management. Age older than 45 years increased a patient's odds of diagnostic pathology and surgery affecting management to 6.01 (95% CI: 2.38-14.09) and 2.88 (95% CI: 1.35-6.17), respectively (Table 6). Biopsy of a radiographic lesion increased a patient's odds of diagnostic pathology and surgery affecting management to 14.09 (95% CI: 5.57-22.57) and 9.34 (95% CI: 1.35-6.17), respectively (Table 6). Using age and operative plan as variables, the percentages of biopsies that led to diagnostic pathology and affected management were tabulated and presented in numerical (see Table 2, Supplemental Content 2, http://links.lww.com/NEU/A539) and graphic forms (Figure 3A). This model illustrates that in a person 45 years of age or younger and without a discrete lesion on MRI, an open brain biopsy will lead to diagnostic pathology in 22.7% and affect management in 13.6% of patients. Age older than 45 years increases the diagnostic yield of a biopsy to 54.1%, affecting management in 35.1%. A discrete lesion on MRI with age 45 years or younger led to 75.0% diagnostic yield with 65.9% biopsy affecting management. Finally, a person older than 45 years of age and with a discrete lesion on biopsy had a 95.2% chance of diagnostic pathology and an 82.5% chance of biopsy affecting management.
TABLE 6.
Multivariate Analyses of Predictors of Effectiveness and Outcomesa
| Predictors of Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Diagnostic pathology | |||
| Age >45 y | 6.01 | 2.38-15.22 | <.001 |
| Biopsy of specific lesion on MRI | 14.09 | 5.57-22.57 | <.001 |
| Affecting management | |||
| Age >45 y | 2.88 | 1.35-6.17 | <.01 |
| Biopsy of specific lesion on MRI | 9.34 | 4.26-20.46 | <.001 |
| Intracranial hemorrhage | |||
| Age >60 y | 2.10 | 1.03-4.31 | <.05 |
| Aspirin use history | 2.26 | 1.01-5.09 | <.05 |
OR, odds ratio; CI, confidence interval; MRI, magnetic resonance imaging.
FIGURE 3.
Correlating age, aspirin (ASA) history, and operative plan with effectiveness and outcome for open brain biopsy. A, percentages of diagnostic pathology (circles) and biopsy affecting management (squares) are plotted relative to predicting variables. Variables included age at the time of operation older than 45 years or 45 years and younger and biopsy of a discrete lesion on magnetic resonance imaging (lesion). B, percentages of intracranial hemorrhage (ICH) (circles) and symptomatic ICH (squares) are plotted relative to predicting variables. Variables included age at the time of operation older than 60 years or 60 years and older and aspirin use on initial consultation.
A multivariate model was also created for postoperative ICH risk assessment. Aspirin use at the time of consultation and age older than 60 years were found to increase the risk of a postoperative ICH (AUC = 0.63)21. Age older than 60 years and aspirin use increased the odds of an ICH by 2.10 (95% CI: 1.03-4.31) and 2.26 (95% CI: 1.01-5.09), respectively (Table 6). Using these 2 variables, the percentages of biopsies that led to radiographic ICH and symptomatic ICH were tabulated and presented in numerical (see Table 3, Supplemental Content 3, http://links.lww.com/NEU/A540) and graphic forms (Figure 3B). Age older than 60 years and use of aspirin on initial consultation predicted the chance of ICH and symptomatic ICH in 61.5% and 30.8% of patients, respectively.
DISCUSSION
This single-center retrospective analysis of variables can help predict effectiveness and outcomes for brain biopsies of the superficial cortex. We found that, overall, superficial brain biopsies led to neoplastic diagnosis in 49% of cases but were nondiagnostic in 29%. Older age, previous cancer diagnosis, and a focal/enhancing lesion on imaging increase likelihood of diagnostic pathology. Our findings corroborate those of other reports suggesting an association between age and diagnostic yield.13,20 Our data show that older age correlates with diagnostic pathology. This is similar to Field et al,13 who showed that in needle biopsies, younger age was associated with a reduced diagnosis rate. In contrast, Burns et al,20 who included only those with normal or indeterminate imaging findings and excluded immunocompromised patients and those with a previous diagnosis of intracranial neoplasms, showed younger age correlated with diagnostic pathology. Biopsy of a specific lesion on imaging and use of intraoperative image-guided surgery increased the likelihood of diagnostic pathology. Previous reports have indicated that angiography has minimal sensitivity for vasculitis.22 In our patients, angiographic signs of vasculitis were not associated with diagnostic pathology.
