SUMMARY
Objective
To investigate longitudinal changes in laminar and spatial distribution of knee articular cartilage magnetic resonance imaging (MRI) T1ρ and T2 relaxation times, in individuals with and without medial compartment cartilage defects.
Design
All subjects (at baseline n = 88, >18 years old) underwent 3-Tesla knee MRI at baseline and annually thereafter for 3 years. The MR studies were evaluated for presence of cartilage defects (modified Whole-Organ Magnetic Resonance Imaging Scoring – mWORMS), and quantitative T1ρ and T2 relaxation time maps. Subjects were segregated into those with (mWORMS ≥2) and without (mWORMS ≤1) cartilage lesions at the medial tibia (MT) or medial femur (MF) at each time point. Laminar (bone and articular layer) and spatial (gray level co-occurrence matrix – GLCM) distribution of the T1ρ and T2 relaxation time maps were calculated. Linear regression models (cross-sectional) and Generalized Estimating Equations (GEEs) (longitudinal) were used.
Results
Global T1ρ, global T2 and articular layer T2 relaxation times at the MF, and global and articular layer T2 relaxation times at the MT, were higher in subjects with cartilage lesions compared to those without lesions. At the MT global T1ρ relaxation times were higher at each time point in subjects with lesions. MT T1ρ and T2 became progressively more heterogeneous than control compartments over the course of the study.
Conclusion
Spatial distribution of T1ρ and T2 relaxation time maps in medial knee OA using GLCM technique may be a sensitive indicator of cartilage deterioration, in addition to whole-compartment relaxation time data.
Keywords: GLCM, Texture, Quantitative MRI, Cartilage defects, Laminar
Introduction
Knee osteoarthritis (OA) most commonly affects the medial compartment1 and degenerative cartilage lesions associated with knee OA have been reported more frequently at the medial compartment of the knee2–4. Early degenerative changes in OA consist of reduction in the proteoglycan content and disruption of the collagen network5. T1ρ and T2 relaxation time mapping magnetic resonance imaging (MRI) techniques, among others, have been proposed for quantitative evaluation of early changes associated with OA in knee hyaline cartilage6–10. An increase in T1ρ and T2 relaxation times indicates loss of proteoglycans and disruption of collagen matrix respectively7–9,11–13. T2 relaxation time has also been inversely correlated with proteoglycan concentration14, suggesting that this metric is sensitive to both collagen and proteoglycan concentration. Previous studies have demonstrated differences between superficial and deep layers of articular cartilage using laminar analyses, for mean T1ρ10 and T215 relaxation times, possibly due to spatial differences in collagen orientation and content throughout the cartilage matrix. It has also been shown that individuals with greater number and severity of cartilage lesions in the medial femur (MF) have higher T1ρ relaxation times at the MF4. However, longitudinal analysis of changes in T1ρ and T2 relaxation times for the superficial and deep layers of articular cartilage, and their association with medial knee cartilage defects, has not been performed.
Haralick et al.16 developed a method of texture analysis based on the gray level co-occurrence matrix (GLCM) that is used to evaluate spatial distribution of pixel intensities in an image along a corresponding angle or direction. Spatial analysis of T1ρ and T2 relaxation times in cartilage has been shown to provide supplementary information about specific patterns of degeneration when compared to standard metrics alone (compartment mean values and standard deviations)17,18. Techniques to flatten regions of interest after image acquisition to more accurately classify tissues with well-defined layers have been proposed19. Carballido-Gamio et al.20 reported significant increases in T1ρ GLCM parameter reproducibility with flattened cartilage maps compared to non-flattened maps. Flattening of T1ρ and T2 cartilage maps allows for quantification of GLCM spatial heterogeneity both along (parallel to the bone–cartilage interface, corresponding to the A–P axis) and through (perpendicular to the bone–cartilage interface, corresponding to the S–I axis) the natural lamina present in articular cartilage. Longitudinal changes in knee articular cartilage GLCM parameters for both T1ρ and T2 relaxation times, using flattened cartilage maps, and their association with cartilage defects, have not been investigated to date.
The goals of this study were to (1) compare global, laminar (bone and articular layer), and flattened texture parameters of T1ρ and T2 relaxation times between medial knee compartments with and without cartilage lesions (cross-sectional), and (2) to compare the changes in global, laminar (bone and articular layer), and flattened texture parameters of T1ρ and T2 relaxation times in medial knee compartments with and without cartilage lesions over 3 years (longitudinal). We hypothesized that longitudinally, knee compartments with cartilage lesions will display elevated T1ρ and T2 relaxation times and will become increasingly more heterogeneous compared to compartments without cartilage lesions.
Materials and methods
Subjects
Patients with OA and control subjects without OA were recruited from UCSF orthopedic surgeons and the communityas part of a natural evolution study on knee OA. The data in this study include ongoing analyses from these previouslycollected data. The inclusion criteria for OA patients were frequent clinical symptoms of OA (including pain, stiffness and dysfunction) and demonstration of typical signs of OA in radiographs [Kellgren–Lawrence (KL)grade>0]21. The controlshad no history of diagnosed OA, clinical OA symptoms, previous knee injuries, or signs of OA on radiographs. Standard standing antero-posterior radiographs of the knee were obtained in all subjects at baseline to determine the KL grade and OA severity22. At baseline, the 88 subjects (41 men, 47 women) that participated in this study had a mean age of 50.1 ± 14 years and a mean BMI of 26.1 ± 4.6 kg/m2.
