Abstract
A fully intact immune system would be expected to hinder the efficacy of oncolytic virotherapy by inhibiting viral replication. Simultaneously, however, it may also enhance antitumor therapy through initiation of pro-inflammatory, antiviral cytokine responses at the tumor site. The aim of the current study was to investigate the role of a fully intact immune system upon the antitumor efficacy of an oncolytic virus. In this respect, injection of oncolytic Vesicular Stomatitis Virus (VSV) into subcutaneous B16ova melanomas in C57Bl/6 mice leads to tumor regression, but it is not associated with viral replicative burst in the tumor. In contrast, intratumoral delivery of VSV induces an acute proinflammatory reaction, which quickly resolves concomitantly with virus clearance. Consistent with the hypothesis that therapy may not be dependent upon the ability of VSV to undergo progressive rounds of replication, a single-cycle VSV is equally effective as a fully replication-competent VSV, whereas, inactivated viruses do not generate therapy. Even though therapy is dependent upon host CD8+ and NK cells, these effects are not associated with IFN-γ-dependent responses against either the virus or tumor. There is, however, a strong correlation between viral gene expression, induction of proinflammatory reaction in the tumor and in vivo therapy. Overall, our results suggest that acute innate antiviral immune response, which rapidly clears VSV from B16ova tumors, is associated with the therapy observed in this model. Therefore, the antiviral immune response to an oncolytic virus mediates an intricate balance between safety, restriction of oncolysis and, potentially, significant immune-mediated antitumor therapy.
Keywords: Oncolytic viruses, Experimental Melanoma, Interferon Type I, Gene Therapy, Virus Replication, Rhabdovirus
Introduction
Oncolytic Vesicular Stomatitis Virus (VSV) is a potent oncolytic agent against a variety of both human and rodent tumors1-5 in both immunodeficient and immunocompetent models.2,3,6-9 Infection of normal cells with VSV, a negative strand Rhabdovirus, induces Type I IFN responses (IFN-α/β), thereby blocking viral replication and extinguishing infection. In contrast, many tumor cells have defects in their IFN response1,4,5 allowing unhindered infection, and lysis of tumors.2,3,6 We have shown that delivery of oncolytic VSV into B16ova melanomas growing in immune competent C57Bl/6 mice leads to tumor regressions and cures of a proportion of animals.10-13 In the context of direct intratumoral injection, VSV recruited multiple immune effectors into the tumors, of which both CD8+ T cells and NK cells were critical for the antitumor therapy.10 These findings showed that tumor destruction in this model may have at least two components. The first is presumably contributed by the conventional effector mechanisms of oncolytic virotherapy, namely direct tumor cell killing as a result of viral infection, replication and lysis.14,15 The second is contributed by immune effectors, including, but probably not exclusively, CD8+ T cells and NK cells. However, from our initial studies, it was unclear whether this immune–mediated component of therapy is based upon innate or adaptive responses to either virus, tumor or both, following the direct injection of VSV into the tumor.
The innate immune response against viral infection can be viewed simultaneously as both a positive contributor to, as well as a negative detractor from, the overall potential of oncolytic virotherapy.14-16 On one hand, a robust antiviral immune response to an oncolytic virus is an important and necessary safety component. Correctly functioning innate and adaptive responses ensure that the virus does not spread systemically and prevents widespread toxicity. Indeed, one of the theoretical cornerstones for the development of many oncolytic viruses has been that an intact antiviral innate immune response is fully operational in normal cells but not in tumor cells.14-17 On the other hand, the antiviral immune response has evolved specifically to sense, shut down, and clear viral infections as rapidly as possible after they become evident in vivo. This activity acts to suppress ongoing viral replication, limit viral-mediated oncolysis and inhibit therapeutic efficacy. As a result, strategies have been devised specifically to suppress the innate response to virus infection at the tumor site to enhance viral replication, spread and oncolysis.14,15,18,19
With respect to the adaptive response induced by oncolytic virotherapy, there is considerable evidence that local tumor destruction can lead to the priming of antitumor T cell responses and that these can enhance tumor clearance in vivo.20 In addition, viral replication within the tumor inevitably leads to generation of antiviral T and B cell responses. However, exactly how the balance between antiviral and antitumor immune priming is affected by different viruses has not been extensively studied. In the case of VSV, we have reported that VSV-associated proteins are immunologically dominant compared to much less immunogenic tumor-associated antigens10. However, oncolytic VSV virotherapy was able to prime specific T cell responses against tumor-associated antigens (TAA) expressed within the B16ova tumors much more efficiently when either the virus itself was engineered to express a TAA,10 or when the precursor frequency of TAA-specific T cells was artificially increased prior to virotherapy by adoptive transfer of T lymphocytes.10 In addition, delivery of VSV to B16ova tumor cells growing in the lymph nodes and spleen induced much more efficient priming of antitumor T cell responses than if VSV-mediated tumor cell killing occurs in a subcutaneous tumor.12,20 Therefore, VSV-mediated oncolysis is able to prime tumor-specific T cell responses under the appropriate conditions. Tumor oncolysis with other viruses, with different kinetics of infection and inherent immunogenicities, may significantly alter the balance between antiviral and antitumor immune priming.20
Therefore, at best, the immune system is currently viewed as a troublesome partner in an uneasy truce with oncolytic virotherapy, contributing to its safety and, possibly, to its efficacy at the level of adaptive T cell immunity to the tumor. At worst, antiviral immunity is seen as an opponent to oncolytic virotherapy, restricting its scope and efficacy at the level of viral clearance before tumor destruction can be achieved14,15,19. In the current report, we describe a tumor model in which the ability of the oncolytic virus to undergo progressive rounds of replication is irrelevant for antitumor therapy. In addition, the innate immune response to intratumoral viral injection, which is itself responsible for very rapid viral clearance, is also the major driver of antitumor therapy. Therefore, immunomodulatory strategies aimed at enhancing the efficacy of oncolytic virotherapy should consider the effects that such approaches may have on both the positive, as well as negative, contributions that antiviral immune effectors (cells and cytokines) can have on antitumor therapy.
Materials and Methods
Cell lines
Murine B16ova melanoma cells (H2-Kb) were derived from B16 cells by transduction with a cDNA encoding for the chicken ovalbumin gene.21 Cell lines were grown in Dulbecco's Modification of Eagle's Medium with 4.5 g/L glucose and L-glutamine without sodium pyruvate (Mediatech, Herndon, VA, USA) supplemented with 10% (v/v) fetal bovine serum (Life Technologies). All cell lines were monitored routinely and found to be free of Mycoplasma infection.10
Viruses
VSV (Replication-competent)
VSV-XN2 is the parental VSV virus (Indiana Serotype) (no transgene) from which all recombinant viruses were derived. This virus serves as the control virus in experiments in which recombinant viruses expressing an additional transgene (GFP or CD40L) are used. VSV-CD40L was constructed from VSV-XN2 as described below, based upon the hypothesis that local expression of CD40L at the site of tumor cell oncolysis would enhance activation of adaptive, tumor specific T cell responses in treated mice. VSV-GFP (Indiana serotype) was a gift from Dr. Glen Barber and was described previously.22 VSV-CD40L was constructed by PCR amplifying the mouse CD40L gene from pCR2.1-CD40L, subsequently this PCR product was digested with the restriction enzymes XhoI and NheI and ligated into the plasmid pVSV-XN2 (genomic plasmid of VSV Indiana serotype and a kind gift from Dr. John Rose of Yale University) to yield pVSV-CD40L. Recombinant VSV-CD40L and the parental VSV-XN2 were recovered based on the method described previously.23,24 Bulk amplification of plaque-purified VSVs were performed by infecting BHK-21 cells (MOI=0.01) for 24 hours. Filtered supernatants were harvested and subjected to 2 rounds of 10% sucrose (10% w/v) in 1X PBS (Mediatech, Inc., Herndon, VA, USA) cushion centrifugation at 27,000 rpm for 1 hour at 4°C. The pelleted virus was resuspended in 1X PBS, aliquoted and kept at −80°C. VSVs were titrated in BHK-21 using standard plaque assay.10
VSV (Single-cycle viruses)
Replication-defective VSV-XN2 and VSV-CD40L were generated by deleting the glycoprotein gene based on a previously published method.25-28 Specifically, the same plasmids used above, i.e. pVSV-XN2 and pVSV-CD40L, were digested with MluI and XhoI to remove the VSV G gene sequence, blunted with T4 DNA polymerase, and ligated with T4 DNA ligase to yield the following plasmids: pVSVXN2ΔG and pVSV-CD40LΔG, respectively. Viruses were recovered by co-transfecting 10 μg of pVSV-XN2ΔG or pVSV-CD40LΔG with 3 μg pBS-N, 5 μg pBS-P, 4 μg pBS-G, and 1 μg pBS-L (pBS plasmids were generously given by Dr. John Rose of Yale University) into BHK-21 cells previously transduced an hour before with a replication-defective vaccinia virus encoding for T7 polymerase (MVA-T7), a kind gift from Dr. Roberto Cattaneo of Mayo Clinic. The recovered viral supernatants were centrifuge-clarified (1200 rpm for 7 minutes), filtered through a 0.2- μm MILLEX® GP Syringe Filter Unit (Millipore, Carrigtwohill, Co., Cork, Ireland), pelleted in 10% sucrose cushion as above, resuspended in 1X PBS, and stored at −80°C.
