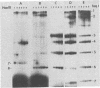
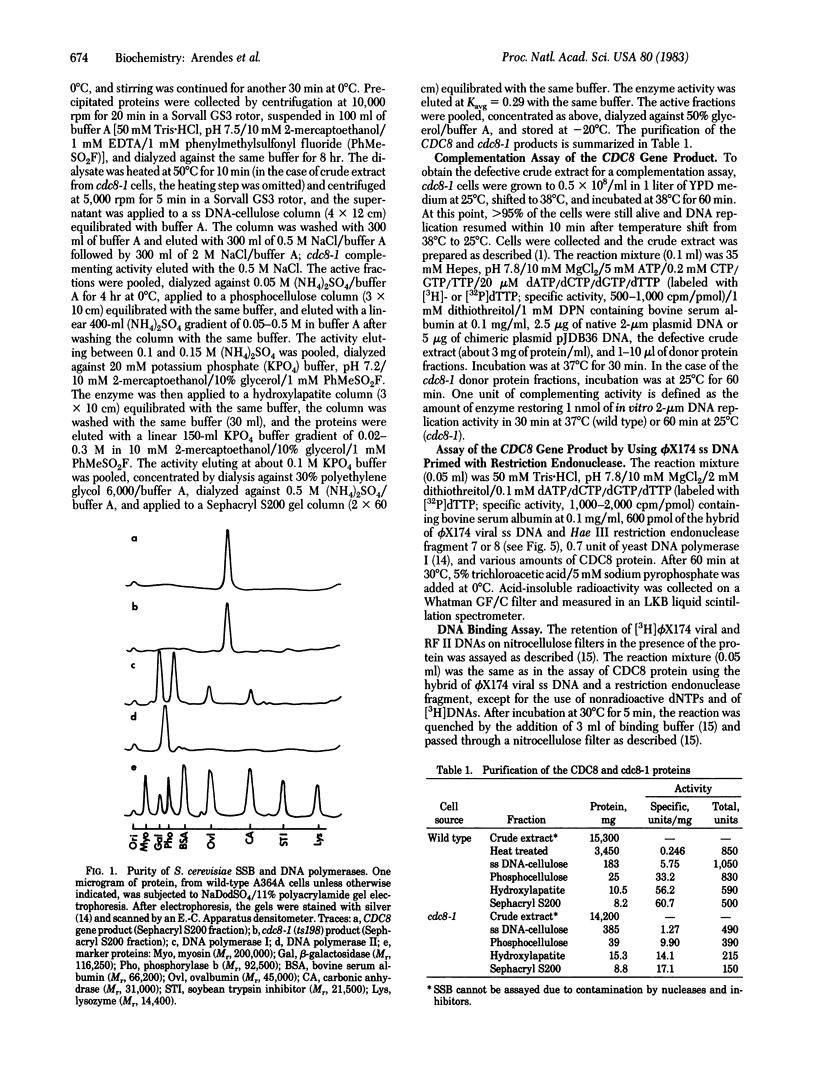
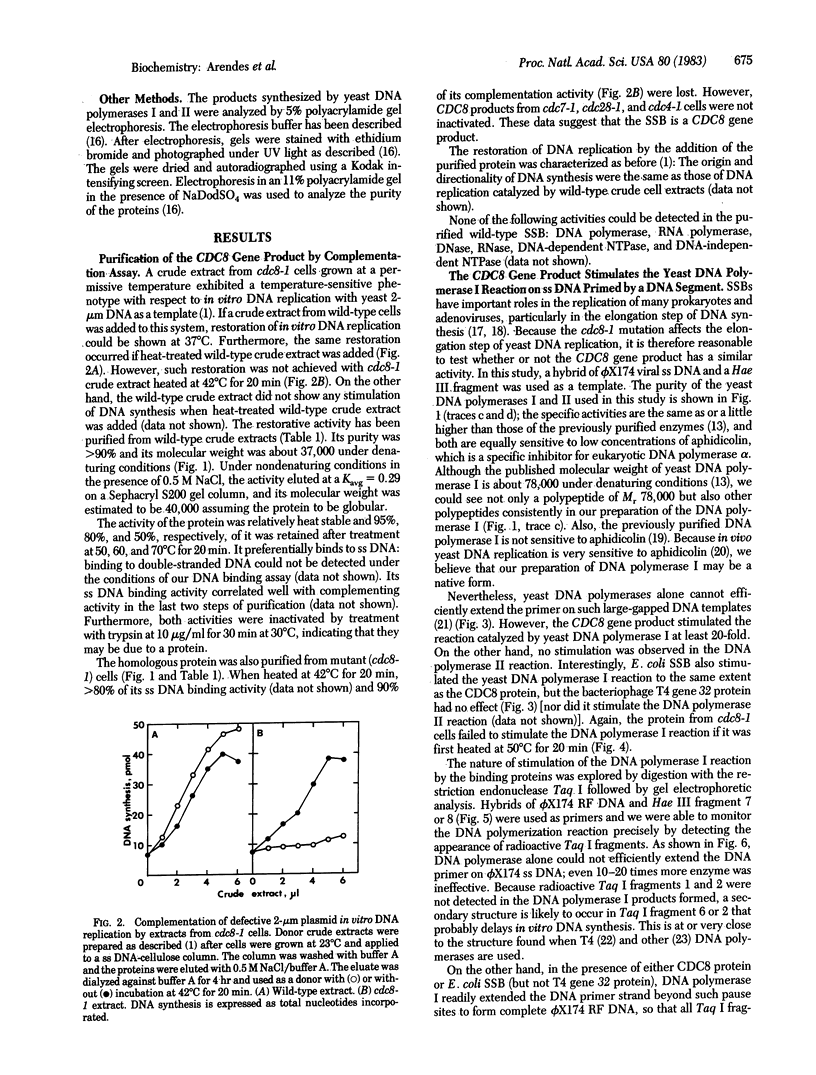
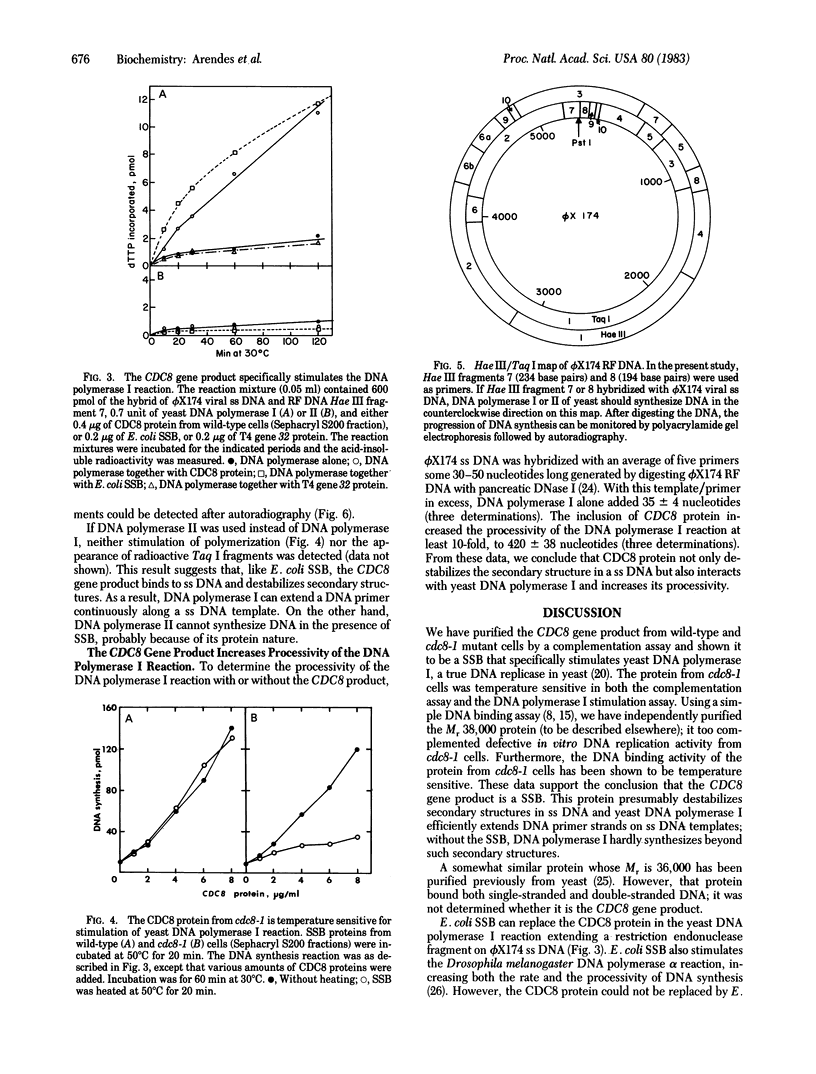
Abstract
Extracts of the yeast Saccharomyces cerevisiae support DNA replication on exogenous yeast 2-microns plasmid DNA templates. A crude extract from a S. cerevisiae cell division cycle mutant, cdc8-1, expressed the temperature-sensitive phenotype since it could be inactivated at 42 degrees C in vitro. This heat-inactivated extract was fully complemented by the addition of either wild-type or cdc8-1 single-stranded DNA binding protein (SSB). restoration by SSB of the activity of the mutant cell extract allowed replication like that of a wild-type crude extract, as shown by bidirectional DNA synthesis from the in vivo origin. The DNA binding protein specifically stimulates the reaction catalyzed by yeast DNA polymerase I, a true DNA replicase, using the hybrid of phi X174 single-stranded DNA and a restriction endonuclease fragment as a template. It also increases processivity of DNA polymerase I at least 10-fold. Escherichia coli SSB, but not T4 gene 32 protein, can substitute for yeast SSB. Both restoration of DNA synthesis in the heated mutant cell extract and stimulation of the DNA polymerase I reaction by SSB from cdc8-1 cells are inactivated at nonpermissive temperatures, suggesting that yeast SSB is the CDC8 gene product.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrara G., Gattoni S., Mercanti D., Tocchini-Valentini G. P. Purification of a DNA-binding protein from Xenopus laevis unfertilized eggs. Nucleic Acids Res. 1977 Aug;4(8):2855–2870. doi: 10.1093/nar/4.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. The effect of template secondary structure on vaccinia DNA polymerase. J Biol Chem. 1979 Aug 25;254(16):7820–7826. [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Chang L. M. Low molecular weight deoxyribonucleic acid polymerase from calf thymus chromatin. I. Preparation of homogeneous enzyme. J Biol Chem. 1973 Jun 10;248(11):3789–3795. [PubMed] [Google Scholar]