This study highlights the importance of diagnostic imaging to predict effectiveness of the open brain biopsy. As shown in Figure 2, the presence of an enhancing lesion on MRI dramatically reduced the incidence of nondiagnostic pathology from 50% to 15%. Furthermore, in patients without an enhancing lesion, neoplasms still accounted for a large percentage of the diagnostic pathology followed closely by vascular abnormalities.
Predictors of morbidity included older age, aspirin use, pre-operative seizures, and a history of cancer. Older age increased the odds of an ICH, possibly due to small-vessel disease and other age-related factors. With clinical details such as patient age, operative plan, and medication list, a clinician can estimate the chances of diagnostic success and hemorrhage for an open brain biopsy. Some patients may be in poor neurological condition without obvious options for treatment, whereby a high risk-benefit ratio may be warranted, but for other patients, observation may be justified. A hemiparetic young woman with an enhancing superficial lesion has a 75% chance of a diagnosis. An elderly man with progressive cognitive decline and no lesion on imaging has a 54% chance of diagnostic pathology. A young, neurologically devastated man without a lesion on imaging has a 23% chance of a diagnosis. The findings shown here and in other studies2,13,16,17,19,20,23 provide hope for achieving a diagnosis for very ill patients and emphasize that for some patients, the clinical picture may strongly justify the risk of biopsy.
This study carries some inherent limitations such as its single-center retrospective nature. Patient selection was affected by the overlap between patients selected for resection and open biopsy. We excluded needle biopsies, which account for a large number of biopsies and likely excluded many pathologies. The definition of an ICH likely overrepresents this complication. Information regarding transfusions was not available for this study. The analysis was not powered strongly enough to evaluate aspirin dose, time held, or platelet function studies.
CONCLUSION
Preoperative variables can be used to predict the potential risks and benefits of performing an open brain biopsy. Variables that correlated with increased effectiveness include increased age, previous cancer diagnosis, focal lesions, lesions with enhancement, and targeting lesions with surgical navigation. Conversely, older age and history of aspirin are associated with an increased risk of postoperative complications. This single-center, retrospective study suggests that using age, operative plan, and history of aspirin use, the models and concepts presented here can aid a physician's clinical judgment to stratify the risks and benefits for open brain biopsy procedures.
Supplementary Material
WHAT IS THIS BOX?
A QR Code is a matrix barcode readable by QR scanners, mobile phones with cameras, and smartphones. The QR Code above links to Supplemental Digital Content from this article.
Acknowledgments
The authors thank the Washington University Department of Neurosurgery for support of this study and departmental colleagues for suggestions and assistance. They acknowledge the support of the Biostatistics Core of Siteman Comprehensive Cancer Center and National Institutes of Health NCI Cancer Center Support Grant P30 CA091842. Finally, they dedicate this study to their patients, who humble and inspire them daily.
The work was supported by internal funding from the Department of Neurosurgery at Washington University School of Medicine.
ABBREVIATIONS
- AUC
area under the curve
- CI
confidence interval
- ICH
intracranial hemorrhage
Footnotes
SANS LifeLong Learning and NEUROSURGERY offer CME for subscribers that complete questions about featured articles. Questions are located on the SANS website (http://sans.cns.org/). Please read the featured article and then log into SANS for this educational offering.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.neurosurgery-online.com).
Disclosures
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.neurosurgery-online.com).