MRI
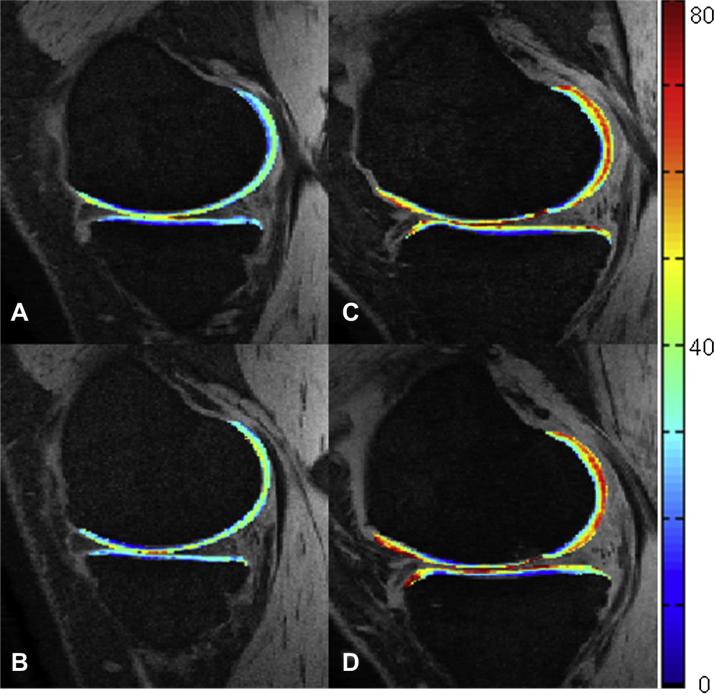
All subjects underwent MR imaging of the knee at baseline, and at 1 year intervals for 3 more years. MR data were acquired on a 3 T Signa HDx MR (GE Healthcare, Piscataway, NJ) scanner with a dedicated 8-channel phased array knee coil. Clinical scoring of cartilage lesions was performed on a sagittal T2 fast-spin echo (FSE) sequence (repetition time (TR)/echo time (TE) = 4300/51 ms, field of view (FOV) = 6–8 cm, matrix = 512 × 256, slice thickness (ST) = 1 mm, echo train length = 9, bandwidth (BW) = 31.25 kHz, NEX = 2, acquisition time = 4 min). A fat-saturated 3D spoiled gradient-echo (SPGR) sequence (TR/TE = 15/6.7 ms, flip angle = 12, FOV = 6–8 cm, matrix = 512 × 512, ST = 1 mm, BW = 31.25 kHz, number of excitations (NEX) = 1, acquisition time = 8 min 30 s) was acquired for the purposes of cartilage segmentation. Cartilage T1ρ and T2 maps were generated using 3D T1ρ mapping techniques20 based on a gradient echo sequence (TR/TE = 9.3/3.7 ms, FOV = 6–8 cm, matrix = 256 × 128, ST = 2 mm, BW = 31.25 kHz, views per segment = 64, Trec = 1.5 s, spin-lock time (TSL) = 0, 10, 40, 80 ms, spin-lock frequency (FSL) = 500 Hz, acquisition time = 13 min)23. T2-weighted images were acquired using sagittal 3D T2 mapping (TR = 3700 ms, TE = 4.1, 14.5, 25, 45.9 ms, FOV = 6–8 cm, matrix = 256 × 128, ST = 2 mm, BW = 31.25 kHz, views per segment = 64, time of recovery (Trec) = 1.5 s, acquisition time = 13 min). Parallel imaging was used on all imaging sequences utilizing Array Spatial Sensitivity Encoding Technique (ASSET) with an acceleration factor of 2. Fig. 1 displays representative T1ρ relaxation time color overlays of baseline and year 2 time points for both groups.
Fig. 1.
Representative sagittal SPGR images with T1ρ relaxation times superimposed on articular cartilage as a color overlay of a healthy control at (A) baseline and (B) at the 2-year follow-up. OA patient at (C) baseline and (D) at the 2-year follow-up. Qualitative OA spatial heterogeneity increases are visible near the anterior portion of the MF/MT. Color scale (right) measured in milliseconds.
Clinical grading
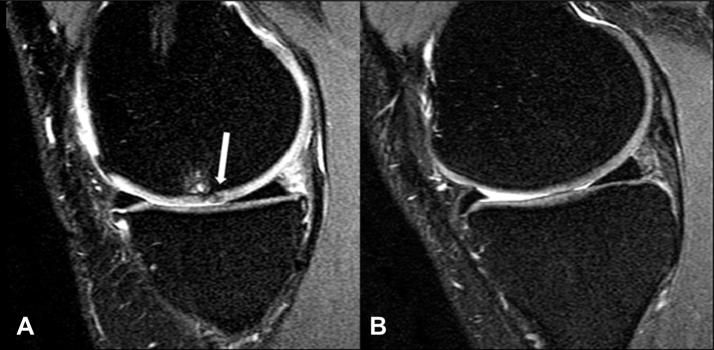
UCSF modified Whole-Organ Magnetic Resonance Imaging Score (mWORMS)24 was used to assess cartilage morphology at each time point, on a sagittal intermediate-weighted FSE fat-saturated image (Fig. 2) by board certified radiologists (TML with 20 and LN with 4 years of experience with musculoskeletal MRI). The radiologists were blinded to subject information and performed separate readings, with a consensus in case of disagreement. Cartilage was graded as follows: 0: normal signal and thickness; 1: normal thickness and elevated signal; 2: partial-thickness focal defect less than 1 cm in width; 2.5: full-thickness focal defect less than 1 cm in width; 3: multiple areas of partial-thickness focal defects mixed with areas of normal thickness or a grade 2 defect wider than 1 cm but less than 75% of the region; 4: diffuse partial thickness loss (≥75% of region); 5: multiple areas of full-thickness cartilage loss less than 1 cm or a full-thickness lesion greater than 1 m but less than 75% of the region; 6: diffuse full-thickness cartilage loss. Subjects were stratified into those with cartilage lesions (mWORMS ≥2) and those without cartilage lesions (mWORMS ≤1) at each time point.
Fig. 2.
Sagittal T2-weighted FSE images displaying (A) a MF osteoarthritic partial-thickness lesion (arrow) associated with underlying bone marrow edema mWORMS grade 2 (0.7 mm) and (B) a healthy control with intact cartilage.
Image processing
Cartilage compartments were segmented on multiple slices semi-automatically in high resolution SPGR images using the in-house software developed with Matlab (Mathworks, Natick, MA, USA) based on edge detection and Bezier splines25. The cartilage compartments analyzed for this study included the MF and medial tibia (MT). T1ρ and T2 maps were reconstructed by fitting T1ρ- and T2-weighted images pixel-by-pixel to the equations below using in-house developed software:
| (1) |
| (2) |
Post-processing of T1ρ and T2 maps for this study was identical to that of previous studies from our group which used the same dataset26,27. MF and MT ROIs were further partitioned into two equal layers: bone (closer to the subchondral bone) and articular (closer to articular surface) lamina automatically using in-house developed software25.
Cartilage T1ρ and T2 maps were flattened before quantification of the GLCM contrast, entropy, and variance parameters in the horizontal (corresponding to the A–P axis) and vertical (corresponding to the S–I axis) directions, for the regions of interest20. Flattening was achieved using a Bezier spline, non-linear warping technique setting the bone–cartilage interface spline as the reference for warped flattening. Relaxation times were analyzed at a one pixel offset. Elevated contrast indicates a greater number of adjacent pixels of differing values. Entropy is a measure of pixel orderliness with elevated entropy indicating a more uniform histogram (i.e., equal numbers of each pixel value). Variance is a measure in reference to how much pixel values vary from the compartment mean. Equations (3)–(5) denote three representative GLCM measurements16.
| (3) |
| (4) |
where
| (5) |
P indicates the probability of pixel values i and j co-occur in an image and N indicates the total number of pixel co-occurrences in each region of interest. A pixel offset of one pixel was chosen based on the fact that approximately three to four pixels span the cartilage thickness. Methods of using these specific representative measurements from each GLCM group have been widely applied in the study of T1ρ and T2 mapping of auricular cartilage18,28–30.