Titration of Single Cycle VSV
Single-cycle VSVs were titered in BHK cells complemented with the VSV-G protein. 6-well plates (>90% confluent) of BHK cells were transfected with pCMV-VSV-G plasmid for 8 hours, washed with PBS, and infected/transduced with serial dilutions of single-cycle VSV for 2 hours, then overlaid with 2% Noble agar. Plaques developed between 24-36 hours.
VSV (Physical and chemical inactivation)
Sucrose-purified VSVs were inactivated using heat, ultraviolet (UV), and formalin. For heat inactivation, VSVs were diluted to a concentration of 1×1010 pfu/mL in 1X PBS, aliquoted in 0.5 mL eppendorf tubes, and heated to 99°C for 20 minutes. Inactivation by UV (λ=254 nm) was performed by exposing 1×1010 pfu/mL (one mL per well in an uncovered 6-well plate) under 120,000 microjoules/cm2 of UV light for 120 minutes using a CL-1000 Ultraviolet Crosslinker (UVP, LLC, Upland, CA, USA). VSV was formalin-inactivated as described previously.29 Briefly, high-titer, purified VSV was mixed with an equal volume of 0.1250% (v/v) formalin (Fisher Scientific, Pittsburgh, PA, USA) in DMEM supplemented with 1% (v/v) FBS at 4°C for 18 hours. Prior to an in vivo experiment, formalin-fixed VSV was diluted in PBS to a final concentration of 1×1010 pfu/mL.
Viral titer determination from in vitro studies
Cultures of either BHK-21 or B16ova melanoma cells were grown overnight in 6 well plates (750,000 cells/well). Cells were washed once with 1X PBS and infected with recombinant VSVs (MOI=1 unless otherwise indicated) in plain DMEM for 1 hour at 37°C in a humidified 5% CO2 incubator. Virus was siphoned out and replaced with regular culture media. Supernatants were harvested at various time points, clarified, filtered, serially-diluted in plain DMEM, and titrated in BHK-21 using a standard plaque assay.10
Viability assays
Overnight cultures of BHK-21 or B16ova melanoma cells (1×104 cells/50μL medium/well) in 96-well plates (three replicate wells per sample) were infected with 50 μL of VSV (MOI=1.0) and incubated at 37°C in a humidified 5% CO2 incubator. At indicated time points, cell viability was assessed using Cell Proliferation Kit I (MTT, Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's recommended protocol.
Survival studies
All mouse in vivo protocols were approved by the Mayo Foundation Institutional Animal Care and Use Committee. Female C57Bl/6 mice were purchased from The Jackson Laboratories (Bar Harbor, ME, USA) at 6-8 weeks of age. To establish subcutaneous tumors, 5×105 B16ova cells suspended in 100μl of 1X PBS were injected into the right flanks of mice. Viral injections (5×108 pfu suspended in 50 μl 1X PBS) were performed intratumorally at indicated time points after tumor seeding. In comparing VSV and TLR-4 agonist, purified lipopolysaccharide or LPS (200 μg per dose) (Sigma-Aldrich, St. Louis, MO, USA) was injected intratumorally three times on days 7, 9, and 11 after subcutaneous implantation of B16ova cells.
Viral titer determination from in vivo studies
Established subcutaneous B16ova melanoma tumors were intratumorally injected with a single dose of 5×108 pfu VSV. At indicated time points, mice were euthanized and tumors were harvested and placed in 2 mL cryotubes and immediately snap-frozen in liquid nitrogen. To determine the viral titers, tumors were homogenized in 1 mL 1X PBS and the supernatants were clarified, serially-diluted in plain DMEM, and titrated in BHK-21 using a standard plaque assay.10
ELISPOT and ELISA analysis for IFN-γ secretion
Spleens or tumor draining lymph nodes were removed from mice at the indicated times. For ELISA, a million cells were plated (unless otherwise indicated) in 24 well plates and incubated at 37°C with the indicated peptides i.e., H-2Kb-restricted peptides TRP-2180-188 SVYDFFVWL, ova SIINFEKL and VSV-N protein-derived RGYVYQGL were synthesized at the Mayo Foundation Core facility. Cell-free supernatants were collected after 48 h and tested by ELISA for IFN-γ (BD OptEIA™ Mouse IFN-γ ELISA Set; BD Biosciences Pharmingen, San Diego, CA, USA). For ELISPOT assays (Mouse Interferon-γ ELISpotPlus, MABTECH AB, Nacka Strand, Sweden) , 1 ×105 cells were plated into each well of a 96-well ELISPOT plate in triplicates and were re-stimulated for 48 hours at 37°C under the different conditions (all peptides were at 5 μg/ml). Peptide-specific IFN-γ positive spots were detected according to the manufacturer's protocol and were quantified by computer assisted image analyzer.10
Flow cytometry
For analysis of phenotype, 1 × 106 cells were washed in 1X PBS containing 0.1 % BSA and 0.01% sodium azide (FACS buffer), re-suspended in 50 μl of FACS buffer, and exposed to fluorochrome-conjugated primary antibodies (anti-CD40LPE, anti-I-Ab-PE, anti-CD45R/B220-PE, anti-CD4-FITC and anti-CD45-PerCP from BD Pharmingen, CA, USA, and anti-VSV-G-FITC from Immunology Consultants Lab, Newberg, OR, USA), for 30 min at 4°C. Cells were then washed and resuspended in 500 μl of PBS containing 4% formaldehyde.10 Cells were subjected to flow cytometry and data were analyzed using CellQuest software (BD Biosciences, San Jose, CA, USA) or FlowJo (Tree Star, Inc., Ashland, OR, USA).
RT-PCR
Total RNA were extracted from either monolayer cultures or tumor samples using RNeasy RNA purification kit (QIAGEN, Valencia, CA, USA) following the manufacturer's suggested protocols. Reverse transcription was performed on 1 μg total RNA/sample using First Strand cDNA synthesis kit (Roche Applied Science, Indianapolis, IN, USA) according to the recommended procedure. The resulting cDNA was used as template for PCR using the following mouse DNA primers: GAPDH-f: 5’-aactttggcattgtggaagg-3’; GAPDH-r: 5’-tgtgagggagatgctcagtg-3’; RANTES-f: 5’-gtgcccacgtcaaggagtat-3’, RANTES-r: 5’-atttcttgggtttgctgtgc-3’; PKR-f: 5’-caaagcaggaggcaagaaac-3’; PKR-r: 5’-gctgactgggaaacaccatt-3’; IFN-β-r: 5’-tcccacgtcaatctttcctc-3’; IFN-β-f: 5’-ataagcagctccagctccaa-3’; VSV-N-r: 5’-agttccgtatctgaacgaggc-3’; VSV-N-f: 5’-acgaagacaaacaaaccattattatcattaa-3’; IRF3-f:5’-caagcttgtgaaggagtacgtg-3’; IRF3-r: 5’-gtactggtcagaggtaagggagatag-3’; IRF7-f: 5’-gtcacactatctgtggctacaacc-3’; IRF7-r: 5’-gtactgcagaacctgaagcaagag-3’; ISG56-f: 5’-catcaccttcctctggctacttac-3’; and ISG56-r: 5’-gtgtgattctacagctcacaggag-3’. PCR products were resolved in 1X TAE-1% agarose gel stained with ethidium bromide.