- Fay P. J., Johanson K. O., McHenry C. S., Bambara R. A. Size classes of products synthesized processively by DNA polymerase III and DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1981 Jan 25;256(2):976–983. [PubMed] [Google Scholar]
- Godson G. N., Boyer H. Susceptibility of the phiX-like phages G4 and G14 to R-EcoRi endonuclease. Virology. 1974 Nov;62(1):270–275. doi: 10.1016/0042-6822(74)90321-3. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Sequential gene function in the initiation of Saccharomyces cerevisiae DNA synthesis. J Mol Biol. 1974 Apr 15;84(3):445–461. doi: 10.1016/0022-2836(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E., Alberts B. M. Two types of replication proteins increase the rate at which T4 DNA polymerase traverses the helical regions in a single-stranded DNA template. J Biol Chem. 1981 Apr 25;256(8):4087–4094. [PubMed] [Google Scholar]
- Hübscher U., Spanos A., Albert W., Grummt F., Banks G. R. Evidence that a high molecular weight replicative DNA polymerase is conserved during evolution. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6771–6775. doi: 10.1073/pnas.78.11.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Edelman G. M. Replication in vitro of the 2-micrometer DNA plasmid of yeast. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1223–1227. doi: 10.1073/pnas.76.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H., Greenberg B. D., Sugino A. Yeast 2-micrometer plasmid DNA replication in vitro: origin and direction. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7261–7265. doi: 10.1073/pnas.78.12.