Abstract
Objective
We tested the hypothesis that concentrated formula begun within the first two weeks of life increases growth in infants born to HIV-infected mothers.
Study Design
HIV-exposed infants from the U.S., Bahamas, and Brazil were randomized in a double-blind controlled trial to receive either a concentrated 87 kcal/100 ml (26 kcal/oz) formula (CF) or standard 67 kcal/100 ml (20 kcal/oz) formula (SF) for 8 weeks. This paper presents results for infants who were not determined to be HIV-infected based on testing at 4 weeks. Primary outcomes were safety, tolerability, and growth in weight and length.
Results
2,097 infants were enrolled, of whom 1998 were uninfected and had study formula dispensed. At weeks 4 and 8, uninfected infants receiving CF showed higher energy intake compared with uninfected infants receiving SF (P<0.001). By week 8, uninfected infants assigned to CF weighed more than infants on SF. There were no consistent differences in measures of tolerability, and rates of discontinuation or perceived formula intolerance were similar between treatment groups.
Conclusions
A concentrated formula is well tolerated and results in increased weight gain compared with SF. Until the HIV-status of an infant is reliably determined, early introduction of a concentrated formula in HIV-exposed children may have beneficial effects on growth. The role of early nutritional intervention remains to be determined for individuals living in countries with endemic malnutrition for whom formula feeding is a viable option.
Introduction
The greatest effect of the global HIV epidemic continues to be among children and young adults living in sub-Saharan Africa. In this region, in 2006, there were 24.7 million adults and children who were HIV infected and of these individuals, 13.3 million were women. (1) Worldwide, over 90% of HIV-infected children live in resource-poor environments. Similar to what was seen early in the pandemic of pediatric AIDS in the United States (U. S.), rates of mortality, infections and chronic malnutrition are extremely high among HIV-infected children. The mucosal immune system of the human gastrointestinal tract is preferentially depleted of CD4+ T lymphocytes following infection by human immunodeficiency virus (HIV) type 1 (HIV-1) (2) and this defect in mucosal immunity increases the host’s susceptibility to gastrointestinal infection and malabsorption. Although the benefits of preventing malnutrition on the impact of the progression of HIV disease is not well established, malnutrition and enteric infection may potentiate the effects of HIV by increasing nutrient malabsorption, metabolic requirements and the severity of immunocompromise. HIV-infected women, who themselves are often undernourished, frequently give birth to infants who, even if not HIV-infected, may be underweight (3). Until infection with HIV is excluded and the HIV status of the baby is determined, providing increased energy intake to the infant may be beneficial.
The Pediatric AIDS Clinical Trials Group (PACTG) initiated a randomized, double-blind controlled clinical trial designed to evaluate the effects on growth of a concentrated formula compared with standard formula (SF) in infants born to HIV-infected women in the U.S., the Bahamas, and Brazil. This study provides a unique evaluation of CF taken during the first two months of life in almost 2,000 HIV-negative infants, and of the longer term safety of this nutritional strategy in a subgroup of the HIV-exposed, uninfected infants who were followed for a year.
Methods
Study Design
This study was a randomized, double-blind, controlled multicenter clinical trial designed to compare the effects on growth in weight and length of 87 kcal/100 ml (26 kcal/oz) concentrated formula (CF) versus standard 67 kcal/100 ml (20 kcal/oz) formula (SF) in infants born to HIV-infected women. The eligible population consisted of infants who were 1.8 kg or more in weight, 17 or fewer days of age, and born to HIV-infected women. Informed consent was obtained for all participants according to local institutional review board and federal guidelines (4) and regulations (5). None of the infants were breast fed. Infants were randomized to receive either CF or SF, with stratification by gestational age (<37 weeks versus 37 or more weeks) and site location (U.S., including Puerto Rico, versus non-U.S. [Brazil or the Bahamas]). Randomization was balanced using a dynamic permuted block design within site. Enrollment commenced July, 1997 and closed April, 2002. Infant HIV infection status was not determined prior to randomization. The primary objective of the study was to compare the two formulas initiated soon after birth and continued for 28 weeks in infants subsequently determined to be infected. Infection status was determined at study week 4 by HIV DNA PCR. At study week 8, infants with a positive DNA test result continued randomized formula for 28 weeks with follow-up for 52 weeks. The primary objective of the study was to have been addressed using data from this cohort of infected infants. Except for a random sample of approximately 1 in 9 uninfected infants who continued in follow-up for 52 weeks after taking SF for 28 weeks, infants with negative or indeterminate week 4 DNA results discontinued randomized formula and follow-up at week 8. The longer-term follow-up of this subgroup of uninfected infants was included to provide an evaluation in uninfected infants of any potential adverse effects of initiating CF formula before HIV infection status could be determined. The study was originally designed to enroll 1,900 infants in anticipation that 10% (190 infants) would be infected. This would have provided about 90% power to detect differences in mean weight among HIV-infected infants between the two formulas of 0.45 kg at 6 months of age. However, since the study was designed, successful prevention of mother-to-child transmission of HIV in the United States reduced transmission rates substantially, and despite expansion of the sample size to include additional sites in the Bahamas and Brazil as well as the U.S., the proportion of HIV-infected infants in the study remained at 2.0%. Therefore, in March 2002, an independent study monitoring committee recommended that the study be terminated on the basis that it would not be able to answer the original question among HIV-infected infants within a reasonable timeframe or sample size. This report therefore focuses on the following valid randomized comparisons: (1) growth and adverse outcomes among uninfected infants during the first 8 weeks of follow-up, when these infants were taking the CF or SF, as randomized; and (2) adverse outcomes in uninfected infants who received 8 weeks of CF followed by 20 weeks of SF, versus SF throughout.
Study Product Formulation
The ingredients for the SF, 67 kcal/100 ml (20 kcal/fluid ounce), and the CF, 87 kcal/100 ml (26 kcal/fluid ounce) are:
Water, nonfat milk, lactose, soy oil, coconut oil, mono-and diglycerides, soy lecithin, ascorbic acid, carrageenen, choline, chloride, ferrous sulfate, taurine, m-Inositol, alpha-tocopheryl acetate, zinc sulfate, niacinamide, calcium pantothenate, cupric sulfate, vitamin A palmitate, thiamine chloride hydrochloride, riboflavin, pyridoxine hydrochloride, folic acid, manganese sulfate, phylloquinone, biotin, sodium selenite, vitamin D3, and cyanocobalamin.
The CF contains a 29% increase each of fat, protein and carbohydrate as well as a 29% increase in vitamins and minerals. The measured osmolality of the CF was 407 mOsm/kg and ash content 0.87% (w/w), corresponding to 219 mOsm/L and 4.7 g/L as fed (diluted).
The composition of the CF conforms with nutrient requirements applicable to commercially available infant formula products as specified by federal law and regulation under the Infant Formula Act of 1980 and its 1986 amendments (21 USC Ch. 9 Section 350a and 21 CFR Ch. 1 Subpart D Section 107.100), including allowable protein concentration (1.8–4.5 g per 100 kcal). These levels are listed together with the relevant characteristics per 100 kcal of both products in Table 1.