Statistics
Survival data from the animal studies were analyzed by Logrank test using GraphPad Prism 4 (GraphPad Software, La Jolla, CA, USA). Immunological assays and other in vitro experiments were analyzed using JMP® Software (SAS Institute, Inc., Cary, NC, USA). Statistical significance was determined at the level of p<0.05.10
Results
In vivo therapy is not associated with a replicative burst in B16ova tumors
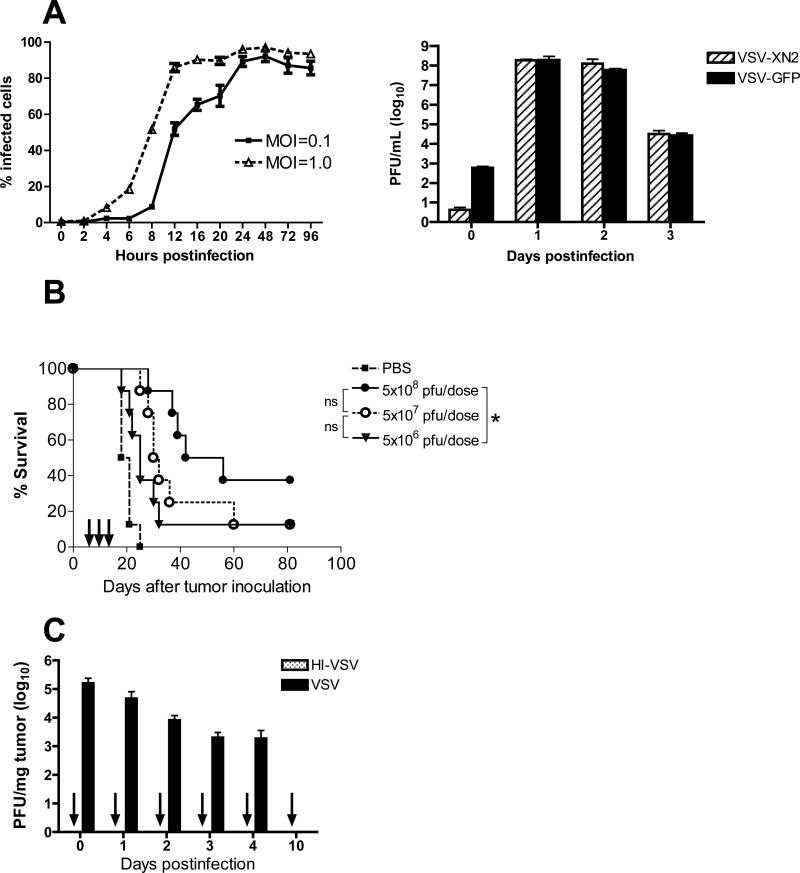
In vitro, VSV replicates rapidly to high titers in B16ova cells (Fig. 1A, right) leading to infection of between 90% to 100% of cells by 24 hours post infection, even at a low MOI (Fig. 1A, left). Direct injection of VSV into established subcutaneous B16ova tumors is also associated with significant therapy compared to either heat-inactivated virus (not shown) or PBS (Fig. 1B). We also observed a partial dose-response of this therapy. Thus, therapy with three intratumoral doses of 5×106, 5×107 or 5×108 pfu were all significantly more effective than control treatment (p=0.0145; p=0.0001 or p<0.0001 respectively) (Fig. 1B) and injections of 5×108 pfu were significantly better than 5×106 pfu (p=0.0244). Although we observed a consistent trend to better outcome with tenfold stepwise increases in dose, these did not reach significance (5×108 pfu vs. 5×107 pfu: p=0.1253; and 5×107pfu vs. 5×106pfu: p=0.2569).
Figure 1. Intratumoral VSV induced an acute proinflammatory reaction at the tumor site.
A. (Left panel) B16ova cells were infected with VSV-GFP and the percentage of infected cells (GFP+ cells) were counted using flow cytometry at various time points. (Right panel) Overnight monolayer cultures of B16ova cells were infected with either VSV-XN2 or VSV-GFP (MOI=1.0). The number of infectious progeny viruses were determined from the culture supernatants harvested daily for 3 days using standard plaque assay in BHK cells. Values are averages of triplicate wells (+ SEM) and representative of two independent experiments. B. Effect of VSV dose-escalation on the survival of C57Bl/6 mice (n=8 per group) bearing subcutaneous B16ova tumors treated with three intratumoral injections of replication-competent VSV. *p<0.05, **p<0.01, ***p<0.001. C. Seven-day old subcutaneous B16ova tumors in C57Bl/6 mice (n=3 mice per group per time point) were infected with a single intratumoral dose of either VSV-GFP or HI-VSV (both using 5×108 pfu), harvested right after injection and on indicated days postinfection. The number of infectious virus was assayed using standard plaque assay. HI-VSV consistently gave no detectable titers (indicated by arrows). Values are averages of three tumors (+ SEM). D. Established B16ova tumors in immunocompetent mice (n=3 mice per group) were injected intratumorally with one dose of VSV (5×108 pfu), the injected tumors and corresponding draining lymph nodes were harvested at indicated times, the total RNA was extracted and used in a ribonuclease protection assay (RPA). The symbol (٭) corresponds to upregulated cytokine mRNA, while each lane corresponds to a sample from one mouse. E. Kaplan-Meier survival graph comparing the therapeutic efficacy of six intratumoral VSV in tumor-bearing C57Bl/6 mice (B6) or IFN-γ knockout mice (IFN-γko). Total of 8 mice per treatment group. *p<0.05, **p<0.01, ***p<0.001.
In contrast to the exponential increase of output virus seen in vitro (Fig. 1A, right), no such replicative amplification was observed following intratumoral injection of established subcutaneous B16ova tumors in vivo (Fig. 1C). In fact, viral titers consistently decreased steadily and by the 10th day postinfection, no infectious VSV could be detected in the tumor. Significantly, although increased doses of input virus improved therapy (Fig. 1B), they were not associated with increased titers recovered from tumors. These data suggest that therapy may not be exclusively associated with levels of viral replication within the tumors.
Intratumoral VSV induces rapid inflammation
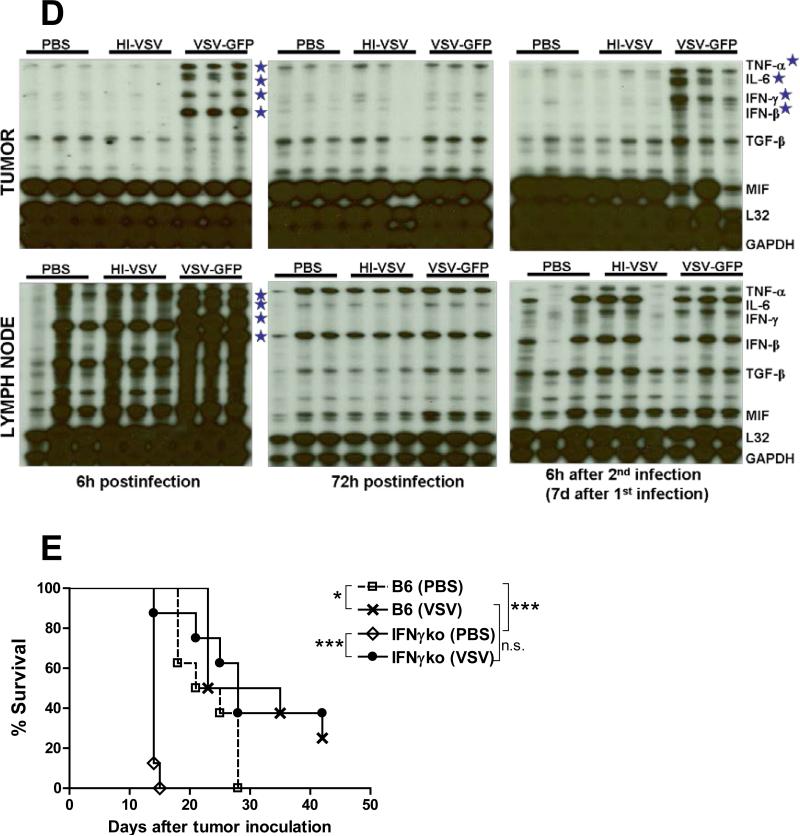
Consistent with an immune-mediated shutdown of virus replication, intratumoral injection of VSV into B16ova tumors was associated with an early-onset and very rapid induction of proinflammatory cytokines at the tumor site and the draining lymph nodes (Fig. 1D, left panels). The four cytokines induced include TNF-α, IL-6, IFN-γ, and IFN-β, all of which have reported antitumor activities.30-33 Importantly, this cytokine profile was almost completely quenched within 3 days of the viral injection (Fig. 1D, middle panels) indicating that the source of the inflammation (i.e. the virus) was probably fully cleared within a relatively short time frame both at the tumor site and the draining lymph nodes. This potent, local proinflammatory reaction was re-induced in tumors with similar kinetics following a subsequent intratumoral VSV injection given 7 days following the first injection, but not in the draining lymph nodes (Fig. 1D, right panels).
Consistent with the hypothesis that viral replication may not be the sole determinant of VSV-mediated therapy in the B16ova model, we have also previously observed that therapy absolutely requires both CD8+ and NK cells.10 For this reason, in separate studies, we hypothesized that improving T cell priming by encoding the strong immune adjuvant CD40L34 in VSV would further enhance antitumor efficacy. However, using either 2, 6 or 9 intratumoral injections, VSV-CD40L never generated significantly better therapy than VSV-GFP (Galivo et al. Submitted). In addition to VSV therapy not being enhanced by co-expression of a molecule which targets CD8+ T cell effector activity, VSV-mediated therapy of B16ova tumors was not affected in host animals genetically deficient for the production of IFN-γ (Fig. 1E).