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Kupfer D. M. Control of Saccharomyces cerevisiae 2microN DNA replication by cell division cycle genes that control nuclear DNA replication. J Mol Biol. 1977 Oct 25;116(2):249–260. doi: 10.1016/0022-2836(77)90215-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae. Microbiol Rev. 1980 Dec;44(4):519–571. doi: 10.1128/mr.44.4.519-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Williamson D. H. Replicating circular DNA molecules in yeast. Cell. 1975 Mar;4(3):249–253. doi: 10.1016/0092-8674(75)90172-5. [DOI] [PubMed] [Google Scholar]
- Plevani P., Badaracco G., Ginelli E., Sora S. Effect and mechanism of action of aphidicolin on yeast deoxyribonucleic acid polymerases. Antimicrob Agents Chemother. 1980 Jul;18(1):50–57. doi: 10.1128/aac.18.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Clivio A., Valentini O., Cobianchi F. DNA binding proteins from calf thymus with an enhanced ability to stimulate DNA polymerase alpha in vitro. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1053–1062. doi: 10.1016/0006-291x(80)90059-5. [DOI] [PubMed] [Google Scholar]
- Roth A. C., Nossal N. G., Englund P. T. Rapid hydrolysis of deoxynucleoside triphosphates accompanies DNA synthesis by T4 DNA polymerase and T4 accessory proteins 44/62 and 45. J Biol Chem. 1982 Feb 10;257(3):1267–1273. [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G., Fay P. J., Bambara R. A., Lehman I. R. Elongation of RNA-primed DNA templates by DNA polymerase alpha from Drosophila melanogaster embryos. J Biol Chem. 1981 Aug 10;256(15):8202–8207. [PubMed] [Google Scholar]
- Weaver D. T., DePamphilis M. L. Specific sequences in native DNA that arrest synthesis by DNA polymerase alpha. J Biol Chem. 1982 Feb 25;257(4):2075–2086. [PubMed] [Google Scholar]
- Wintersberger U. Absence of a low-molecular-weight DNA polymerase from nuclei of the yeast, Saccharomyces cerevisiae. Eur J Biochem. 1974 Dec 16;50(1):197–202. doi: 10.1111/j.1432-1033.1974.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Brewer B. J., Fangman W. L. Replication of each copy of the yeast 2 micron DNA plasmid occurs during the S phase. Cell. 1979 Aug;17(4):923–934. doi: 10.1016/0092-8674(79)90332-5. [DOI] [PubMed] [Google Scholar]