Table 1.
| Infant Formula Nutrient Levels | |||||
|---|---|---|---|---|---|
| Per 100 kcal | Per fluid oz or 100 ml as fed (diluted) | ||||
| Recommended | |||||
| Minimum | Maximum | Both formulas (* and **) | Standard formula* | Concentrated formula** | |
| Nutrient | |||||
| Protein, g | 1.8 | 4.5 | 2.14 | 0.43 | 0.55 |
| Fat, g | 3.3 | 6 | 5.4 | 1.08 | 1.38 |
| % calories | 30 | 54 | 48.6 | 48.6 | 48.6 |
| Carbohydrate, g | 10.7 | 2.14 | 2.74 | ||
| Water, g | 100**/133* | 26.6 | 25.64 | ||
| Linoleic Acid, mg | 300 | 1300 | 260 | 333.33 | |
| % calories | 2.7 | 11.7 | 11.7 | 11.7 | |
| Vitamins | |||||
| Vit A. IU | 250 | 750 | 300 | 60 | 76.92 |
| Vit D, IU | 40 | 100 | 60 | 12 | 15.38 |
| Vit E. IU | 0.7 | -- | 3 | 0.6 | 0.77 |
| Vit K, mcg | 4 | -- | 8 | 1.6 | 2.05 |
| Thiamine, mcg | 40 | -- | 100 | 20 | 25.64 |
| Riboflavin, mcg | 60 | -- | 150 | 30 | 38.46 |
| Vit B6, mcg | 35 | -- | 60 | 12 | 15.38 |
| Vit B12, mcg | 0.15 | -- | 0.25 | 0.05 | 0.06 |
| Niacin, mcg | 250 | -- | 1050 | 210 | 269.23 |
| Folic Acid, mcg | 4 | -- | 15 | 3 | 3.85 |
| Biotin, mcg | 1.5 | -- | 4.4 | 0.88 | 1.13 |
| Pantothenic Acid, mcg | 300 | -- | 450 | 90 | 115.38 |
| Vit C, mg | 8 | -- | 9 | 1.8 | 2.31 |
| Choline, mg | 7 | -- | 16 | 3.2 | 4.1 |
| Inositol, mg | 4 | -- | 4.7 | 0.94 | 1.21 |
| Minerals | |||||
| Calcium, mg | 60 | -- | 73 | 14.6 | 18.72 |
| Magnesium, mg | 6 | -- | 6 | 1.2 | 1.54 |
| Iron, mg | 0.15 | 3.0 | 1.8 | 0.36 | 0.46 |
| Zinc, mg | 0.5 | -- | 0.75 | 0.15 | 0.19 |
| Manganese, mcg | 5 | -- | 5 | 1 | 1.28 |
| Copper, mcg | 60 | -- | 90 | 18 | 23.08 |
| Iodine, mcg | 5 | 75 | 9 | 1.8 | 2.31 |
| Sodium, mg | 20 | 60 | 27 | 5.4 | 6.92 |
| Potassium, mg | 80 | 200 | 105 | 21 | 26.92 |
| Chloride, mg | 55 | 150 | 64 | 12.8 | 16.41 |
| Phosphorus, mg | 30 | -- | 56 | 11.2 | 14.36 |
The 67 kcal/100 ml [20 kcal/oz] (as fed) liquid concentrate study formula product is similar in manufacture and composition to previously commercially available Similac with iron (Ross) infant formula.
67 kcal/100 ml [20 kcal/oz]
87 kcal/100 ml [26 kcal/oz]
Evaluation of Infection Status
For the purposes of this study, a child was considered to be uninfected if there was not a single positive viral test (culture, HIV-1 DNA, or HIV-1 RNA at any age, or neutralizable ICD-p24 antigen after 28 days of age). The study team reviewed results of all diagnostic HIV tests for any infant who had at least one positive or indeterminate test result reported in the study database. The team considered as infected any child who had two different positive results, unless there was contrary information to confirm that these two test results were both false positives. The number of children who were determined to be HIV-infected (n=41) was too small for meaningful analysis and these children are not considered further in this report.
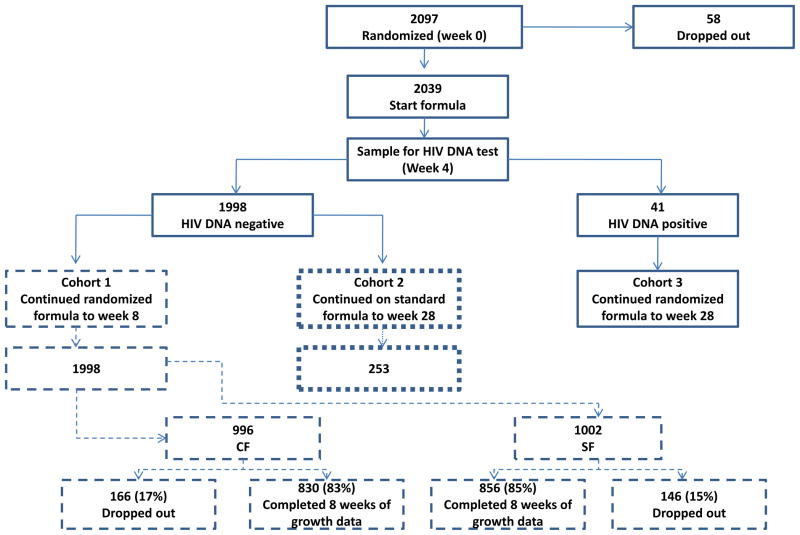
Cohorts for Analysis (Figure 1)
Figure 1.
Schema of study design with numbers of patients enrolled. The number of infected HIV-infants in Cohort 3 was too small for meaningful analysis and so this Cohort is excluded from this report.
Based on the design of the study, two main cohorts of uninfected children are defined:
Cohort 1 (Uninfected, 8 weeks): All infants not determined to be infected who had study formula dispensed. These infants received their randomized formula for a minimum of 8 weeks. Other than those infants in Cohort 2 who were followed for 52 weeks and included in Cohort 1, infants in this cohort were discontinued from the study at week 8. This cohort included infants who were presumed to be uninfected at week 8, as well as infants for whom a determination was not made (for example, because they were lost to follow-up or HIV test information was not available by then). Thus, there is a possibility that a very small proportion of this cohort could in fact be HIV-infected due to missing or false-negative test results (considered likely to be much less than 1% of the total number of subjects in this cohort). There were 1,998 infants in this cohort, including 212 infants enrolled at non-U.S. sites.
Cohort 2 (Uninfected, 52 weeks): Infants not determined to be infected who were followed for 52 weeks. About 12% of infants in Cohort 1 received their randomized formula for 8 weeks, then SF from week 8 to week 28 (except for same birth siblings of an infected infant who continued on their randomized formula), and were followed for 52 weeks. The 253 infants in this cohort, including 24 infants enrolled at non-U.S. sites, were randomly selected.
Evaluation of Growth
Study examiners were instructed regularly at PACTG meetings by anthropometric trainers in use of standard methods, techniques, and equipment for measurement of infant length, weight, and occipito-frontal head circumference. Weight and length were measured at study entry and at 4, 8, 16, 28 and 52 weeks after entry. These measures were converted to age- and gender-adjusted z-scores based upon the 2000 National Center for Health Statistics pediatric growth reference. (6) (7) Gender-adjusted weight-for-length z-scores were obtained using the same growth reference; these excluded a number of infants for whom weight-for-length z-score could not be calculated because the length was below the minimal length of 45 cm for which standards exist.