Statistical analysis
Independent two-tail Student's t tests were carried out to compare differences in subject age and BMI for compartments in the presence and absence of cartilage lesions at baseline. Similarly, chi-square tests were employed to calculate gender differences between the two groups. For cross-sectional statistics, a linear regression model was fit to each outcome, adjusting for age, gender and BMI. To evaluate whether lesion and control groups changed differentially over time, we utilized Generalized Estimating Equations (GEEs) to accommodate the repeated measures. All analyses were conducted in SAS 9.3 (SAS Institute, Cary, NC).
Results
Subject characteristics
Age, BMI and gender distribution at each time point for both groups are presented in Table I. Subjects with lesions tended to be older and heavier. Overall, there were 27 subjects with lesions in both MF and MT compartments, eight subjects with a lesion in the MF but not in the MT compartment, 0 subject with a lesion in the MT but not in the MF compartment, and 53 subjects without a lesion in either MF or MT compartments.
Table I.
Age, BMI, and gender distribution for the groups. P values from independent samples t-tests for age and BMI, and from chi-square tests for gender distribution
| Baseline (n = 88) |
1 Year (n = 60) |
2 Year (n = 38) |
3 Year (n = 27) |
|||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 53) | Lesion (n = 35) | Control (n = 37) | Lesion (n = 23) | Control (n = 28) | Lesion (n = 10) | Control (n = 15) | Lesion (n = 12) | |
| MF | ||||||||
| Age (years) | 43.9 (12.3) | 59.5 (11) | 45.3 (12.4) | 55 (10.7) | 45.3 (12) | 57.5 (7) | 47.8 (13.6) | 51.3 (10.4) |
| P-value | <0.0001 | 0.002 | 0.001 | 0.461 | ||||
| BMI (kg/m2) | 25.1 (4.6) | 27.5 (4.4) | 24.8 (4.4) | 26.9 (4.1) | 24 (3.1) | 26.4 (6.3) | 22.6 (2.7) | 24.4 (3.6) |
| P-value | 0.021 | 0.074 | 0.279 | 0.173 | ||||
| Gender (F:M) | 26:27 | 21:14 | 16:21 | 12:11 | 10:18 | 4:6 | 8:7 | 6:6 |
| P-value | 0.385 | 0.500 | 0.810 | 0.863 |
| Control (n = 61) | Lesion (n = 27) | Control (n = 43) | Lesion (n = 17) | Control (n = 29) | Lesion (n = 9) | Control (n = 20) | Lesion (n = 7) | |
|---|---|---|---|---|---|---|---|---|
| MT | ||||||||
| Age (years) | 44.4 (11.9) | 61.9 (9.9) | 46 (11.8) | 56.5 (11.5) | 46.1 (11.9) | 56.2 (9.5) | 49.2 (12.5) | 49.9 (12.1) |
| P-value | <0.0001 | 0.004 | 0.018 | 0.898 | ||||
| BMI (kg/m2) | 25.3 (4.5) | 27.8 (4.8) | 24.9 (4.1) | 27.5 (4.5) | 24.1 (3.1) | 26.2 (6.6) | 23.3 (2.9) | 23.7 (4.3) |
| P-value | 0.022 | 0.050 | 0.396 | 0.830 | ||||
| Gender (F:M) | 30:30 | 16:11 | 20:23 | 8:9 | 11:18 | 3:6 | 12:8 | 2:5 |
| P-value | 0.422 | 0.970 | 0.802 | 0.148 |
The bold indicates significance at P < 0.05.
MF
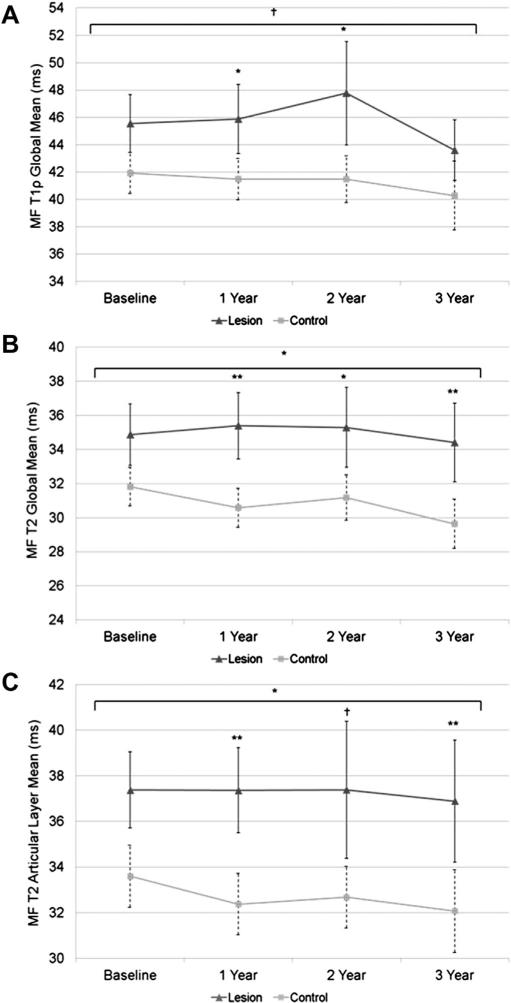
Mean values (95% confidence intervals (CI), estimated model differences) for T1ρ and T2 global, laminar, and GLCM texture data for MF are shown in Table II. For the global T1ρ relaxation times, the subjects with lesions displayed higher T1ρ at year 1 and 2 (P < 0.05) but not at baseline and year 3. For laminar T1ρ the subjects with lesions had higher articular layer T1ρ at year 1 (P = 0.015) and higher deep layer T1ρ at year 3 (P = 0.001). For the GLCM measures at baseline, the subjects with lesion had higher contrast, entropy, and variance in both directions (P < 0.05). At year 1, the subjects with lesions had higher vertical contrast (P = 0.03) as well as higher entropy and variance in both directions (P < 0.05). At year 2, the subjects with lesions had higher horizontal entropy (P = 0.02), higher contrast and variance in both directions (P < 0.05). At year 3, there were no differences between the groups for any of the GLCM measures. Longitudinal change in global mean T1ρ relaxation time between the two groups approached a significant difference (P = 0.056) (Table IV). The lesion group global mean displayed increasingly longer relaxation time until year 2, experiencing the largest drop-off from year 2 to year 3 (Fig. 3). Meanwhile, the control cartilage group experienced a slight yet consistent decrease in global mean T1ρ relaxation time (roughly 2 ms throughout the course of the study) (Fig. 3).
Table II.