Single-cycle VSV
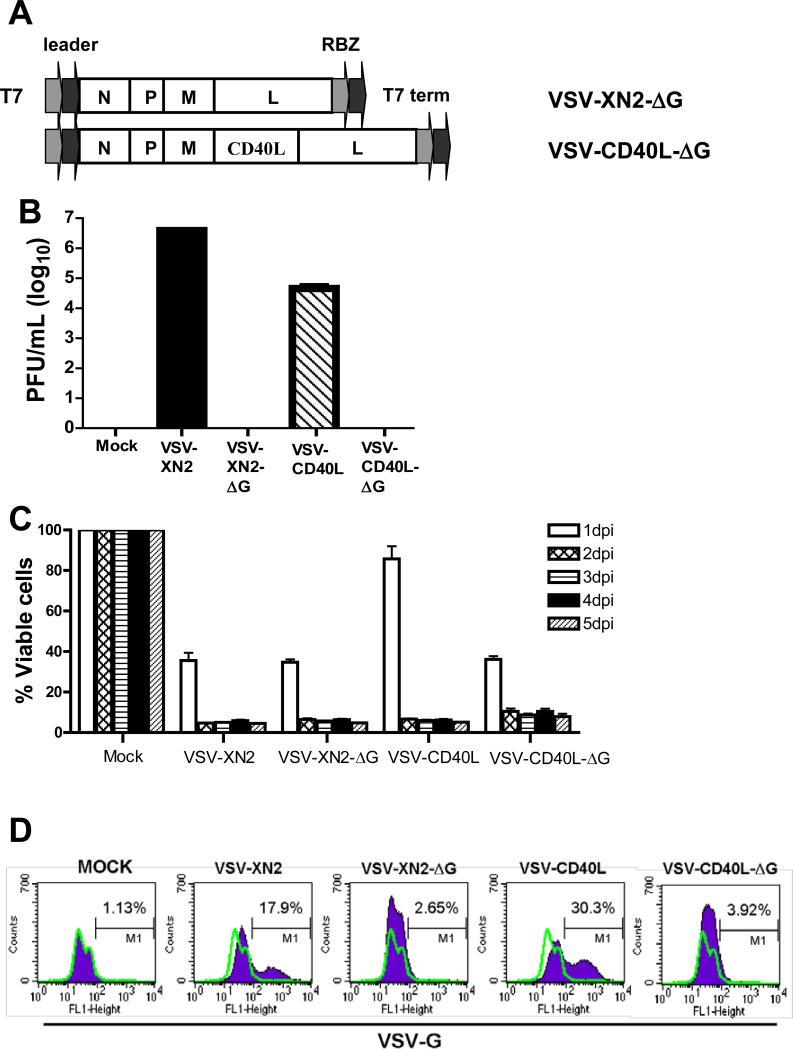
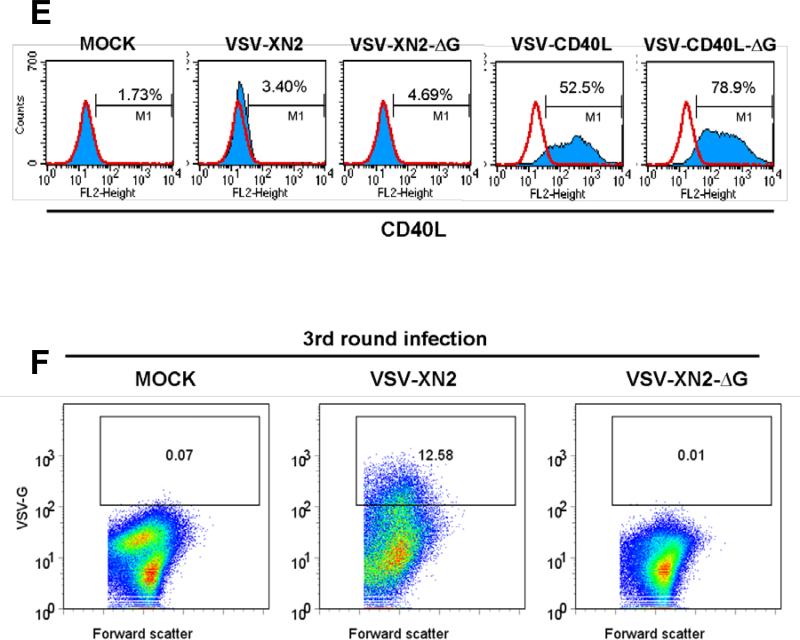
Taken together, these data suggest that VSV-mediated therapy of B16ova tumors is associated with neither a large replicative burst of intratumoral virus nor with the levels of virus recovered from tumors. Although therapeutic efficacy is critically dependent upon host immune cells, it is not dependent upon IFN-γ-mediated effector mechanisms. Moreover, intratumoral virus is associated with rapid induction of pro-inflammatory cytokines which could potentially have significant antitumor activities. Therefore, we tested whether a virus with no capacity to produce infectious progeny viruses would show similar therapeutic efficacy in vivo as a fully replication competent VSV by generating single-replication cycle VSV vectors (VSV-XN2-ΔG and VSVCD40L-ΔG) in which the gene encoding the VSV-G glycoprotein was deleted (Fig. 2A). As expected, no infectious VSV particles were recovered from culture supernatants of B16ova cells 24 hours following infection with single-cycle VSVs (MOI=0.01) (Fig. 2B). Direct infection of B16ova cells with both VSV-XN2-ΔG and VSV-CD40L-ΔG led to both significant cytotoxicity in vitro as a result of viral gene expression (Fig. 2C), and to expression of the CD40L transgene, but no transfer of expression of the VSV-G protein even at very high MOI of infection with the single cycle viruses (Fig.2D/E). In contrast, direct infection with the replication competent counterparts led to cytoxicity, transgene expression and transfer of VSV-G (Figs.2B-D). However, upon exposure of fresh target cells to supernatants harvested from BHK cells infected with the delta-G viruses, expression of neither the transgene, nor of VSV-G (Fig.2F), nor any cytotoxicity, could be detected, although all three were readily transferred through multiple passages from supernatants of cells initially infected with replication competent viruses(Fig.2F). Taken together, the data in Figs.2B-F, confirm the replication defective, single cycle nature of the delta-G viruses.
Figure 2. Characteristics of replication-defective recombinant VSV in B16ova melanoma cells in vitro.
A. cDNA representing the viral genomes of recombinant VSVs flanked by T7 RNA polymerase leader, T7 terminator, and hepatitis virus delta ribozyme (RBZ). VSV-XN2-ΔG and VSV-CD40L-ΔG were generated by removal of the G segment from VSV-XN2 and VSV-CD40L, respectively. B. 2×106 B16ova cells were infected with VSVs (MOI=0.01) for 24 hours and viral titers were measured in the supernatants. Values are averages of duplicate samples ± SEM. C. Using 96-well plates, 5×103 B16ova melanoma cells were infected with VSVs at an MOI of 1.0. The number of viable cells was measured using MTT assay at the indicated time points postinfection (hpi). Values are averages of triplicate samples (+ SEM) and representative of 2 independent experiments. D/E. B16ova cells were infected with replication competent (VSV-XN2 or VSV-CD40L) or single cycle (VSV-XN2-ΔG or VSVCD40L-ΔG) viruses in vitro at either low (replication-competent, MOI=0.001) or high (single-cycle, MOI>100) viral concentrations. 12 hours postinfection, cells were analyzed for expression of either viral VSV-G protein (D) or the CD40L transgene (E). F. Fresh BHK cells were exposed to undiluted supernatants harvested 48 hours postinfection from BHK cells infected with either replication competent VSV-XN2 (MOI=0.01), or single-cycle VSV-XN2-ΔG (MOI>100). Virus was allowed to expand in these cultures for 48hrs. Supernatants were harvested again and used to infect fresh cultures of BHK cells. 24hrs later, cells were analyzed for expression of the VSV-G protein by flow cytometry.