Statistical Analysis
A modified intention-to-treat approach to analysis was used, including only those infants who were randomized and for whom study formula was dispensed. Growth parameter analyses were specified in the protocol and used standard linear regression to estimate the difference in mean weight or length between the two formulas, adjusted for baseline weight or length, gender, and the two stratification factors used in the randomization (gestational age at birth and site location). Unadjusted comparisons between CF and SF using two-sample t-tests are presented also. Fisher’s exact test was used to evaluate differences in categorical outcomes between the two formula groups. Wilcoxon’s test was used to compare distributions of relative and absolute CD4+ T-lymphocyte counts between the two formula groups.
Results
Characteristics of Infants
Table 2 shows infant baseline characteristics and demonstrates the comparability of the two randomized treatment groups in each cohort. In Cohort 1, 52% of infants were male, median age at randomization was 8 days, and 4% of infants were from multiple births. Overall, 59% were black, non-Hispanic/Latino, 30% were Hispanic/Latino, and 10% were white non-Hispanic/Latino. At U.S. sites, those proportions were 64%, 24%, and 11%. In contrast, all Bahamian infants were black, non-Hispanic/Latino, and 97% of Brazilian infants were Hispanic/Latino. The randomized treatment groups in each cohort were comparable with regard to baseline growth parameters (Table 2b). Mean weight, length, and head circumference (and associated z-scores) in the 1,998 uninfected Cohort 1 infants were 3.14 kg (−0.59), 49.3 cm (−0.66) and 34.3 cm (−0.54). Infants enrolled at U.S. sites had somewhat lower mean weight and length (mean z-score) at entry as those enrolled at non-U.S. sites: 3.15 versus 3.03 kg (−0.56 versus −0.79), 49.4 versus 48.7 cm (−0.63 versus −0.91); but the same mean head circumference (mean z-score) and 34.3 versus 34.3 cm (−0.54 versus −0.54).
Table 2.
| (a) Baseline demographic characteristics of the two cohorts of infants (Cohort 1: All infants not determined to be infected. These infants received their randomized formula for a minimum of 8 weeks. Other than those infants in Cohort 2 who were followed for 52 weeks and included in Cohort 1, infants in Cohort 1 were discontinued from the study at week 8; Cohort 2: Infants determined not to be infected who were followed for 52 weeks) | ||||||
|---|---|---|---|---|---|---|
| Cohort 1(Uninfected, 8 wk) | Cohort 2 (Uninfected, 52 wk) | |||||
|
| ||||||
| Characteristic | Concentrated * (n=996) | Standard ** (n=1002) | All Infants (n=1998) | Concentrated * (n=127) | Standard ** (n=126) | All Infants (n=253) |
| Sex – no. (%) | ||||||
| Males | 528 (53) | 513 (51) | 1041 (52) | 68 (54) | 69 (55) | 137 (54) |
| Females | 468 (47) | 489 (49) | 957 (48) | 59 (46) | 57 (45) | 116 (46) |
| Age-days | ||||||
| Mean ± SD | 7.6 ± 4.2 | 7.3 ± 4.1 | 7.5 ± 4.2 | 7.5 ± 3.9 | 7.0 ± 4.0 | 7.2 ± 3.9 |
| 25th percentile | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Median | 8.0 | 8.0 | 8.0 | 7.0 | 7.0 | 7.0 |
| 75th percentile | 11.0 | 11.0 | 11.0 | 11.0 | 10.0 | 10.0 |
| Race or ethnic group – no. (%) | ||||||
| White, non-Hispanic | 94 (9) | 108 (11) | 202 (10) | 13 (10) | 13 (10) | 26 (10) |
| Black, non-Hispanic | 585 (59) | 592 (59) | 1177 (59) | 68 (54) | 76 (60) | 144 (57) |
| Hispanic | 309 (31) | 283 (28) | 592 (30) | 46 (36) | 34 (27) | 80 (32) |
| Other | 8 (1) | 19 (2) | 27 (1) | 0 (0) | 3 (2) | 3 (1) |
| Country – no. (%) | ||||||
| U.S. | 888 (89) | 898 (90) | 1786 (89) | 112 (88) | 117 (93) | 229 (91) |
| Brazil | 93 (9) | 81 (8) | 174 (9) | 13 (10) | 7 (6) | 20 (8) |
| Bahamas | 15 (2) | 23 (2) | 38 (2) | 2 (2) | 2 (2) | 4 (2) |
| Infants from multiple births-no. (%) | 39 (4) | 36 (4) | 75 (4) | 31 (24) | 32 (25) | 63 (25) |
| (b) Descriptive statistics (mean ± SD) for baseline growth parameters among the two cohorts | ||||||
|---|---|---|---|---|---|---|
| Cohort 1(Uninfected, 8 wk) | Cohort 2 (Uninfected, 52 wk) | |||||
|
| ||||||
| Characteristic | Concentrated* (n=996) | Standard ** (n=1002) | All Infants (n=1998) | Concentrated * (n=127) | Standard ** (n=126) | All Infants (n=253) |
| Weight (kg) | 3.13 ± 0.52 | 3.15 ± 0.54 | 3.14 ± 0.53 | 2.95 ± 0.58 | 3.05 ± 0.55 | 3.00 ± 0.57 |
| Length (cm) | 49.33 ± 2.64 | 49.27 ± 2.67 | 49.30 ± 2.65 | 48.69 ± 3.00 | 48.98 ± 2.87 | 48.84 ± 2.94 |
| Head Circumference (cm) | 34.32 ± 1.58 | 34.33 ± 1.64 | 34.32 ± 1.61 | 33.93 ± 1.88 | 34.24 ± 1.57 | 34.09 ± 1.74 |
| Weight for age/gender Z-Score | −0.61 ± 0.95 | −0.56 ± 1.00 | −0.59 ± 0.98 | −0.93 ± 1.04 | −0.73 ± 0.97 | −0.83 ± 1.01 |
| Length for age/gender Z-Score | −0.64 ± 1.21 | −0.68 ± 1.25 | −0.66 ± 1.23 | −0.91 ± 1.34 | −0.76 ± 1.26 | −0.83 ± 1.30 |
| Head Circumference for age/gender Z-Score | −0.55 ± 0.90 | −0.53 ± 0.93 | −0.54 ± 0.92 | −0.76 ± 1.11 | −0.56 ± 0.85 | −0.67 ± 0.99 |
26 kcal/oz (87 kcal/100 ml)
20 kcal/oz (67 kcal/100 ml)
SD=standard deviation
26 kcal/oz (87 kcal/100 ml)
20 kcal/oz (67 kcal/100 ml)
SD=standard deviation
Completeness of Follow-up
Of the 1,998 Cohort 1 uninfected infants, 830/996 infants (83%) assigned CF and 856/1,002 infants (85%) assigned SF completed 8 weeks of follow-up including growth measurements at 8 weeks, but not all of these infants completed 8 weeks of study formula. Of the remaining 312 Cohort 1 infants (16%), 3 died (0.2%), 141 (7%) did not attend the clinic for growth measurements at 8 weeks but did have safety follow-up to after 8 weeks, and 168 (8%) were lost to follow-up prior to 8 weeks; the percentages were similar for both formulae.