MF mean values (95% CI, estimated differences) of each variable cross-sectionally (linear regression models adjusting for age, gender, BMI)
| T1ρ Global (ms) | Estimated difference | 95% CI | P-value | T1ρ Articular layer (ms) | Estimated difference | 95% CI | P-value | T1ρ Bone layer (ms) | Estimated difference | 95% CI | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Control | 41.93 | 0.811 | –1.926, 3.5 | 0.56 | 47.16 | 1.082 | –1.94, 4.1 | 0.48 | 36.43 | 0.387 | –2.7, 3.4 | 0.8 |
| Lesion | 45.55 | 51.62 | 39.31 | ||||||||||
| 1 Year | Control | 41.48 | 3 | 0.553, 5.5 | 0.017 | 47.37 | 3.4 | 0.69, 6.2 | 0.015 | 35.26 | 2.5 | –0.61, 5.5 | 0.11 |
| Lesion | 45.88 | 52.55 | 38.99 | ||||||||||
| 2 Year | Control | 41.48 | 3.9 | 0.664, 7.2 | 0.02 | 47.40 | 3.4 | –0.25, 7.0 | 0.067 | 35.28 | 3.8 | –0.59, 8.2 | 0.088 |
| Lesion | 47.77 | 54.20 | 41.01 | ||||||||||
| 3 Year | Control | 40.28 | 2.3 | –0.274, 4.9 | 0.077 | 47.37 | 1.321 | –2.69, 5.3 | 0.5 | 32.82 | 4.60 | 2.1, 7.2 | 0.001 |
| Lesion | 43.60 | 50.04 | 37.24 | ||||||||||
| T1ρ Contrast-horizontal | Estimated difference | 95% CI | P-value | T1ρ Contrast-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 10.62 | 23 | 8.6, 37 | 0.002 | 81.84 | 41 | 8.4, 73 | 0.014 | ||||
| Lesion | 35.32 | 148.90 | |||||||||||
| 1 Year | Control | 9.43 | 16 | –3.76, 36 | 0.11 | 81.23 | 36 | 4, 69 | 0.028 | ||||
| Lesion | 30.09 | 137.36 | |||||||||||
| 2 Year | Control | 6.22 | 22 | 4.8, 40 | 0.015 | 85.65 | 81 | 29, 134 | 0.0034 | ||||
| Lesion | 33.88 | 202.60 | |||||||||||
| 3 Year | Control | 9.51 | 10.2 | –7.38, 28 | 0.24 | 95.41 | –5.678 | –37.66, 26 | 0.72 | ||||
| Lesion | 22.37 | 100.51 | |||||||||||
| T1ρ Entropy-horizontal | Estimated difference | 95% CI | P-value | T1ρ Entropy-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 5.26 | 0.211 | 0.039, 0.382 | 0.017 | 5.55 | 0.225 | 0.07, 0.34 | 0.004 | ||||
| Lesion | 5.56 | 5.84 | |||||||||||
| 1 Year | Control | 5.21 | 0.258 | 0.102, 0.414 | 0.0016 | 5.61 | 0.251 | 0.11, 0.40 | 0.001 | ||||
| Lesion | 5.54 | 5.89 | |||||||||||
| 2 Year | Control | 5.13 | 0.271 | 0.057, 0.484 | 0.015 | 5.64 | 0.13 | –0.10, 0.36 | 0.25 | ||||
| Lesion | 5.52 | 5.83 | |||||||||||
| 3 Year | Control | 5.28 | 0.047 | –0.183, 0.277 | 0.68 | 5.74 | 0.031 | –0.22, 0.28 | 0.8 | ||||
| Lesion | 5.41 | 5.84 | |||||||||||
| T1ρ Variance-horizontal | Estimated difference | 95% CI | P-value | T1ρ Variance-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 72.35 | 85 | 55, 116 | <0.0001 | 66.05 | 77 | 48, 106 | <0.0001 | ||||
| Lesion | 185.54 | 167.22 | |||||||||||
| 1 Year | Control | 75.92 | 73 | 43, 103 | <0.0001 | 68.04 | 57 | 32, 82 | <0.0001 | ||||
| Lesion | 167.96 | 141.72 | |||||||||||
| 2 Year | Control | 80.14 | 127 | 67, 187 | 0.0001 | 71.43 | 85 | 39, 130 | 0.0006 | ||||
| Lesion | 232.83 | 183.11 | |||||||||||
| 3 Year | Control | 97.18 | 32 | –43.08, 107 | 0.39 | 85.56 | 24 | –31.89, 80 | 0.38 | ||||
| Lesion | 148.24 | 125.27 | |||||||||||
| T2 Global mean (ms) | Estimated difference | 95% CI | P-value | T2 Articular layer (ms) | Estimated difference | 95% CI | P-value | T2 Bone layer (ms) | Estimated difference | 95% CI | P-value | ||
| Baseline | Control | 31.82 | 1.349 | –0.929, 3.6 | 0.24 | 33.60 | 1.753 | –0.699, 4.2 | 0.16 | 29.93 | 1.003 | –0.97, 3.2 | 0.42 |
| Lesion | 34.88 | 37.38 | 32.33 | ||||||||||
| 1 Year | Control | 30.58 | 3.8 | 1.781, 1.551 | 0.0004 | 32.37 | 3.7 | 1.551, 5.8 | 0.001 | 28.67 | 4 | 1.52, 6.4 | 0.002 |
| Lesion | 35.39 | 37.37 | 33.32 | ||||||||||
| 2 Year | Control | 31.18 | 2.6 | 0.162, 5.1 | 0.038 | 32.68 | 2.4 | –0.048, 4.9 | 0.054 | 29.60 | 2.9 | –0.30, 6.1 | 0.074 |
| Lesion | 35.30 | 37.39 | 33.16 | ||||||||||
| 3 Year | Control | 29.64 | 4.1 | 1.74, 6.5 | 0.0016 | 32.07 | 3.7 | 0.985, 6.4 | 0.0098 | 27.05 | 2.3, 6.8 | 0.0004 | |
| Lesion | 34.41 | 36.89 | 31.80 | 4.60 | |||||||||
| T2 Contrast-horizontal | Estimated difference | 95% CI | P-value | T2 Contrast-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 10.66 | 11.9 | –2.284, 26 | 0.099 | 59.12 | 63 | 28, 99 | 0.0007 | ||||
| Lesion | 25.26 | 139.79 | |||||||||||
| 1 Year | Control | 4.08 | 8.4 | 3.5, 13.3 | 0.0012 | 53.76 | 42 | 19.1, 65 | 0.0006 | ||||
| Lesion | 13.60 | 100.70 | |||||||||||
| 2 Year | Control | 3.35 | 9.9 | 3.5, 16.3 | 0.0035 | 48.85 | 47 | 8.4, 86 | 0.019 | ||||
| Lesion | 13.92 | 113.73 | |||||||||||
| 3 Year | Control | 3.16 | 2.2 | 0.299, 4 | 0.025 | 45.14 | 16.2 | 1.878, 31 | 0.028 | ||||
| Lesion | 5.70 | 66.65 | |||||||||||
| T2 Entropy-horiizontal | Estimated difference | 95% CI | P-value | T2 Entropy-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 5.09 | 0.094 | –0.05, 0.24 | 0.19 | 5.48 | 0.056 | –0.11, 0.22 | 0.49 | ||||
| Lesion | 5.32 | 5.61 | |||||||||||
| 1 Year | Control | 4.90 | 0.25 | 0.09, 0.41 | 0.003 | 5.45 | 0.134 | –0.01, 0.28 | 0.064 | ||||
| Lesion | 5.27 | 5.66 | |||||||||||
| 2 Year | Control | 4.84 | 0.166 | –0.05, 0.38 | 0.13 | 5.48 | –0.087 | –0.26, 0.09 | 0.31 | ||||
| Lesion | 5.13 | 5.48 | |||||||||||
| 3 Year | Control | 4.86 | 0.107 | –0.07, 0.29 | 0.23 | 5.47 | 0.083 | –0.10, 0.28 | 0.36 | ||||
| Lesion | 5.07 | 5.66 | |||||||||||
| T2 Variance-horizontal | Estimated difference | 95% CI | P-value | T2 Variance-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 73.12 | 78 | 39, 117 | 0.0001 | 67.09 | 50 | 22, 78 | 0.0006 | ||||
| Lesion | 186.63 | 146.76 | |||||||||||
| 1 Year | Control | 68.57 | 61 | 30, 92 | 0.0002 | 61.54 | 47 | 22, 73 | 0.0005 | ||||
| Lesion | 139.81 | 116.40 | |||||||||||
| 2 Year | Control | 63.52 | 101 | 32, 169 | 0.0056 | 57.00 | 59 | 11.9, 107 | 0.016 | ||||
| Lesion | 180.03 | 136.00 | |||||||||||
| 3 Year | Control | 64.19 | 18.1 | –6.19, 42 | 0.14 | 56.86 | 12.3 | –5.936, 31 | 0.18 | ||||
| Lesion | 92.75 | 78.23 |
The bold indicates significance at P < 0.05.