Single cycle VSV are effective antitumor agents
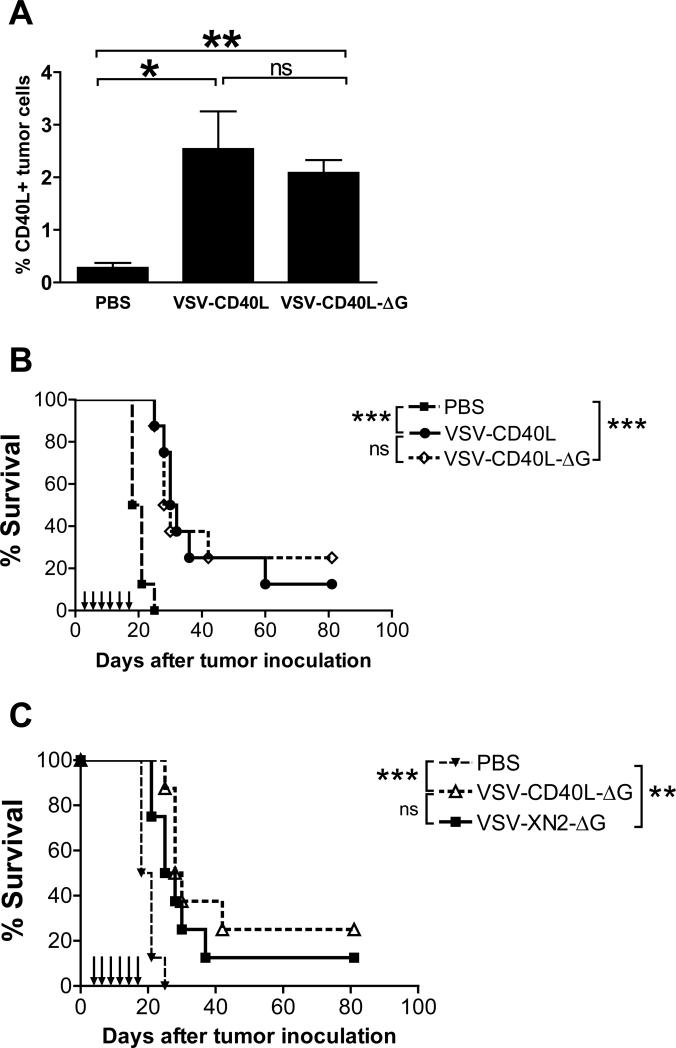
Levels of transduction of tumors with the CD40L transgene were indistinguishable following a single intratumoral injection of VSV-CD40L-ΔG or VSV-CD40L (Fig. 3A). This confirmed both that these viruses express their genes in vivo and that there is very little viral spread in B16ova tumors following the initial infection with a replication-competent virus. Intratumoral injections of either replication competent VSV-CD40L or single-cycle VSV-CD40L-ΔG virus consistently resulted in a significant prolongation in survival relative to PBS-treated control groups (p=0.0001 in Fig. 3B). However, there was never any significant difference between the therapeutic efficacy of replication-competent VSV-CD40L compared to single cycle VSV-CD40L-ΔG (Fig. 3B, p=0.8910) or between VSV-XN2 and VSV-XN2-ΔG (Supplemental Figure). Similar to replication-competent VSVs, VSV-CD40L-ΔG did not augment the oncolytic efficacy of the empty VSV-XN2-ΔG (p=0.2745) (Fig. 3C), confirming that the therapy is probably not operating through priming of adaptive T cell responses. These data show that direct viral oncolysis beyond at least a single round of infection has only a very limited contribution to therapeutic efficacy.
Figure 3. Live replication-defective recombinant VSVs delayed the growth of established subcutaneous B16ova tumors in immunocompetent mice.
A. VSV-injected B16ova tumors (n=3 per treatment group) were harvested four days after injection, dissociated to obtain single cell suspensions, and assayed for CD40L expression using flow cytometry. B. Seven-day old subcutaneous B16ova tumors were injected intratumorally six times with 5×107 pfu of either VSV-CD40L or VSV-CD40L-ΔG. Tumor growth and overall survival were monitored (n=8 per treatment group). C. Kaplan-Meier survival plot of subcutaneous B16ova tumor-bearing C57Bl/6 mice treated with six intratumoral injections (5×107 pfu/dose) of VSV-XN2-ΔG or VSV-CD40L-ΔG. *p<0.05, **p<0.01, ***p<0.001.
Immune responses to single-cycle VSV
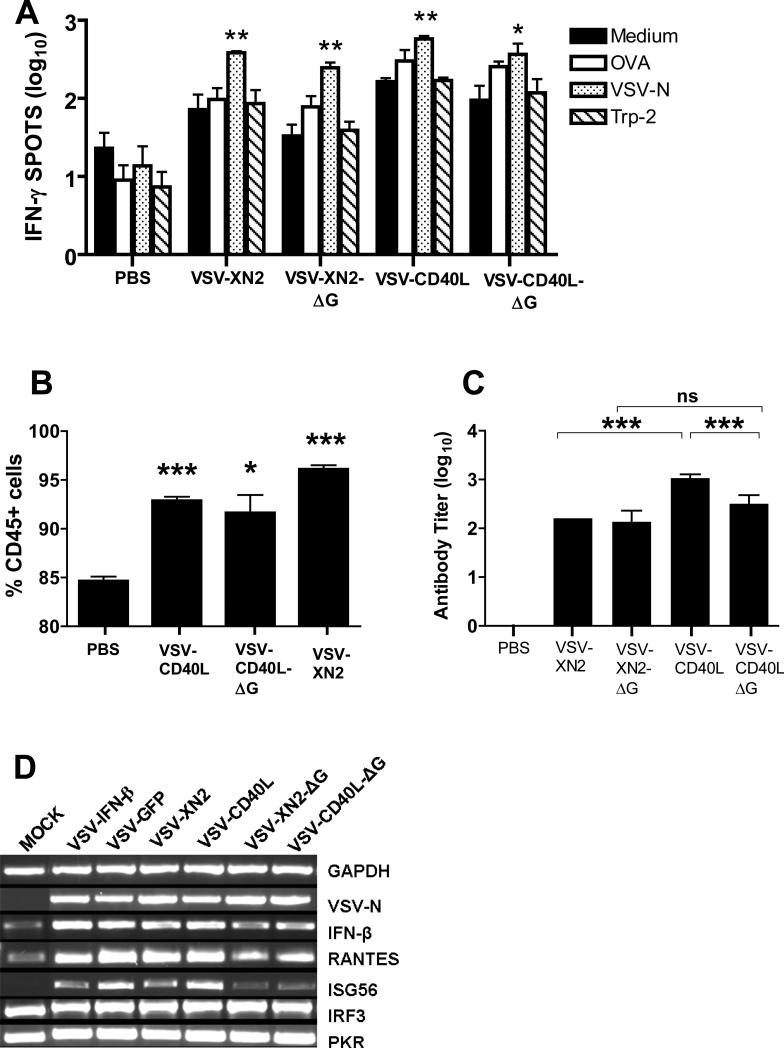
Similar to replication-competent viruses, single-cycle viruses induced generally elevated levels of T cell activation 7 days following intratumoral VSV injection (Fig. 4A). Although these T cell responses were associated with specificity against VSV-derived epitopes, they were not specific for the tumor associated TRP-2 or OVA antigens (Fig. 4A). Likewise, both replication-competent and single-cycle viruses increased the numbers of CD45+ cells in the tumor-draining lymph nodes to similar extents, and were all significantly higher than the PBS group (VSV-CD40L: p<0.0001; VSV-CD40L-ΔG: p=0.0105; VSV-XN2: p<0.0001) (Fig. 4B). Both types of virus induced comparable levels of serum antibody titer, although replication-competent VSV-CD40L significantly augmented levels of antiviral antibody compared to either VSV-XN2 (p<0.0001) or VSV-CD40L-ΔG (p<0.0001) (Fig. 4C). However, this enhanced antiviral antibody response was not associated with increased therapy (Figs. 3B,C). Finally, both replication-competent and single-cycle VSVs rapidly induced interferon regulated genes within the tumor microenvironment, although induction of IFN-β, RANTES, and ISG56 genes were consistently somewhat lower by replication–defective viruses compared to the replication competent viruses (Fig. 4D). Expression of CD40L did not quantitatively influence these effects (Fig. 4D).
Figure 4. Effects of infecting B16ova with single-cycle recombinant VSVs on the adaptive and innate immune responses.
A. IFN-γ ELISPOT assay of splenocytes harvested seven days after the third intratumoral virus injection. Two replicates of 1×105 splenocytes were plated in 96-well ELISPOT plates and cultured for 48 hours in the presence of the indicated peptides. B. Inguinal draining lymph nodes from mice treated with a single injection of intratumoral VSVs were harvested 4 days postinfection and assessed the frequency of CD45+ populations via flow cytometry. Three inguinal lymph nodes were pooled into a single sample per treatment group. Flow cytometric analysis of CD45+ populations were done in quadruplicates. C. From the same groups of mice in (a), blood was collected and the average serum antibody titer against VSV was determined (n=3 per group). *p<0.05, **p<0.01, ***p<0.001. D. RT-PCR was performed on total RNA from B16ova cells in vitro following 8 hours of infection with the following viruses using an MOI=1.0.
The nearly identical immune signatures of both types of virus following intratumoral injection support the hypothesis that the antiviral innate response is a major contributor to the antitumor effects in the B16ova model.