Of the 253 uninfected infants in Cohort 2, 67% completed 52 weeks of follow-up, including 65% and 68% among those randomized to receive CF and SF, respectively, during the first 8 weeks. The remaining 33% did not complete all 52 weeks of follow-up, including 6% who were inadvertently discontinued at 8 weeks and 27% who were lost to follow-up or the parent/guardian withdrew the infant from follow-up.
Intake of Study Formula
Of the 1,998 Cohort 1 uninfected infants, 809 (81%) assigned CF and 818 (82%) assigned SF completed 8 weeks of study formula feeds. A total of 186 (19%) infants assigned CF and 182 infants (18%) assigned SF discontinued study formula prematurely for reasons other than death (P=0.77). Intolerability was the most common reason for discontinuation in both groups (12% for CF versus 11% for SF). The other infants who discontinued study formula early were lost to follow-up or their parent/guardian requested discontinuation for reasons not related to intolerability; the percentages were similar for both formulae.
The mean volume of study formula consumed was recorded in a log of the baby’s diet that was completed by the primary caregiver for the 24-hour period on the day before study visits at weeks 4 and 8. The mean volume was significantly lower for infants assigned CF compared with those assigned SF at both weeks 4 and 8 (762 ml versus 810 ml [25.4 versus 27.0 ounces] for CF versus SF at week 4, P=0.002; 867 ml versus 954 ml [28.9 versus 31.8 ounces] at week 8, P<0.001). These mean volumes correspond to higher mean energy intakes in Cohort 1 for CF versus SF at week 4 (654 versus 540 kcals, P<0.001) and at week 8 (741 versus 636 kcals, P<0.001).
Mortality
Three infants died while taking study formula. Two infants died of sudden infant death syndrome (SIDS), one on each of the study formulas, CF and SF. The cause of death in the other infant who died while taking SF was group B streptococcal infection. All deaths of infants who participated in this and any PACTG study are required to be reported. In addition, deaths that occur after discontinuation of study treatment and formal study follow-up may be reported and the study team reviewed all the reported deaths. In the uninfected cohort, there were five reported deaths among infants assigned CF, compared with three among those assigned SF. No death on or off study was considered by the study team to be related to use of study formula.
Tolerability
To evaluate tolerability, caregivers were asked about the frequency of vomiting, “spit-up”, and colic, as well as the regularity and consistency of stools. Among uninfected infants there were no clinically meaningful differences in measures of tolerability between the two study formulas. Differences between CF and SF with regard to regurgitation, colic, stool frequency, and stool consistency were small and did not consistently favor one formula over the other. For both CF and SF, the median numbers at study weeks 4 and 8 of vomiting episodes per week were zero, spit-up episodes per day one, colic episodes per week zero, and average daily stool frequency two.
Serious Adverse Experience Reports
Reporting of serious adverse experiences (SAEs) as defined according to 21 CFR 312.32 that occurred during the study was required. These were monitored closely by the study team and no concerns were identified. Overall, there were 19 reports affecting 14 infants assigned CF and 15 reports affecting 15 infants assigned SF (P=1.00).
Changes in Mean Growth
Figure 2 shows mean weight (together with associated z-scores) at baseline and study weeks 4 and 8, for the two treatment groups in Cohort 1. Infants assigned CF had significantly higher weight at week 8 than those assigned SF (P=0.16 at week 4 and P=0.010 at week 8; Figure 2a). The difference in mean weight at week 8 was 0.09 kg (95% confidence interval: 0.02 to 0.16 kg). In the protocol-specified primary analysis that included adjustment for baseline weight, gender, site (U.S. versus non-U.S.), and gestational age (<37 versus 37 or more weeks), the difference in mean weight at week 4 and at week 8, while small, was highly significant (P<0.001 for both). At week 8, the adjusted difference reflected a 0.11 kg higher mean weight for CF (95% CI: 0.06 to 0.16 kg). Figure 2b shows corresponding results for weight for age z-scores (WAZ). Mean WAZ at baseline was −0.61 for the CF and −0.56 for the SF group, increasing to 0.05 and −0.07, respectively, at week 8. Thus, the means in the two groups at week 8 are very close to the mean of zero in the population used to generate the growth reference. At week 8, the observed difference in mean WAZ was 0.12 (95% confidence interval: 0.02 to 0.22; P=0.017). In the adjusted analysis, the WAZ difference was 0.15 higher for CF (95% confidence interval: 0.07 to 0.23; P<0.001).
Figure 2.
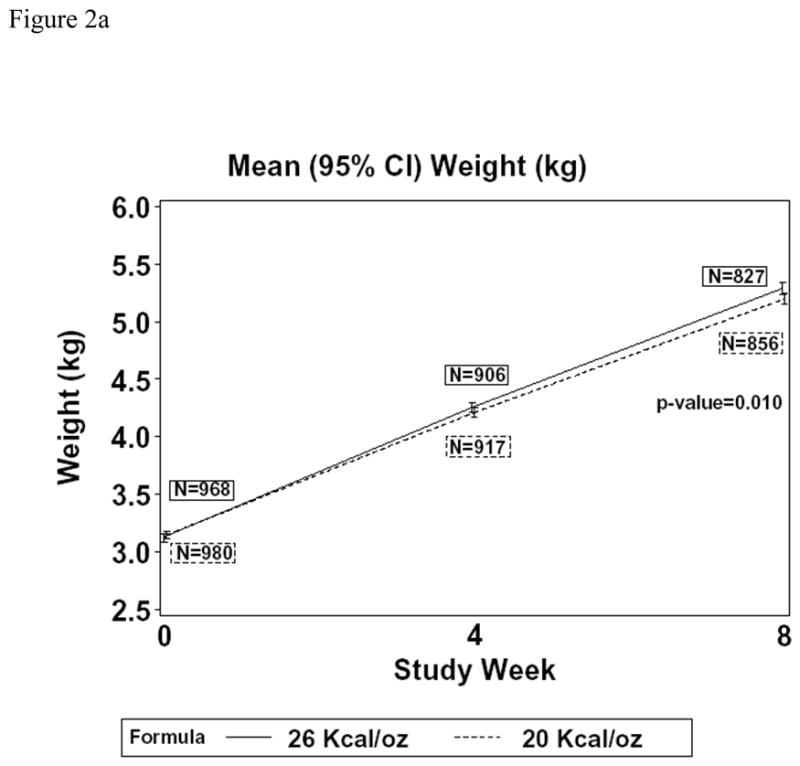
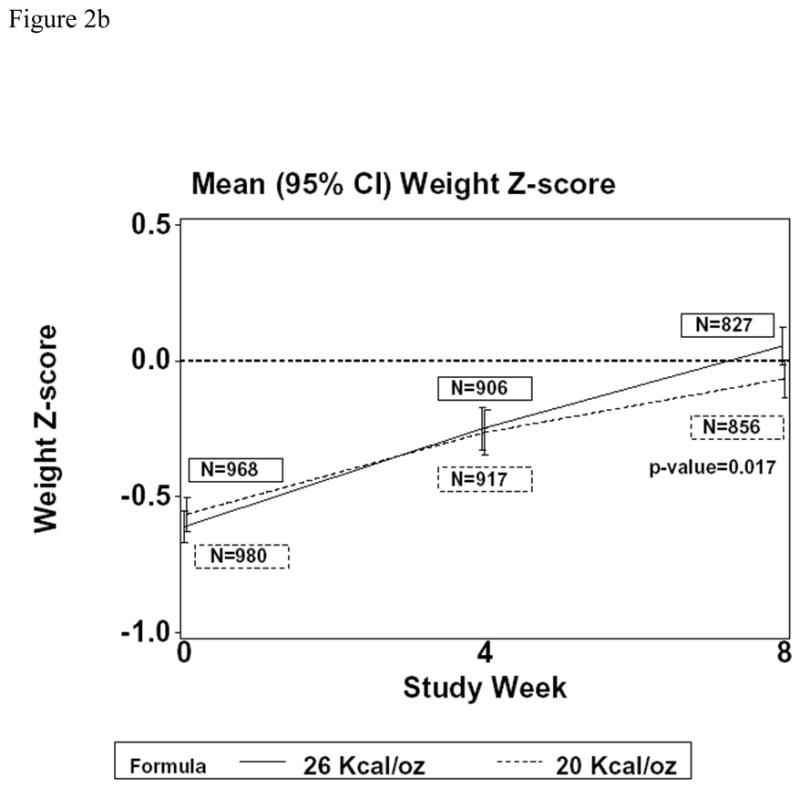
Changes in weight [Figure 2a (kg); Figure 2b (weight for age z-score)] over the first eight weeks in children fed CF or SF