REFERENCES
- 1.Hall WA. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82(9):1749–1755. doi: 10.1002/(sici)1097-0142(19980501)82:9<1756::aid-cncr23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman HH, Catalano LW., Jr Diagnostic brain biopsy: a series of 50 cases and a review. Neurosurgery. 1979;4(2):129–136. doi: 10.1227/00006123-197902000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Woodworth GF, McGirt MJ, Samdani A, et al. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J Neurosurg. 2006;104(2):233–237. doi: 10.3171/jns.2006.104.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Kim JE, Kim DG, Paek SH, Jung HW. Stereotactic biopsy for intracranial lesions: reliability and its impact on the planning of treatment. Acta Neurochir (Wien) 2003;145(7):547–554. doi: 10.1007/s00701-003-0048-8. discussion 554-555. [DOI] [PubMed] [Google Scholar]
- 5.Apuzzo ML, Chandrasoma PT, Cohen D, Zee CS, Zelman V. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20(6):930–937. doi: 10.1227/00006123-198706000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein M, Parrent AG. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81(2):165–168. doi: 10.3171/jns.1994.81.2.0165. [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka D, Firlik AD, Lunsford LD. Complications of stereotactic brain surgery. Neurol Clin. 1998;16(1):35–54. doi: 10.1016/s0733-8619(05)70366-2. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni AV, Guha A, Lozano A, Bernstein M. Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. J Neurosurg. 1998;89(1):31–35. doi: 10.3171/jns.1998.89.1.0031. [DOI] [PubMed] [Google Scholar]
- 9.Lunsford LD, Martinez AJ. Stereotactic exploration of the brain in the era of computed tomography. Surg Neurol. 1984;22(3):222–230. doi: 10.1016/0090-3019(84)90003-x. [DOI] [PubMed] [Google Scholar]
- 10.Olivi A, Weingart JD, Liauw J, Raza SM. Frame and frameless stereotactic brain biopsy. In: Winn HR, editor. Youmans Neurological Surgery. 6th ed. Vol. 2. Elsevier; Philadelphia, PA: 2011. pp. 1254–1260. [Google Scholar]
- 11.Ostertag CB, Mennel HD, Kiessling M. Stereotactic biopsy of brain tumors. Surg Neurol. 1980;14(4):275–283. [PubMed] [Google Scholar]
- 12.Voges J, Schröder R, Treuer H, et al. CT-guided and computer assisted stereotactic biopsy. Technique, results, indications. Acta Neurochir (Wien) 1993;125(1-4):142–149. doi: 10.1007/BF01401842. [DOI] [PubMed] [Google Scholar]
- 13.Field M, Witham TF, Flickinger JC, Kondziolka D, Lunsford LD. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg. 2001;94(4):545–551. doi: 10.3171/jns.2001.94.4.0545. [DOI] [PubMed] [Google Scholar]
- 14.Skolasky RL, Dal Pan GJ, Olivi A, et al. HIV-associated primary CNS lymorbidity and utility of brain biopsy. J Neurol Sci. 1999;163(1):32–38. doi: 10.1016/s0022-510x(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 15.Josephson SA, Papanastassiou AM, Berger MS, et al. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg. 2007;106(1):72–75. doi: 10.3171/jns.2007.106.1.72. [DOI] [PubMed] [Google Scholar]
- 16.Schott JM, Reiniger L, Thom M, et al. Brain biopsy in dementia: clinical indications and diagnostic approach. Acta Neuropathol. 2010;120(3):327–341. doi: 10.1007/s00401-010-0721-y. [DOI] [PubMed] [Google Scholar]
- 17.Schuette AJ, Taub JS, Hadjipanayis CG, Olson JJ. Open biopsy in patients with acute progressive neurologic decline and absence of mass lesion. Neurology. 2010;75(5):419–424. doi: 10.1212/WNL.0b013e3181eb5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren JD, Schott JM, Fox NC, et al. Brain biopsy in dementia. Brain. 2005;128(pt 9):2016–2025. doi: 10.1093/brain/awh543. [DOI] [PubMed] [Google Scholar]
- 19.Hulette CM, Earl NL, Crain BJ. Evaluation of cerebral biopsies for the diagnosis of dementia. Arch Neurol. 1992;49(1):28–31. doi: 10.1001/archneur.1992.00530250032011. [DOI] [PubMed] [Google Scholar]
- 20.Burns JD, Cadigan RO, Russell JA. Evaluation of brain biopsy in the diagnosis of severe neurologic disease of unknown etiology. Clin Neurol Neurosurg. 2009;111(3):235–239. doi: 10.1016/j.clineuro.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York, NY: 2001. pp. 230–264. [Google Scholar]
- 22.Kadkhodayan Y, Alreshaid A, Moran CJ, et al. Primary angiitis of the central nervous system at conventional angiography. Radiology. 2004;233(3):878–882. doi: 10.1148/radiol.2333031621. [DOI] [PubMed] [Google Scholar]
- 23.Javedan SP, Tamargo RJ. Diagnostic yield of brain biopsy in neurodegenerative disorders. Neurosurgery. 1997;41(4):823–828. doi: 10.1097/00006123-199710000-00011. discussion 828-830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.