Table IV.
Longitudinal interactions for variables approaching or displaying significantly divergent interactions using GEE models. Data adjusted for age, gender, BMI
| Variable | 95% CI | Estimated difference | P-value |
|---|---|---|---|
| MF T1ρ global mean (ms) | –0.019, 1.596 | 0.788 | 0.056 |
| MF T2 global mean (ms) | 0.03, 1.576 | 0.803 | 0.042 |
| MF T2 articular layer mean (ms) | 0.027, 1.6 | 0.813 | 0.043 |
| MT T2 global mean (ms) | –0.073, 2.706 | 1.317 | 0.063 |
| MT T2 articular layer mean (ms) | 0.474, 3.482 | 1.978 | 0.0099 |
| MT T2 horizontal entropy | 0.002, 0.117 | 0.059 | 0.043 |
| MT T1ρ vertical entropy | 0.017, 0.212 | 0.114 | 0.021 |
| MT T1ρ horizontal entropy | 0.058, 0.21 | 0.134 | 0.0006 |
The bold indicates significance at P < 0.05.
Fig. 3.
Global mean T1ρ and T2 relaxation times (A and B) and mean articular layer T2 relaxation times (C) in the MF. Single asterisk indicates P < 0.05, double asterisk indicates P < 0.01, and cross indicates P = 0.07–0.051 (approaching significance). Longitudinal significance between the groups is denoted above the horizontal bracket.
For MF global T2, the subjects with lesions had higher relaxation times at years 1, 2 and 3 (P < 0.05) (Table II). For laminar T2, the subjects with lesions had higher articular and deep layer T2 relaxation times at years 1 and 3 (P < 0.05). For T2 GLCM measures at baseline, the subjects with lesions had higher vertical contrast (P = 0.0007), and higher variance in both directions (P < 0.05) (Table II). At year 1, the subjects with lesions had higher contrast and variance in both directions (P < 0.05) and higher horizontal entropy (P = 0.003). At year 2, the subjects with lesions had higher contrast and variance in both directions (P < 0.05). At year 3, the subjects with lesions had higher contrast in both directions (P < 0.05). Global T2 relaxation time displayed significant longitudinal changes between lesion and control cartilage groups (P = 0.042) (Table IV). Lesion group global T2 relaxation time remained relatively constant throughout the study, fluctuating less than 1 ms from baseline to year 3, while control compartment global mean T2 relaxation time longitudinally decreased more than 2 ms (Fig. 3). Articular layer T2 relaxation time for lesion and control compartment groups also showed significantly different longitudinal changes (P = 0.043). Similarly to global mean T2, lesion group articular T2 fluctuated very little throughout the course of the study (less than 0.5 ms) while the control group decreased roughly 1.5 ms throughout all time points (Fig. 3) (Table IV).
MT
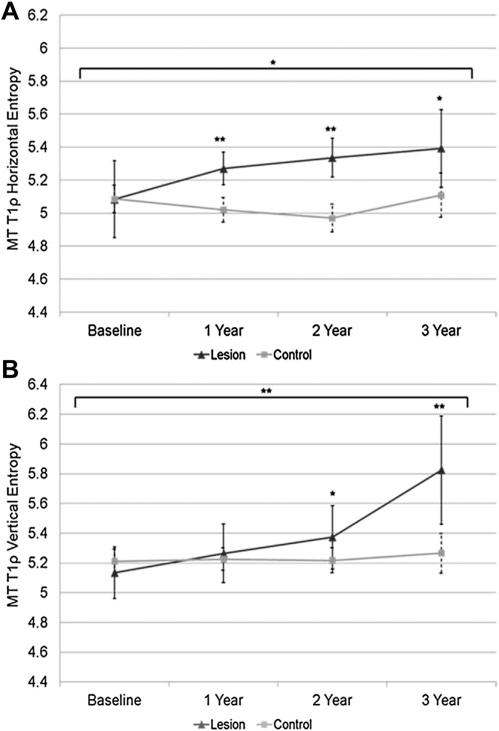
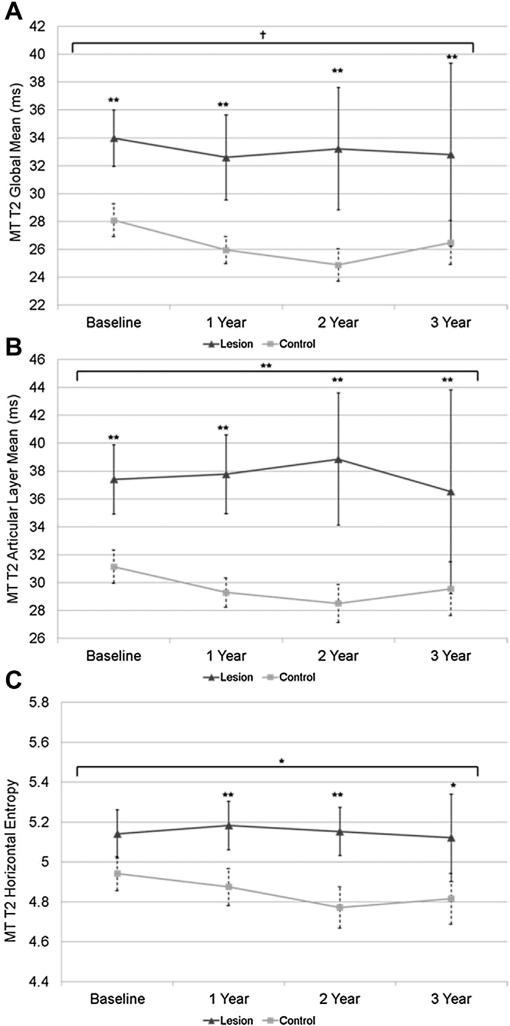
Mean values (95% CI, estimated model differences) for T1ρ and T2 global, laminar, and GLCM texture data for MT are shown in Table III. For global and laminar T1ρ relaxation times, the subjects with MT lesions had higher values for all parameters at all time points (P < 0.05). For T1ρ GLCM measures, at baseline and years 1 and 2, the subjects with lesions had higher contrast and variance in both directions (P < 0.05). Horizontal entropy was higher at years 1, 2 and 3, and vertical entropy was higher at years 2 and 3 (P < 0.05) (Table III). Subjects with lesions in the MT compartment also showed an increase in horizontal entropy (P = 0.021) and vertical entropy (P = 0.0006) over time compared to subjects without lesions (Table IV) (Fig. 4).