There were no significant differences between study formulas with regard to mean length or mean length for age z-score (LAZ) at week 4 or week 8, in either unadjusted or adjusted analyses. At week 8, the observed difference in mean length was 0.11 cm longer for CF (95% confidence interval: −0.14 to 0.28 cm; P=0.49). Mean LAZ showed no difference in either group between baseline and week 8. At baseline, mean LAZ was −0.64 for CF and −0.68 for SF, and −0.72 and −0.75, respectively, at week 8. The observed difference (CF minus SF) in mean LAZ at week 8 was 0.03 (95% confidence interval: −0.08 to 0.14; P=0.59) and, in adjusted analysis, was 0.01 (95% confidence interval: −0.08 to 0.11; P=0.79). The analysis of weight-for-length z-score (WLZ) did not demonstrate a significant difference between infants who were followed for eight weeks. The mean WLZ at baseline in the cohort on CF was −0.55 compared with −0.46 in infants fed SF. At eight weeks the mean WLZ was 0.51 for the group receiving CF compared with 0.40 for infants fed SF. The observed difference in mean WLZ at week 8 was 0.11 in favor of CF (95% confidence interval: −0.01 to 0.22; P=0.06) and, in adjusted analysis, was 0.15 in favor of CF (95% confidence interval: 0.03 to 0.27; P=0.01).
The results for mean head circumference and associated z-score were very similar to those for length. No significant differences between the two formulas in terms of mean head circumference or mean head circumference z-score (HCZ) were seen at week 4 or week 8 in either unadjusted or adjusted analyses. At week 8, the difference in mean head circumference was 0.00 cm (95% confidence interval: −0.14 to 0.14 cm; P=0.97), and in the adjusted analysis, it was −0.01 cm favoring SF (95% confidence interval: −0.12 to 0.10 cm; P=0.92). Mean HCZ showed no difference in either group between baseline and week 8. At baseline, mean HCZ was −0.55 for CF and −0.53 for SF, and at week 8 was −0.54 and −0.51, respectively. The observed difference in mean HCZ at week 8 was −0.03 in favor of SF (95% confidence interval: −0.12 to 0.07; P=0.58) and, in adjusted analysis, was −0.03 (95% confidence interval: −0.11 to 0.05; P=0.53).
Change in Growth Z-scores Among Children in the Extremes on the Distribution of Growth Parameters
One potential concern in undertaking the study was the possibility of an adverse effect from CF that would lead to a higher proportion of uninfected infants with poor growth or, conversely, a higher proportion with excessive growth compared with those who received SF. This was investigated by comparing the proportions in each treatment group of Cohort 1 infants who had weight, length, or head circumference below the 10th percentile or above the 90th percentile for their age and gender. For all three measures, there was no significant difference between the two formulas in the proportions of infants below the 10th percentile or above the 90th percentile (Table 3). For weight, the proportion below the 10th percentile declined for both CF and SF at week 8. There was a similar increase in the proportion above the 90th percentile for CF and SF at week 8 compared with entry. For length and head circumference, there was little change in the proportion below the 10th percentile or above the 90th percentile at week 8 compared with week 0 for either formula. Similarly, when infants in Cohort 2 were analyzed at 28 weeks, the proportion of infants at the extremes of the percentile distribution (<10th or > 90th percentiles) did not differ between infants fed CF or SF.
Table 3.
Extremes of Growth Distribution by Study Formula at Entry and 4 and 8 weeks
| Proportion of Infants with Growth Z-scores <10th percentile or >90th percentile | |||||||
|---|---|---|---|---|---|---|---|
| Measure and Study Week | N | CF | N | SF | Fisher’s Exact p-value | ||
| <10th percentile N (percent) | >90th percentile N (percent) | <10th percentile N (percent) | >90th percentile N (percent) | ||||
| WAZ | |||||||
| 0 | 968 | 240 (25) | 29 (3) | 980 | 254 (26) | 37 (4) | 0.51 |
| 4 | 906 | 160 (18) | 99 (11) | 917 | 191 (21) | 94 (10) | 0.22 |
| 8 | 827 | 70 (8) | 92 (11) | 856 | 93 (11) | 88 (10) | 0.23 |
| LAZ | |||||||
| 0 | 962 | 267 (28) | 47 (5) | 970 | 279 (29) | 40 (4) | 0.67 |
| 4 | 902 | 340 (38) | 55 (6) | 913 | 346 (38) | 60 (7) | 0.91 |
| 8 | 827 | 246 (30) | 39 (5) | 853 | 263 (31) | 26 (3) | 0.20 |
| HCZ | |||||||
| 0 | 961 | 193 (20) | 23 (2) | 969 | 192 (20) | 30 (3) | 0.65 |
| 4 | 903 | 212 (23) | 65 (7) | 912 | 208 (23) | 68 (7) | 0.93 |
| 8 | 823 | 171 (21) | 21 (3) | 850 | 190 (22) | 29 (3) | 0.40 |
CF=concentrated (26 kcal/oz, 87 kcal/100 ml) formula
SF=standard (20 kcal/oz, 67 kcal/100 ml) formula
WAZ=weight-for-age z-score
LAZ=length-for-age z-score
HCZ=head circumference-for-age z-score
Discussion
Nutritional compromise and malnutrition are major problems faced by HIV-infected children (8) (9), especially those living in sub-Saharan Africa.(10) In Africa, studies have demonstrated that formula feeding decreases maternal-infant transmission of HIV-1(11), but breastfeeding among women infected with HIV-1 is associated with reduced mortality among infants and children.(12) The benefits and the risks of breastfeeding must be considered in each community and the nutrient requirements that provide optimal opportunity for growth should be evaluated. In Europe, where HIV-infected children are generally less socio-economically disadvantaged, The European Collaborative Study reported that uninfected children had normal growth patterns from an early age and that differences in growth velocities between infected and uninfected children increased after two years of age for length and after four years of age for weight.(13) New knowledge is required in different socioeconomic groups to determine whether underweight HIV-infected infants in the first eight weeks of life can tolerate, and benefit from, increased calorie formula feeding.