Viral gene expression is required for therapy
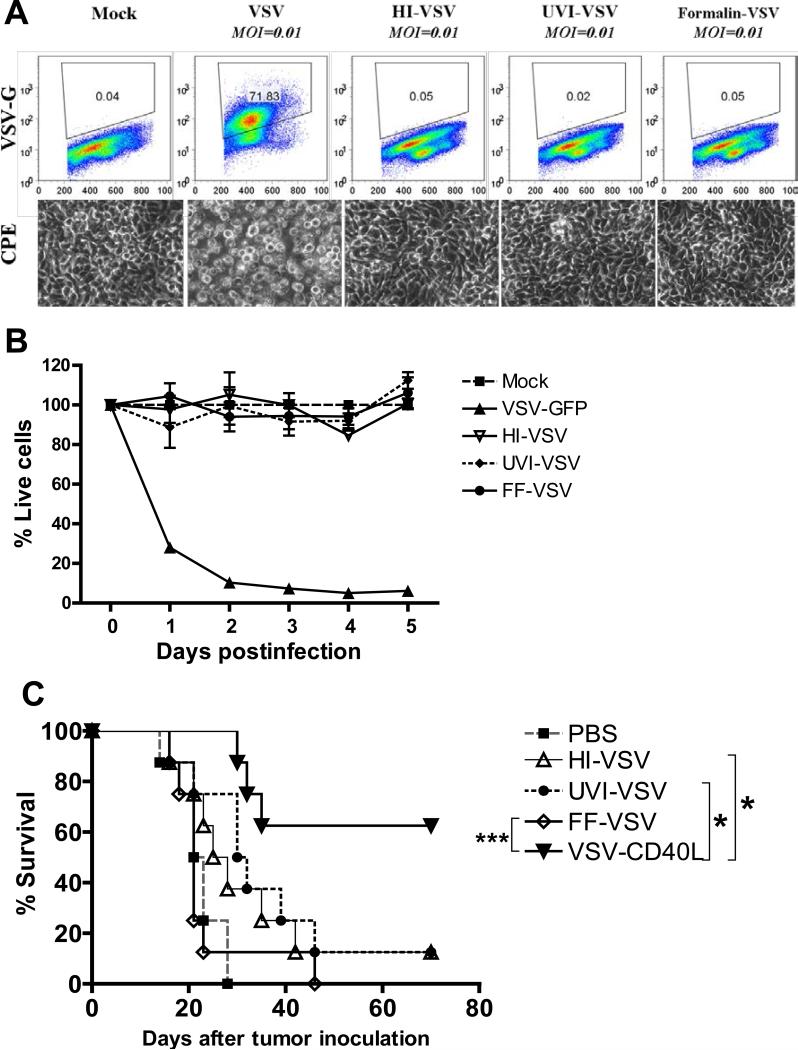
Next, we investigated whether viral antigen load alone is sufficient to trigger the antiviral innate response associated with antitumor therapy, or whether viral gene expression is required. To this end, we generated inactivated viral preparations which would contain similar antigen loads but are incapable of even a single cycle of infection and viral gene expression. Viral inactivation by heat (HI), exposure to ultraviolet (UVI), or by formalin-fixation (FF) was confirmed by the inability of B16ova cells exposed to different inactivated viral preparations in vitro to express viral glycoprotein G (Fig. 5A, top panels), to exhibit CPE (Fig. 5A, bottom panels) or to be killed in vitro (Fig. 5B). Intratumoral injection of any of the three inactivated viral preparations was, however, ineffective in generating antitumor therapy compared to the replication competent viruses VSV or VSV-CD40L (Fig. 5C). These results indicate that live, viable virus which can express its genome is required for antitumor effects of VSV.
Figure 5. The effects of physical and chemical inactivation of replication-competent VSV on the efficacy of VSV virotherapy.
A. Overnight cultures of 2×106 B16ova cells were infected with VSVs (MOI=0.01) for 24 hours. Top panels show dot plots of B16ova cells depicting surface expression of VSV-G. Representative photographs showing cytopathic effects (CPE) (bottom panels) after 24 hours. HI: Heat-inactivated VSV; UVI: ultraviolet-inactivated VSV; FF: Formalin-fixed VSV. B. Using 96-well plates, 5×103 B16ova melanoma cells were infected with either live or inactivated VSVs at an MOI of 1.0. The number of viable cells was measured using MTT assay at the indicated time points postinfection. Values are averages of triplicate samples (± SEM) and representative of 2 independent experiments. C. Kaplan-Meier survival plot of subcutaneous B16ova tumor-bearing C57Bl/6 mice treated with six intratumoral injections of either live VSV or inactivated forms of VSV (5×108 pfu/injection). *p<0.05, **p<0.01, ***p<0.001.
Antitumor VSV virotherapy is associated with an acute inflammatory response
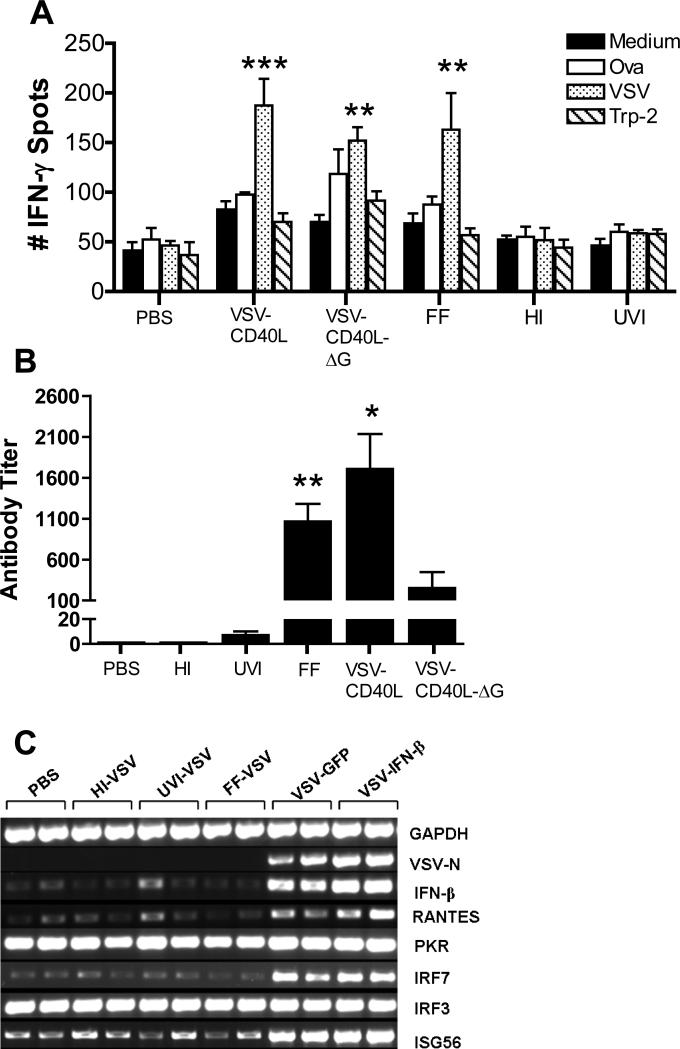
We investigated the key correlates between viral properties and in vivo antitumor efficacy. Only mice treated with live replication-competent virus, single-cycle replication defective virus or with formalin-fixed inactivated virus generated both specific antiviral T cell responses and elevated levels of non-specific T cell activation compared to PBS treated mice (splenocytes pulsed with medium alone: VSV-CD40L: **p=0.0025; VSVCD40L-ΔG: *p=0.0214; FF-VSV: *p=0.0268; HI-VSV: p=0.3303; UVI-VSV: p=0.6529) (Fig. 6A). FF-VSV also induced an antiviral antibody response comparable to that induced by replication-competent virus (Fig. 6B). A good correlation was observed between viral forms associated with in vivo antitumor therapy (live replication-competent or live single-cycle viruses) and their ability to direct viral gene expression (Fig. 6C) and to induce expression of IFN-β and other interferon-responsive genes such as RANTES, ISG56 and IRF7 in vivo (Fig. 6C). However, induction of other IRGs such as PKR and IRF3 were not correlated with the ability of different viral types to induce therapy (Fig. 6C).
Figure 6. Immune responses after intratumoral injection of live and inactivated forms of VSV and therapeutic efficacy of intratumoral TLR agonist in B16ova tumors.