Table III.
MT mean values (95% CI, estimated differences) of each variable cross-sectionally (linear regression models adjusting for age, gender, BMI)
| T1ρ Global (ms) | Estimated difference | 95% CI | P-value | T1ρ Articular layer (ms) | Estimated difference | 95% CI | P-value | T1ρ Bone layer (ms) | Estimated difference | 95% CI | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Control | 34.57 | 5.5 | 2.5, 8.6 | 0.0005 | 41.02 | 4.5 | 1.056, 8 | 0.011 | 28.04 | 5.9 | 2.2, 9.6 | 0.0024 |
| Lesion | 40.66 | 47.28 | 33.75 | ||||||||||
| 1 Year | Control | 34.52 | 8 | 4.2, 11.9 | 0.0001 | 41.13 | 10.1 | 5.4, 14.9 | <0.0001 | 27.68 | 5.8 | 1.579, 10 | 0.0079 |
| Lesion | 42.40 | 51.55 | 33.47 | ||||||||||
| 2 Year | Control | 35.38 | 6.6 | 2.7, 10.6 | 0.0017 | 42.30 | 6.3 | 1.864, 10.8 | 0.0069 | 28.33 | 5.1 | 0.4, 9.8 | 0.034 |
| Lesion | 41.46 | 49.23 | 32.21 | ||||||||||
| 3 Year | Control | 33.58 | 6.2 | 2.6, 9.7 | 0.0018 | 40.33 | 8.3 | 2.3, 14.4 | 0.0091 | 26.71 | 5.8 | 1.55, 10 | 0.0099 |
| Lesion | 38.11 | 47.38 | 30.12 | ||||||||||
| T1ρ Contrast-horizontal | Estimated difference | 95% CI | P-value | T1ρ Contrast-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 13.14 | 28 | 15, 41 | <0.0001 | 106.24 | 118 | 63, 173 | <0.0001 | ||||
| Lesion | 42.96 | 260.12 | |||||||||||
| 1 Year | Control | 13.68 | 55 | 18.8, 91 | 0.0035 | 94.21 | 183 | 112, 253 | <0.0001 | ||||
| Lesion | 64.90 | 307.70 | |||||||||||
| 2 Year | Control | 8.59 | 50 | 19.4, 81 | 0.0023 | 106.12 | 110 | 33, 187 | 0.0064 | ||||
| Lesion | 57.93 | 256.48 | |||||||||||
| 3 Year | Control | 13.83 | –2.187 | –10.35, 6 | 0.58 | 98.72 | –7.996 | –40.63, 25 | 0.62 | ||||
| Lesion | 10.87 | 97.71 | |||||||||||
| T1ρ Entropy-horizontal | Estimated difference | 95% CI | P-value | T1ρ Entropy-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 5.09 | –0.045 | –0.279, 0.188 | 0.7 | 5.21 | –0.07 | –0.28, 0.14 | 0.51 | ||||
| Lesion | 5.08 | 5.14 | |||||||||||
| 1 Year | Control | 5.02 | 0.248 | 0.101, 0.395 | 0.0014 | 5.23 | 0.058 | –0.125, 0.24 | 0.53 | ||||
| Lesion | 5.27 | 5.26 | |||||||||||
| 2 Year | Control | 4.97 | 0.355 | 0.188, 0.522 | 0.0001 | 5.22 | 0.187 | –0.006, 0.38 | 0.05 | ||||
| Lesion | 5.34 | 5.37 | |||||||||||
| 3 Year | Control | 5.11 | 0.267 | 0.024, 0.51 | 0.033 | 5.27 | 0.524 | 0.207, 0.841 | 0.0024 | ||||
| Lesion | 5.39 | 5.82 | |||||||||||
| T1ρ Variance-horizontal | Estimated difference | 95% CI | P-value | T1ρ Variance-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 97.44 | 124 | 70, 179 | <0.0001 | 84.71 | 89 | 44, 134 | 0.0002 | ||||
| Lesion | 262.71 | 212.65 | |||||||||||
| 1 Year | Control | 95.14 | 201 | 138, 264 | <0.0001 | 87.72 | 159 | 104, 215 | <0.0001 | ||||
| Lesion | 315.53 | 257.53 | |||||||||||
| 2 Year | Control | 100.03 | 133 | 79, 187 | <0.0001 | 86.38 | 120 | 71, 169 | <0.0001 | ||||
| Lesion | 258.85 | 226.33 | |||||||||||
| 3 Year | Control | 103.72 | 30 | –3.777, 64 | 0.079 | 95.90 | 24 | –13.87, 62 | 0.2 | ||||
| Lesion | 139.75 | 122.61 | |||||||||||
| T2 Global mean (ms) | Estimated difference | 95% CI | P-value | T2 Articular layer (ms) | Estimated difference | 95% CI | P-value | T2 Bone layer (ms) | Estimated difference | 95% CI | P-value | ||
| Baseline | Control | 28.09 | 3.7 | 1.216, 6.3 | 0.0042 | 31.15 | 4.1 | 1.301, 6.9 | 0.0045 | 24.97 | 3.6 | 0.017, 7.2 | 0.049 |
| Lesion | 33.97 | 37.40 | 30.76 | ||||||||||
| 1 Year | Control | 25.96 | 6.6 | 4, 9.2 | <0.0001 | 29.30 | 7.9 | 5.4, 10.4 | <0.0001 | 22.56 | 5.5 | 1.765, 9.3 | 0.0047 |
| Lesion | 32.60 | 37.77 | 27.74 | ||||||||||
| 2 Year | Control | 24.89 | 7.9 | 4.8, 11 | <0.0001 | 28.52 | 9.3 | 5.9, 12.6 | <0.0001 | 21.17 | 6.8 | 3.2, 10.5 | 0.0006 |
| Lesion | 33.22 | 38.86 | 27.88 | ||||||||||
| 3 Year | Control | 26.50 | 7.3 | 3.8, 10.8 | 0.0003 | 29.56 | 6.9 | 2.2, 11.6 | 0.0056 | 23.28 | 8 | 3.7, 12.3 | 0.0009 |
| Lesion | 32.80 | 36.53 | 29.30 | ||||||||||
| T2 Contrast-horizontal | Estimated difference | 95% CI | P-value | T2 Contrast-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 15.86 | 23 | 6.6, 40 | 0.0066 | 115.95 | 123 | 52, 194 | 0.001 | ||||
| Lesion | 41.70 | 262.36 | |||||||||||
| 1 Year | Control | 11.06 | 14.2 | 8, 20 | <0.0001 | 80.42 | 173 | 106, 240 | <0.0001 | ||||
| Lesion | 25.00 | 285.26 | |||||||||||
| 2 Year | Control | 6.96 | 11.6 | 2.1, 21 | 0.019 | 73.21 | 127 | 68, 186 | 0.0001 | ||||
| Lesion | 23.57 | 241.23 | |||||||||||