Endemic, chronic malnutrition results in immune dysregulation, especially in T cell function and may, in the undernourished child, increase the risk for formula intolerance and lower rates of growth. In the HIV-infected child or the child born to an undernourished HIV-infected mother, this secondary insult to the developing immune system exacerbates the impact of HIV disease on immunoregulation and may potentiate immunodeficiency. The malnourished, HIV-infected child is now more susceptible to enteric and opportunistic infections that increase metabolic demands and complete a cycle resulting in progressive wasting disease. (14) Antiretroviral medications and nutritional support are effective at breaking this cycle, and early nutritional intervention may be beneficial.
Fomon hypothesizes that the potential for growth of fat-free mass is genetically determined for each infant. (15) The intake of protein is determined by the energy requirement of the infant, and as the rate of growth declines in infancy, the protein requirement per 100 kcal decreases as well. Protein intake averaging 2.14 g/kg/day in the first eight weeks of life decreases to 1.65 g/kg/day in weeks eight to 16, and seems to be adequate. A protein-energy ratio of 1.85 g/100 kcal is required to provide a protein intake of 2.14 g/kg/day and is appropriate to maintain growth of normal male infants in the first two months of life. Formulas containing lower protein-energy ratios of 1.53 g/100 kcal are not adequate. (16) The protein-energy ratio of both formulas in this study was 2.14 g/100 kcal and, based on the results, was sufficient to enhance growth.
Undernutrition early in life may result in detrimental outcomes later in life including increased mortality from infectious disease, impaired growth, stunting, and failure to attain educational potential. (17) Nutritional supplementation early in life may reduce these undesirable outcomes. (18) (19) Beneficial intergenerational effects on growth over three years were observed by providing protein-energy supplements to mothers living in villages in Guatemala. Offspring of mothers receiving moderate energy supplementation without protein did not grow as well. (20) These observations suggest that improved maternal and/or infant nutrition in the first two years of life results in improved infant growth. In contrast, Morley and colleagues found that feeding small for gestational age infants, especially infant girls, a nutrition enriched formula containing about 20% more protein with respect to energy than standard formula, resulted in significant neurodevelopmental disadvantage at nine months of age. These observations were not seen at 18 months of age, but suggest that caution should be used in providing nutrition enriched formula to small for gestational age infants. (21)
Short-term feeding studies in the newborn period suggest that differences in food consumption may be directly related to the infant’s tendency to limit energy intake. In previous studies, infants less than 16 weeks of age who were fed a formula of 67 kcal/100 ml were compared with infants fed a formula with 133 kcal/100 ml. In male infants during the first six weeks of life, infants receiving the more concentrated formula consumed a smaller volume, more kcals and gained more weight than infants receiving the lower caloric density formula.(22) In a later study, infants were fed 100 kcal/100 ml or 54 kcal/100 ml for 16 weeks. In the first six weeks, those infants receiving the lower caloric density formula ingested 75% of the energy intake of the infants in the 100 kcal/100 ml group. During the next 10 weeks of the study, the infants receiving the less calorically dense formula increased their intake to about 90% of the intake measured in the children receiving 100 kcal/100 ml formula. In these studies, children who drank the less calorically dense formula gained less weight. (23)
In this study, the use of CF formula as an early nutritional intervention strategy in infants born to HIV-infected mothers was evaluated to determine safety, tolerability, and efficacy. Similar formulas can readily be prepared by reconstituting powdered or liquid concentrate infant formula using less water. This nutritional strategy may be relevant in resource limited communities. Discontinuation rates for both the CF and SF were very similar. There were no clinically relevant differences between the two formulas with regard to tolerability.
There was also no evidence of differences in serious adverse experiences, mortality, or CD4+ T-cell levels. There were five reported deaths among infants assigned CF, compared with three among those assigned SF. The 1997 overall U.S. infant mortality rate was 7.2/1,000, 6/1,000 for white children and 14/1,000 for black children. (24) These rates are similar to the approximately 4/1,000 infant mortality rate observed among the uninfected children in this study. There were two infants who died of SIDS, one on each of the study formulas. The 1997 U.S. SIDS infant mortality rate was 77.1/100,000. In our study, the SIDS infant mortality rate was about 1/1,000 (or 100/100,000), similar to the reported U.S. 1997 rate. (24) Thus, there do not appear to be any safety or tolerability issues identified by this study concerning use of 87 kcal/100 ml (26 kcal/oz) formula for eight weeks in uninfected infants born to HIV-infected mothers.
By week 8, uninfected infants assigned either CF or SF had essentially normal mean WAZ compared with the reference population. Moreover, there were significant differences in terms of mean weight, WLZ, and WAZ outcomes at week 8, with higher values among infants assigned CF. Of note, the observed 14–17% increase in energy intake with CF resulted in approximately 5% increase in weight. Although the differences were small, these observations, as one might expect, suggest that uninfected infants born to an HIV-infected mother are able to consume increased kcals and gain weight more rapidly than infants receiving SF.
After eight weeks, there were no significant differences between the two formulas in mean length, WLZ, head circumference, or HCZ. This might be expected, as changes in weight often precede changes in length, and most infants were maintained on CF for only eight weeks. At week 8, the mean length and HCZ were approximately 0.7 and 0.5 standard deviations below the average in the reference population, respectively, for both formulas, whereas at this same time, weight achieved “normal” levels relative to the reference population. Sub-analysis to determine factors that might explain the observed differences will be the subject of a separate report.
Growth is a critical indication of nutritional health in children, especially children with chronic illness. Protein-energy requirements in the first six months of life for children born to HIV-infected mothers are not known. Current recommendations support the use of standard infant formula. The adequacy of breast milk replacement in KwaZulu Natal, South Africa was evaluated by Papathakis and Rollins, who concluded that commercial infant formula meets the nutritional needs of HIV-infected children.(25) In the present study, uninfected infants who were born to HIV-infected mothers and who received CF ingested less volume of formula than those children who received SF. Nevertheless, children receiving CF consumed more kcals than children who were randomized to receive SF. Tolerability was equivalent in both groups, and there was no increase in adverse events in the group receiving CF. These data show that CF fed to infants in the first eight weeks of life can safely provide protein-energy requirements sufficient to maintain and possibly to enhance growth.