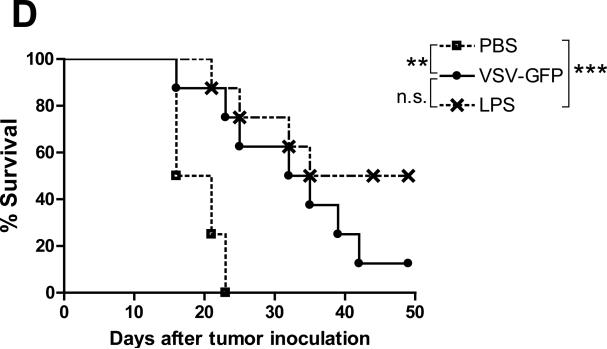
A. Subcutaneous B16ova tumors were infected with 3 daily injections of either live or inactivated VSV (5×107 pfu/injection). Eight days after the last virus, spleens were harvested, dissociated and incubated with one of the four antigens indicated. Total IFN-γ spots (1×105 splenocytes/48h) were measured using ELISPOT (n=3 mice/group). B. At the time of sacrifice (day 17 after tumor challenge), blood was also extracted and serum neutralizing antibody titer was determined. *p<0.05, **p<0.01, ***p<0.001. C. RT-PCR for type I interferon-responsive genes was performed on total RNA from subcutaneous B16ova tumors—harvested 8 hours postinfection—given a single injection of 5×108 pfu either live or inactivated VSV. D. Kaplan-Meier survival plot of B16ova tumor-bearing C57Bl/6 mice (n=8 per group) treated with three (3) intratumoral injections of either VSV-GFP (5×108 pfu/dose) or 200 μg of lipopolysaccharide (LPS). *p<0.05, **p<0.01, ***p<0.001.
These data suggest that the ability to generate antitumor therapy is associated with viral gene expression (but not progressive replication) and its subsequent activation of an acute proinflammatory response within the tumor. A prediction from these results would be that an acute inflammatory agent may, therefore, be just as efficacious as a fully replication-competent oncolytic virus upon direct intratumoral injection. Consistent with this, treatment of B16ova tumors with the TLR-4 agonist lipopolysaccharide (LPS) (at the maximum tolerated dose of 200 μg) was consistently as effective, if not more so, than a similar regimen of replication-competent VSV at its maximum tolerated dose (Fig. 6D).
Discussion
We show here that, at least in the VSV/B16ova/C57Bl/6 model, the therapeutic efficacy of a fully replication competent oncolytic virus does not depend upon its ability to replicate in and through the tumor. In contrast, antitumor therapy results not from viral replication, but from the antiviral immune response, which leads to significant levels of tumor cell killing. These data suggest that there may even be negative therapeutic outcomes in some models if immune suppressive interventions are used which are directed at enhancing viral replication, but which simultaneously diminish immune mediated, anti-viral tumor clearance mechanisms. As such, the results presented here have important implications for the design of future strategies aimed at improving the efficacy of virotherapy in immune competent patients. These data further highlight the inevitable and persistent friction that exists in reconciling the role played by the immune system in mediating the efficacy, inhibition, and safety of oncolytic virotherapy.
Unlike the case in vitro, no sustained replicative burst of virus was detected in B16ova tumors injected with VSV in vivo (Fig. 1C). It may be that active replication does occur within these tumors but is counterbalanced by even more rapid clearance of newly-synthesized virus. However, we believe that it is more likely that replication of VSV within B16ova tumors does not occur at significant levels because it is shut down rapidly by an acute, potent innate immune response (Fig. 1C). This inflammatory response is activated by viral gene expression (Fig. 6C) and disappears within days, presumably correlating with clearance of the inflammatory stimulus (i.e. the virus) (Fig.1C). The dose-response of therapy with increasing levels of input virus was not associated with corresponding increases in viral titers recovered from injected tumors. Therefore, therapy may not be exclusively associated with levels of viral replication within the tumors.
Consistent with this lack of dependence upon viral replication, VSV-mediated therapy of B16ova tumors is critically dependent upon intact host immune cells, including, but probably not exclusively, CD8+ and NK cell compartments.10 However, VSV therapy was neither enhanced by co-expression of a molecule which targets CD8+T cell effector activity (Galivo et al. Submitted) nor was it diminished in mice genetically deficient for the production of IFN-γ (Fig. 1E). Overall, these data suggest that an adaptive T cell response is not a major component of VSV virotherapy of B16ova melanoma in C57Bl/6.
Based on these findings, we hypothesized that the antitumor therapy observed in this model is predominantly due to the host immune response against the invading virus, as opposed to progressive viral replication and oncolysis of tumor cells. One prediction of such a hypothesis is that a virus with diminished capacity to replicate would show similar therapeutic efficacy in vivo as a fully replication-competent VSV. Consistent with this hypothesis, there was no difference between the ability of fully replication-competent VSV, or replication-incompetent, single-cycle VSV to generate antitumor therapy (Fig. 3B). Therefore, ongoing production of infectious VSV progeny is not required in this model for tumor therapy, suggesting that direct viral oncolysis beyond at least a single round of infection has only a very limited contribution to efficacy. Interestingly, both single-cycle and fully replication-competent VSV induced nearly identical immune signatures following intratumoral injection as they relate to T cell, antibody and rapidly induced innate inflammatory immune responses (Fig. 4A-D). This is consistent with their indistinguishable therapeutic efficacy (Fig. 3B, Supplementary Figure) and supports the hypothesis that the antiviral innate response, rather than progressive viral replication and oncolysis, is the major contributor to the antitumor effects in the B16ova model.
We were initially surprised that we did not observe large differences between the ability of replication-competent and single-cycle viruses to induce interferon-regulated genes within the tumor microenvironment. However, in the light of our other data presented here, this is consistent with the fact that the replication-competent viruses are severely restricted in their ability to replicate in vivo in the tumor; hence, we believe that the similar levels of interferon response generated by both type of vector simply reflects the fact that there is minimal progressive replication due to its rapid immune-mediated shutdown in vivo. Our original rationale for the inclusion of CD40L in VSV was to increase the generation of adaptive, tumor specific T cell responses following viral mediated oncolysis. The fact that the CD40L encoding virus also generated very similar levels of interferon responsive gene expression in tumors is, once again, consistent with the hypothesis that minimal viral replication occurs because of immune-mediated shutdown.
Despite the ability of formalin-fixed VSV to elicit both a generalized T cell activation, an antiviral T cell response, and neutralizing anti-VSV antibodies, at levels similar to those generated by replication competent/incompetent viruses (Fig. 6A,B), FF-VSV could neither generate antitumor therapy (Fig. 5C) nor the acute proinflammatory innate response (Fig. 6C). Our experiments with various forms of inactivated virus also indicated that live, viable virus, which can express its genome (Fig. 5A and 6C), is required for antitumor effects of VSV. In addition, only live viable VSV optimally induced expression of IFN-β and other interferon-responsive genes such as RANTES, ISG56 and IRF7 in vivo (Fig. 6C). Finally, injection of a potent inflammatory agent such as LPS induced almost identical antitumor therapy as intratumoral injection of fully replication-competent oncolytic VSV (Fig. 6D). Although the innate immune signaling pathways induced by VSV and LPS probably differ, these data are consistent with the mechanism of action of VSV as being not different from that of a non-specific immune adjuvant in this model.
An important future direction will be to identify the immune mediators of the antitumor therapy. Significantly, many of the cytokines induced intratumorally by VSV injection (Fig. 1D) are themselves associated with antitumor activity31,35,36 either through direct cytotoxic effects or through the activation of further immune effectors. Experiments are currently underway to dissect the critical components of the innate response to VSV using mice deficient in immune signaling pathways, as well as in expression of effector cytokines. It may eventually be possible to selectively boost those cytokine components involved in enhancing tumor cell killing while inhibiting those responses involved in suppressing viral replication. If these components can be functionally separated, it would be possible to manipulate the antiviral innate response to optimize both immune-mediated and oncolytic therapy.
We do not believe that the predominance of the immune-mediated, versus oncolytic, antitumor effector mechanisms that we have characterized in the C57Bl/6-B16ova model, is necessarily applicable to all experimental models of either VSV or other oncolytic viruses. For example, a notable difference between our model and others is the lack of the replicative burst that is observed in vivo. In studies in which VSV serves as a potent oncolytic agent against a rat model of hepatocellular carcinoma, amplification of the input viral dose is clearly seen as a result of replication within the tumor37,38. We hypothesize that differences between the innate response of different tumor cell lines to viral infection will heavily influence the ability of the corresponding tumors to support viral replication, persistence and amplification in vivo. In addition, several other factors may affect the outcome of intratumoral VSV injection, including the genetic strain of the host, the anatomical location of the tumor and the local immune context in which it is growing.
Overall, our results raise further tensions in the already highly-strained relationship between oncolytic virotherapy and the immune system. A robust antiviral immune response to an oncolytic virus in normal, but not tumor, cells is an important and necessary safety component. In contrast, the antiviral immune response acts to suppress ongoing viral replication, limit virus-mediated oncolysis and inhibit therapeutic efficacy. In reality, not all tumor cells have completely defective responses to antiviral type I interferons and there is likely to be a wide spectrum in the magnitude and extent of the responses of different tumor cells to (oncolytic) viral infection. It may be, therefore, that a significant innate response to the virus either by the tumor cells themselves, or by the infiltrating stromal cells, contributes to bystander killing of tumor cells through direct or indirect immune mediated mechanisms. We believe that this is the case in the B16ova model described here. Therefore, the antiviral immune response can, simultaneously, be a) a critical mediator of antitumor therapy, b) a potent inhibitor of viral replication and spread in the tumor and c) an essential safety barrier preventing systemic toxicity.