| 3 Year | Control | 7.05 | 12.7 | 3.8, 22 | 0.0073 | 108.58 | 68 | –33.1, 169 | 0.18 | ||||
| Lesion | 21.21 | 172.17 | |||||||||||
| T2 Entropy-horizontal | Estimated difference | 95% CI | P-value | T2 Entropy-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 4.94 | 0.146 | –0.033, 0.326 | 0.11 | 5.18 | –0.005 | –0.23, 0.22 | 0.97 | ||||
| Lesion | 5.14 | 5.14 | |||||||||||
| 1 Year | Control | 4.88 | 0.313 | 0.135, 0.491 | 0.0009 | 5.15 | 0.154 | –0.05, 0.35 | 0.13 | ||||
| Lesion | 5.18 | 5.31 | |||||||||||
| 2 Year | Control | 4.77 | 0.372 | 0.162, 0.582 | 0.0011 | 5.19 | 0.242 | –0.02, 0.51 | 0.071 | ||||
| Lesion | 5.15 | 5.38 | |||||||||||
| 3 Year | Control | 4.82 | 0.25 | 0.051, 0.45 | 0.017 | 5.17 | 0.226 | –0.02, 0.47 | 0.068 | ||||
| Lesion | 5.12 | 5.46 | |||||||||||
| T2 Variance-horizontal | Estimated difference | 95% CI | P-value | T2 Variance-vertical | Estimated difference | 95% CI | P-value | ||||||
| Baseline | Control | 108.51 | 137 | 77, 197 | <0.0001 | 96.96 | 106 | 54, 158 | 0.0001 | ||||
| Lesion | 287.60 | 198 | 238.24 | ||||||||||
| 1 Year | Control | 82.89 | 143, 254 | <0.0001 | 73.23 | 152 | 108, 196 | <0.0001 | |||||
| Lesion | 304.81 | 240.65 | |||||||||||
| 2 Year | Control | 75.31 | 118 | 66, 171 | <0.0001 | 64.60 | 96 | 54, 139 | <0.0001 | ||||
| Lesion | 229.27 | 190.26 | |||||||||||
| 3 Year | Control | 89.83 | 110 | 43, 177 | 0.0026 | 77.43 | 92 | 37, 147 | 0.0022 | ||||
| Lesion | 205.07 | 172.35 |
The bold indicates significance at P < 0.05.
Fig. 4.
Mean T1ρ entropy (A and B) in the MT. Single asterisk indicates P < 0.05, double asterisk indicates P < 0.01. Longitudinal significance between the groups is denoted above the horizontal bracket.
Global, bone and articular layer T2 relaxation times were higher in subjects with lesions in the MT compartment at each time points (Table III). Subjects with lesions had greater contrast in the horizontal direction at each time point, and greater contrast in the vertical direction at each time point except year 3 (Table III). Subjects with lesions also had higher T2 variance in both directions at each time point compared to subjects without lesions. Horizontal MT T2 entropy in compartments with lesions was higher at year 1 (P = 0.001), year 2 (P = 0.001), and year 3 (P = 0.017) but was not significantly different at baseline. As observed in MF, articular layer T2 relaxation time in MT showed significantly different longitudinal trends between the lesion and control compartment groups (P = 0.01) caused by increases in articular layer T2 for the lesion group and decreases in the control group (Table IV) (Fig. 5). Similar longitudinal trends approaching significance were observed for global T2 relaxation time (P = 0.06) although for this variable control compartment T2 decreased while lesion T2 remained relatively constant. Additionally, T2 horizontal entropy of the two groups changed differently with time. T2 entropy in compartments with lesions increased slightly, then decreased slightly from year 2 to year 3, while control compartments experienced a longitudinal decrease (P = 0.043) (Fig. 5) (Table IV).
Fig. 5.
Global mean and articular T2 relaxation times (A and B) and mean T2 entropy in the MT. Single asterisk indicates P < 0.05, double asterisk indicates P < 0.01, and cross indicates P = 0.07–0.051 (approaching significance). Longitudinal significance between the groups is denoted above the horizontal bracket.
Discussion
In this study we investigated longitudinal changes in global, laminar and flattened texture parameters of articular cartilage T1ρ and T2 relaxation times in medial knee compartments with and without cartilage lesions. It is established that the prevalence of cartilage lesions due to OA is greater in the medial knee joint31,32. In the MF, baseline cross-sectional T1ρ global mean values were not significantly different between the two groups, but the lesion group T1ρ was significantly more heterogeneous. This trend is consistent with the other reports29,33,34 of higher spatial variation of T2 values in people with knee OA compared to controls, which predicts clinical deterioration over the long term. Additionally, there was no significant difference in global mean MF T1ρ relaxation times or GLCM texture measurements between the two groups at the year 3 time point, suggesting prolonged cartilage degeneration may reduce the capacity of the tissue to bind to motion-restricted water molecules.