Increasing evidence supports a link between nutritional events during gestation and early infancy. During this critical period of development, nutrition may affect neurodevelopment, growth, and body composition, and the subsequent development of chronic diseases such as diabetes mellitus. Lucas used the term “programming” to describe factors in infancy that affect long-term health outcomes.(26) Insulin resistance, type 2 diabetes, and hypertension are associated with low birth weight in infants suspected of having intrauterine growth retardation.(27–31) Development of obesity later in life is directly related to high birth weight.(32, 33) Early postnatal nutrition may impact on subsequent development of insulin resistance and obesity. Accelerated weight gain in infancy may increase the risk of adiposity/obesity later in life.(34, 35) The role of leptin in these observational studies remains to be determined.(36)
At the time a decision is made about nutritional support for an infant born to an HIV-infected mother, the HIV status of the baby may not be determined. This study suggests that concentrated formula is tolerated in potentially HIV-infected children and that weight gain is greater than in children fed standard formula. However, as stated in the World Health Organization UNICEF report in 2004, HIV and Infant Feeding: Framework for Priority Action, Guidelines and Related Tools, “When replacement feeding is acceptable, feasible, affordable, sustainable and safe, avoidance of all breastfeeding is recommended. Otherwise EBF (exclusive breast feeding) is recommended for the first months of life.” (37) This study was not designed to follow long term growth of these infants, but future studies evaluating the effects of early nutritional intervention should consider these factors. The long term deleterious effects on intelligence of under-nutrition in the first year of life are well known, but the effects of over-nutrition in the first year of life are not as well established. Future studies of early nutritional intervention in HIV-infected children should include careful monitoring of outcomes related to under- as well as to overnutrition. The observations in this study suggest that in HIV-exposed, uninfected infants, concentrated formula feeding in the first eight weeks of life results in increased weight gain. Providing CF early in life may be beneficial to infants at risk for undernutrition. Though we are unable to determine from this study whether increased growth can be sustained by early nutritional intervention, CF appears to be well tolerated in this age group.
Conclusion
The findings in this large randomized controlled study show that early weight gain is enhanced in HIV-exposed infants given a concentrated formula for eight weeks, with no differences in adverse outcomes compared with standard formula. These observations are relevant for healthcare providers as well as agencies that fund programs supporting nutrition of HIV-infected children in resource-poor environments. Children who are gaining weight well would not require such intervention, but this study does show that concentrated formula can be safe and can benefit those infants in need of additional caloric support. Caution should be used in extrapolating these data to small for gestational age infants because of the potential for accelerated weight gain and obesity.
Acknowledgments
The authors would like to express their gratitude to all the children and their families who participated in this study, and acknowledge the contributions and support of the entire protocol 247 team.
The following sites and individuals of the PACTG contributed to this study:
Princess Margaret Hospital, Bahamas - P. Gomez, P. McNeil, M. Jervis; Federal University of Rio de Janeiro -R. Oliveira, M. Chermont; Hospital dos Servidores do Estado, Rio de Janeiro - E. Custodio Joao, J. Menezes; Universidade Federal de Minas Gerais, Belo Horizonte - J. Pinto, F. Kakehasi; Universidade de Sao Paulo Hospital das Clinicas da Faculdade de Medicina de Ribeirao Preto - M. Mussi-Pinhata, M. de Lima Issac; Instituto de Infectologia Emilio Ribas, Sao Paulo - M. Della Negra, W. Queiroz; Univ. of Med. & Dentistry of NJ - P. Palumbo, L. Bettica; St. Joseph’s Hospital and Medical Center - N. Hutcheon; Cooper Hospital - University Medical Center - A. Feingold; Children’s Hospital of Boston - K. McIntosh, K. Bertelsen; Boston Medical Center - S. Pelton; UCLA Medical Center – Y. Bryson; Cedars-Sinai Medical Center, Los Angeles - P. Brunell; Long Beach Memorial Med. Center - A. Deveikis; Harbor-UCLA Medical Center - M. Keller, K. Zangwill, J. Hayes, A. Gagajena; Johns Hopkins University, Baltimore - A. Ruff, S. Marvin; University of Maryland - P. Vink, J. Farley, M. MacFadden; Texas Children’s Hospital, Houston - W. Shearer, K. Owl, M. Dobmeier, M. Paul, C. Hanson; Chicago Children’s Memorial Hospital - R. Yogev; The University of Chicago Children’s Hospital - J. Englund; The Columbia Presbyterian Medical Center, New York City - A. Gershon, A. Higgins, M. Foca; University of Miami - G. Scott, C. Goldberg, M. Bissainthe, C. Mitchell; Jackson Memorial Hospital, Miami - M. J. O’Sullivan; New York University Bellevue Hospital - W. Borkowsky, T. Hastings, M. Mintor, N. Deygoo; UCSD Medical Center, San Diego - S. Spector; Phoenix Children’s Hospital - J. Piatt; Duke University Medical Center, North Carolina - R. McKinney, Jr., Y. Choi, L. Ferguson, J. Swetnam; University of North Carolina at Chapel Hill - W. Lim; Metropolitan Hospital Center, New York City - M. Bamji, I. Pathak, S. Manwani; Harlem Hospital, New York City - E. Abrams, D. Calo, M. Fere, S. Champion; King’s County Hospital, Brooklyn NY- H. Mendez, D. Swindell; North Shore University Hospital, NY - S. Pahwa; Cornell University, NY - R. Johan-Liang; University of Illinois College of Medicine, Chicago - K. Rich, K. Hayani, J. Camacho; Emory University Hospital, Atlanta - S. Nesheim; San Juan City Hospital, Puerto Rico - E. Jimenez, M. Acevedo, M. Gonzales, C. Martinez Betancoult, F. Pabon; Robert Wood Johnson Medical School, NJ - S. Gaur; St. Peter’s Hospital, NJ - M. Lake; Ramon Ruiz Arnau University Hospital, Puerto Rico - W. Figueroa; Medical University of South Carolina -G. Johnson; Yale University School of Medicine, New Haven - W. Andiman, D. Schroeder, S. Romano, M.J. Aquino-de Jesus; SUNY Upstate Medical University, Syracuse - C. Cunningham; State University of New York at Stony Brook - S. Nachman, M. Davi, C. Seifert, S. Muniz; Children’s Hospital of Michigan, Detroit - E. Moore; Children’s Hospital at Albany Medical Center - N. Wade; Children’s Medical Center of Dallas - J. Squires; Howard University Hospital, Washington DC - S. Rana, P. Yu, S. Dangol, J. Roa; Los Angeles County Medical Center/USC - A. Kovacs, J. Homans, Y. Rodriquez; University of Florida Health Science Center, Jacksonville - M. Rathore; Children’s Hospital, Univ. Colorado Health Sciences Center, Denver - M. Levin, E. McFarland, C. Salbenblatt, E. Barr; Columbus Children’s Hospital, Ohio - M. Brady; North Broward Hospital District, FL - S. Widmayer; University of Florida, Gainesville -J. Sleasman, R. Lawrence, C. Delany; University of Rochester Medical Center, NY - F. Gigliotti; University of Mississippi Medical Center - H. Gay; Medical College of Virginia - S. Lavoie; Palm Beach County Health Department, FL - G. Stiebel-Chin; Washington University School of Medicine, St. Louis - K. McGann; University of South Florida - P. Emmanuel; Arnold Palmer Hospital for Children and Women - C. Lamprecht; University of Texas Health Science Center, Galveston - T. Doran; University of South Carolina School of Medicine - T. Reid; Ponce University Hospital, Puerto Rico -R. Delgado-Ayala; Caguas Regional Hospital, Puerto Rico - I. Rivera-Matos; Mayaguez Clinical Center, Puerto Rico - I. Esquilin; Univ. of Puerto Rico, San Juan - I. Febo Rodriquez, L. Lugo, I. Heyer, C. Martinez; Seattle Children’s Hospital & Medical Center - L. Frenkel; Bronx Lebanon Hospital Center, New York City - R. Kairam; Children’s Hospital, Washington, D.C. - J. Sever; Tulane Univ., Charity Hospital of New Orleans -R. Van Dyke; Baystate Medical Center, Springfield MA - B. Stechenberg; Connecticut Children’s Medical Center - J. Salazar; University of Alabama at Birmingham - R. Pass; Sacred Heart Children’s/Cms of Florida - R. Pass; Medical College of Georgia - W. Foshee, C. Mani, C. White, B. Kiernan; University of South Alabama - M. Mancao; The Medical Center of Columbia, Georgia - R. Stauffer.