Perception of the immune response to viral infection purely as limiting viral replication and efficacy has led to strategies aimed at local inhibition of the innate response to viral infection in efforts to increase oncolysis.16,19,39 These approaches are rational and have shown success. However, we suggest that consideration be given to the exact type of suppressive mechanisms that are used and their likely impact on extinguishing the possible positive impact on antitumor therapy that such immune re-activities may have.
In summary, we show here that the antitumor therapy associated with intratumoral VSV injection in the B16ova model is not dependent upon the ability of the virus to undergo progressive rounds of replication. In contrast, therapy is most closely correlated with viral gene expression which induces a pro-inflammatory reaction, an effect which is similar to that induced by a non-specific immune adjuvant injected directly into the tumor. These data further suggest that the nature and extent of the antiviral immune response to oncolytic virus infection mediates an intricate balance between safety against systemic virus toxicity, restriction of viral replication/oncolysis, and potentially significant immune-mediated antitumor therapy. Therefore, strategies aimed at improving the efficacy of oncolytic virotherapy through modulation of antiviral immunity should consider all three of these possible effects in order to maximize the overall therapeutic outcome.
Supplementary Material
Acknowledgments
We thank Toni Higgins for expert secretarial assistance. This work was supported by the Richard Schulze Family Foundation, the Mayo Foundation and by NIH Grants CA107082-02, CA130878-01, and CA66726-12.
References
- 1.Balachandran S, Barber G. Vesicular stomatitis virus therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran S, Porosnicu M, Barber GN. Oncolytic Activity of Vesicular Stomatitis Virus Is Effective against Tumors Exhibiting Aberrant p53, Ras, or Myc Function and Involves the Induction of Apoptosis. J Virol. 2001;75:3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebert O, Harbaran S, Shinozaki K, Woo SLC. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2004;12:350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 4.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 5.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 6.Lichty BD, Stojdl DF, Taylor RA, Miller L, Frenkel I, Atkins H, et al. Vesicular Stomatitis Virus: A Potential Therapeutic Virus for the Treatment of Hematologic Malignancy. Human Gene Therapy. 2004;15:821–831. doi: 10.1089/hum.2004.15.821. [DOI] [PubMed] [Google Scholar]
- 7.Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, et al. Effects of Intravenously Administered Recombinant Vesicular Stomatitis Virus (VSVΔM51) on Multifocal and Invasive Gliomas. J Natl Cancer Inst. 2006;98:1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 8.Shinozaki K, Ebert O, Woo SLC. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology. 2005;41:196–203. doi: 10.1002/hep.20536. [DOI] [PubMed] [Google Scholar]
- 9.Stojdl DF, Abraham N, Knowles S, Marius R, Brasey A, Lichty BD, et al. The Murine Double-Stranded RNA-Dependent Protein Kinase PKR Is Required for Resistance to Vesicular Stomatitis Virus. J Virol. 2000;74:9580–9585. doi: 10.1128/jvi.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic Immunovirotherapy for Melanoma Using Vesicular Stomatitis Virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 11.Kottke T, Diaz RM, Kaluza K, Pulido J, Galivo F, Wongthida P, et al. Use of Biological Therapy to Enhance Both Virotherapy and Adoptive T-Cell Therapy for Cancer. Mol Ther. 2008;16:1910–1918. doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 13.Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirn DH, McCormick F. Replicating viruses as selective cancer therapeutics. Molecular Medicine Today. 1996;2:519–527. doi: 10.1016/s1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- 15.Parato KA, Senger D, Forsyth PAJ, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 16.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends in Molecular Medicine. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ries SJ, Brandts CH. Oncolytic viruses for the treatment of cancer: current strategies and clinical trials. Drug Discovery Today. 2004;9:759–768. doi: 10.1016/S1359-6446(04)03221-0. [DOI] [PubMed] [Google Scholar]
- 18.Altomonte J, Wu L, Chen L, Meseck M, Ebert O, Garcia-Sastre A, et al. Exponential Enhancement of Oncolytic Vesicular Stomatitis Virus Potency by Vector-mediated Suppression of Inflammatory Responses In Vivo. Mol Ther. 2007;16:146–153. doi: 10.1038/sj.mt.6300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 20.Prestwich RJ, Errington F, Ilett EJ, Morgan RSM, Scott KJ, Kottke T, et al. Tumor Infection by Oncolytic Reovirus Primes Adaptive Antitumor Immunity. Clin Cancer Res. 2008;14:7358–7366. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linardakis E, Bateman A, Phan V, Ahmed A, Gough M, Olivier K, et al. Enhancing the Efficacy of a Weak Allogeneic Melanoma Vaccine by Viral Fusogenic Membrane Glycoprotein-mediated Tumor Cell-Tumor Cell Fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]
- 22.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically Engineered Vesicular Stomatitis Virus in Gene Therapy: Application for Treatment of Malignant Disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, et al. A Vesicular Stomatitis Virus Recombinant Expressing Granulocyte-Macrophage Colony-Stimulating Factor Induces Enhanced T-Cell Responses and Is Highly Attenuated for Replication in Animals. J Virol. 2005;79:15043–15053. doi: 10.1128/JVI.79.24.15043-15053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boritz E, Gerlach J, Johnson JE, Rose JK. Replication-Competent Rhabdoviruses with Human Immunodeficiency Virus Type 1 Coats and Green Fluorescent Protein: Entry by a pH-Independent Pathway. J Virol. 1999;73:6937–6945. doi: 10.1128/jvi.73.8.6937-6945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia SU, Simon ID, Rose JK. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology. 2008;376:165–172. doi: 10.1016/j.virol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Publicover J, Ramsburg E, Rose JK. A Single-Cycle Vaccine Vector Based on Vesicular Stomatitis Virus Can Induce Immune Responses Comparable to Those Generated by a Replication-Competent Vector. J Virol. 2005;79:13231–13238. doi: 10.1128/JVI.79.21.13231-13238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnell MJ, Johnson JE, Buonocore L, Rose JK. Construction of a Novel Virus That Targets HIV-1-Infected Cells and Controls HIV-1 Infection. Cell. 1997;90:849–857. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann MF, Kundig TM, Kalberer CP, Hengartner H, Zinkernagel RM. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas R, Kresse M, Latta M, Wendel A. Tumor Necrosis Factor: How to Make a Killer Molecule Tumor-Specific? Current Cancer Drug Targets. 2005;5:381–392. doi: 10.2174/1568009054863627. [DOI] [PubMed] [Google Scholar]
- 31.Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM. Interferon: Cellular Executioner or White Knight? Current Medicinal Chemistry. 2007;14:1279–1289. doi: 10.2174/092986707780597907. [DOI] [PubMed] [Google Scholar]
- 32.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, et al. IFN-[gamma] mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 33.Robinson BW, Mukherjee SA, Davidson A, Morey S, Musk AW, Ramshaw I, et al. Cytokine gene therapy or infusion as treatment for solid human cancer. J Immunother. 1998;21:211–217. doi: 10.1097/00002371-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi T, Miyazawa N, Moore MAS, Crystal RG. Tumor Regression Induced by Intratumor Administration of Adenovirus Vector Expressing CD40 Ligand and Naive Dendritic Cells. Cancer Res. 2000;60:6391–6395. [PubMed] [Google Scholar]
- 35.Terenzi F, Hui DJ, Merrick WC, Sen GC. Distinct Induction Patterns and Functions of Two Closely Related Interferon-inducible Human Genes, ISG54 and ISG56. J Biol Chem. 2006;281:34064–34071. doi: 10.1074/jbc.M605771200. [DOI] [PubMed] [Google Scholar]
- 36.Ullrich E, Ménard C, Flament C, Terme M, Mignot G, Bonmort M, et al. Dendritic cells and innate defense against tumor cells. Cytokine & Growth Factor Reviews. 2008;19:79–92. doi: 10.1016/j.cytogfr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Ebert O, Shinozaki K, Huang T-G, Savontaus MJ, Garcia-Sastre A, Woo SLC. Oncolytic Vesicular Stomatitis Virus for Treatment of Orthotopic Hepatocellular Carcinoma in Immune-competent Rats. Cancer Res. 2003;63:3605–3611. [PubMed] [Google Scholar]
- 38.Shinozaki K, Ebert O, Kournioti C, Tai Y-S, Woo SLC. Oncolysis of Multifocal Hepatocellular Carcinoma in the Rat Liver by Hepatic Artery Infusion of Vesicular Stomatitis Virus. Mol Ther. 2004;9:368–376. doi: 10.1016/j.ymthe.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.