Longitudinally, we discovered that lesion group MT T1ρ and T2 relaxation times became progressively more heterogeneous than healthy control compartments, as measured by GLCM entropy. Longitudinal changes in MT T1ρ GLCM entropy were significantly different between the groups in both the horizontal and vertical directions. MT T1ρ entropy progressively increased in the lesion group and remained constant in the control group. Qazi et al. studied heterogeneity of T1-weighted images of OA and control patients using entropy calculated from histogram signal intensities. They described increases in entropy as a widening bandwidth of pixel signal intensity values and a reduction of the more dominant pixel values seen in homogenous histograms. Our results suggest that over time MT cartilage with lesions will develop a progressively more diverse array of T1ρ values when compared with control compartments. The longitudinal significance of this relationship in both the horizontal and vertical directions supplement previous studies displaying increasing entropy in T1ρ values in OA cartilage compared to controls18, and show the utility of using this metric to supplement global mean T1ρ values. MT T2 horizontal entropy in control cartilage became increasingly homogeneous over time while entropy in the lesion group remained higher (significantly higher at years 1, 2 and 3). This relationship displayed significant longitudinal differences in voxel heterogeneity between groups. These results are consistent with previous longitudinal studies that displayed elevated medial knee OA mean T2 values along with increased entropy30,35.
This study has several limitations. Firstly, the study focused on investigating the relationship between medial knee cartilage lesions and quantitative MR parameters of cartilage composition. Hence, the findings are not generalizable to the whole knee and pertain to individuals with cartilage lesions in the medial compartment, which are more common than lesions in the lateral compartment. Future studies would need to be done to investigate these relationships for lateral knee cartilage lesions. Secondly, there was a significant reduction in follow-up data collection due to late enrollment and subject attrition that may have limited the power to investigate differences at the year 2 and 3 time points, especially in the lesion group MT (n = 7 year 3). However, even with the limited sample size, we observed a large number of significant differences between the groups.
In summary, T1ρ and T2 MRI provide some promising methods by which the classification of biochemical changes in medial knee joint OA is possible. MF T1ρ and T2 global mean values were not significantly different at baseline, but GLCM contrast and variance were significantly higher in the lesion group indicating that GLCM calculations may provide a heightened level of sensitivity which may be undetectable via global mean analysis alone. MT T1ρ and T2 entropy displayed progressive, longitudinal increases in the lesion group. Thus the longitudinal evolution of cartilage T1ρ and T2, and the heterogeneity of these measures may be different at different stages of OA, and are strongly dependent on compartment and cartilage layer. The results presented here underscore the potential of using flattened T1ρ and T2 cartilage GLCM calculations along with laminar analysis to provide a more detailed characterization of longitudinal biochemical and structural changes in medial osteoarthritic knee articular cartilage.
Acknowledgments
The authors would like to thank Julio Carballido-Gamio and Subburaj Karupppasamy for their technical support, and T Munoz and M Guan for their assistance in subject recruitment and consent. This research was supported by NIHRO1-AR46905.
Footnotes
Author contributions
Conception and design: Schooler, Kumar, Link, Majumdar; Acquisition of data: Schooler, Kumar; Analysis and interpretation of the data: Schooler, Kumar, Nardo, McCulloch, Li, Link, Majumdar; Statistical expertise: McCulloch; Drafting of article or critical revision of the article for important intellectual content: Schooler, Kumar, Nardo, McCulloch, Li, Link, Majumdar; Final approval of the article: Schooler, Kumar, Nardo, McCulloch, Li, Link, Majumdar.
Conflicts of interest No author has any conflict of interest to disclose.
References
- 1.Dearborn JT, Eakin CL, Skinner HB. Medial compartment arthrosis of the knee. Am J Orthop (Belle Mead NJ) 1996;25:18–26. [PubMed] [Google Scholar]
- 2.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50:476–87. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 3.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souza RB, Feeley BT, Zarins ZA, Link TM, Li X, Majumdar S. T1rho MRI relaxation in knee OA subjects with varying sizes of cartilage lesions. Knee. 2013;20:113–9. doi: 10.1016/j.knee.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohmander LS. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J Anat. 1994;184(Pt 3):477–92. [PMC free article] [PubMed] [Google Scholar]
- 6.Binks DA, Hodgson RJ, Ries ME, Foster RJ, Smye SW, McGonagle D, et al. Quantitative parametric MRI of articular cartilage: a review of progress and open challenges. Br J Radiol. 2013;86:20120163. doi: 10.1259/bjr.20120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–23. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29:324–34. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–68. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 10.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–53. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503–9. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 14.Wong CS, Yan CH, Gong NJ, Li T, Chan Q, Chu YC. Imaging biomarker with T1rho and T2 mappings in osteoarthritis – in vivo human articular cartilage study. Eur J Radiol. 2013;82:647–50. doi: 10.1016/j.ejrad.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–5. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 16.Haralick RM, Shanmuga K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;SMC-3:610–21. [Google Scholar]
- 17.Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, et al. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteoarthritis Cartilage. 2008;16:584–90. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61:1310–8. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drury HA, Van Essen DC, Anderson CH, Lee CW, Coogan TA, Lewis JW. Computerized mappings of the cerebral cortex: a multiresolution flattening method and a surface-based coordinate system. J Cogn Neurosci. 1996;8:1–28. doi: 10.1162/jocn.1996.8.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Carballido-Gamio J, Link TM, Majumdar S. New techniques for cartilage magnetic resonance imaging relaxation time analysis: texture analysis of flattened cartilage and localized intraand inter-subject comparisons. Magn Reson Med. 2008;59:1472–7. doi: 10.1002/mrm.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med. 2008;59:298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Carballido-Gamio J, Link TM, Li X, Han ET, Krug R, Ries MD, et al. Feasibility and reproducibility of relaxometry, morpho-metric, and geometrical measurements of the hip joint with magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008;28:227–35. doi: 10.1002/jmri.21411. [DOI] [PubMed] [Google Scholar]
- 26.Subburaj K, Kumar D, Souza RB, Alizai H, Li X, Link TM, et al. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40:2134–41. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar D, Schooler J, Zuo J, McCulloch CE, Nardo L, Link TM, et al. Trabecular bone structure and spatial differences in articular cartilage MR relaxation times in individuals with posterior horn medial meniscal tears. Osteoarthritis Cartilage. 2013;21:86–93. doi: 10.1016/j.joca.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years – data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20:727–35. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res (Hoboken) 2013;65:23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls – data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52:2033–9. doi: 10.1002/art.21148. [DOI] [PubMed] [Google Scholar]
- 32.Zamber RW, Teitz CC, McGuire DA, Frost JD, Hermanson BK. Articular cartilage lesions of the knee. Arthroscopy. 1989;5:258–68. doi: 10.1016/0749-8063(89)90139-4. [DOI] [PubMed] [Google Scholar]
- 33.Dray NWA, Prasad PV, Sharma L, Burstein D. International Society of Magnetic Resonance in Medicine. Miami, FL: 1995. T2 in an OA population: metrics for reporting data. [Google Scholar]
- 34.Urish KL, Keffalas MG, Durkin JR, Miller DJ, Chu CR, Mosher TJ. T2 texture index of cartilage can predict early symptomatic OA progression: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2013;21:1550–7. doi: 10.1016/j.joca.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65:1184–94. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]