This work was supported by the Pediatric AIDS Clinical Trials Group (PACTG) of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, the Pediatric/Perinatal HIV Clinical Trials Network of the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, the Statistical and Data Management Center of the PACTG (NIAID cooperative agreement AI-41110), Ross Products Division of Abbott Laboratories, and NICHD contracts number N01-HD-3-3162 and N01-HD-3-3345.
References
- 1. [Accessed 8 June 2008];UNAIDS AIDS epidemic update. 2006 Dec; Available at: http://www.unaids.org/en/HIV_data/epi2006.
- 2.Johnson RP. How HIV Guts the Immune System. New Engl J Med. 2008;358(21):2287–9. doi: 10.1056/NEJMcibr0802134. [DOI] [PubMed] [Google Scholar]
- 3.Iroha EO, Ezeaka VC, Akinsulie AO, Temiye EO, Adetifa IM. Maternal HIV infection and intrauterine growth: a prospective study in Lagos, Nigeria. West Afr J Med. 2007;26:121–5. [PubMed] [Google Scholar]
- 4.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. OPRR Reports. Office of Protection from Research Risks; Washington, D.C: Apr 18, 1986. The Belmont report: ethical principles and guidelines for the protection of human subjects of research. 1979. [PubMed] [Google Scholar]
- 5.Code of Federal Regulations. Protection of human subjects. Office of Protection from Research Risks; Washington, D.C: 1992. Title 45 CFR Part 46, Protection of Human Subjects, revised June 18, 1991. OPRR Reports. [Google Scholar]
- 6.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. 2000. [PubMed] [Google Scholar]
- 7.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Jirapinyo P, Brewster D, Succi RC, Guarino A, Heyman M, Winter H, et al. HIV disease: Working Group Report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2002;35 (Suppl 2):S134–42. doi: 10.1097/00005176-200208002-00011. [DOI] [PubMed] [Google Scholar]
- 9.Wittenberg D, Benitez CV, Canani RB, Hadigan C, Perin NM, Rabinowitz S, et al. HIV Infection: Working Group Report of the Second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 (Suppl 2):S640–6. doi: 10.1097/00005176-200406002-00010. [DOI] [PubMed] [Google Scholar]
- 10.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005;21(1):96–9. doi: 10.1016/j.nut.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Magoni M, Bassani L, Okong P, Kituuka P, Germinario EP, Giuliano M, et al. Mode of infant feeding and HIV infection in children in a program for prevention of mother-to-child transmission in Uganda. Aids. 2005;19(4):433–7. doi: 10.1097/01.aids.0000161773.29029.c0. [DOI] [PubMed] [Google Scholar]
- 12.Taha TE, Kumwenda NI, Hoover DR, Kafulafula G, Fiscus SA, Nkhoma C, Chen S, Broadhead RL. The impact of breastfeeding on the health of HIV-positive mothers and their children in sub-Saharan Africa. Bull World Health Organ. 2006;84(7):546–54. doi: 10.2471/blt.05.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The European Collaborative Study. Height, Weight, and Growth in Children Born to Mothers With HIV-1 Infection in Europe. Pediatrics. 2003;111:52–60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- 14.Miller TL, Evans SJ, Orav EJ, Morris V, McIntosh K, Winter HS. Growth and body composition in children infected with the human immunodeficiency virus-1. Am J Clin Nutr. 1993;57(4):588–92. doi: 10.1093/ajcn/57.4.588. [DOI] [PubMed] [Google Scholar]
- 15.Fomon SJ. Assessment of growth of formula-fed infants: evolutionary considerations. Pediatrics. 2004;113(2):389–93. doi: 10.1542/peds.113.2.389. [DOI] [PubMed] [Google Scholar]
- 16.Fomon SJ, Ziegler EE, Nelson SE, Rogers RR, Frantz JA. Infant formula with protein-energy ratio of 1. 7 g/100 kcal is adequate but may not be safe. J Pediatr Gastroenterol Nutr. 1999;28(5):495–501. doi: 10.1097/00005176-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Brown JL, Pollitt E. Malnutrition, poverty and intellectual development. Sci Am. 1996;274(2):38–43. doi: 10.1038/scientificamerican0296-38. [DOI] [PubMed] [Google Scholar]
- 18.Rivera JA, Martorell R, Ruel MT, Habicht JP, Haas JD. Nutritional supplementation during the preschool years influences body size and composition of Guatemalan adolescents. J Nutr. 1995;125(4 Suppl):1068S–1077S. doi: 10.1093/jn/125.suppl_4.1068S. [DOI] [PubMed] [Google Scholar]
- 19.Pollitt E, Gorman KS, Engle PL, Martorell R, Rivera J. Early supplementary feeding and cognition: effects over two decades. Monogr Soc Res Child Dev. 1993;58(7):1–99. discussion 111–8. [PubMed] [Google Scholar]
- 20.Stein AD, Barnhart HX, Hickey M, Ramakrishnan U, Schroeder DG, Martorell R. Prospective study of protein-energy supplementation early in life and of growth in the subsequent generation in Guatemala. Am J Clin Nutr. 2003;78(1):162–7. doi: 10.1093/ajcn/78.1.162. [DOI] [PubMed] [Google Scholar]
- 21.Morley R, Fewtrell MS, Abbott RA, Stephenson T, MacFadyen U, Lucas A. Neurodevelopment in children born small for gestational age: a randomized trial of nutrient-enriched versus standard formula and comparison with a reference breastfed group. Pediatrics. 2004;113(3 Pt 1):515–21. doi: 10.1542/peds.113.3.515. [DOI] [PubMed] [Google Scholar]
- 22.Fomon SJ, Filer LJ, Jr, Thomas LN, Rogers RR, Proksch AM. Relationship between Formula Concentration and Rate of Growth of Normal Infants. J Nutr. 1969;98(2):241–54. doi: 10.1093/jn/98.2.241. [DOI] [PubMed] [Google Scholar]
- 23.Fomon SJ, Filer LJ, Thomas LN, Anderson TA, Nelson SE. Influence of formula concentration on caloric intake and growth of normal infants. Acta Pediatr Scand. 1975;64:172–81. doi: 10.1111/j.1651-2227.1975.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald MF, Arkinson JO. Infant Mortality Statistics from the 1997 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 1999;47(23):1–23. [PubMed] [Google Scholar]
- 25.Papathakis PC, Rollins NC. Are WHO/UNAIDS/UNICEF-recommended replacement milks for infants of HIV-infected mothers appropriate in the South African context? Bull World Health Organ. 2004;82(3):164–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004;363(9421):1642–5. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 27.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–50. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 28.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94(6):1310–5. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 29.McKeigue PM, Lithell HO, Leon DA. Glucose tolerance and resistance to insulin-stimulated glucose uptake in men aged 70 years in relation to size at birth. Diabetologia. 1998;41(10):1133–8. doi: 10.1007/s001250051042. [DOI] [PubMed] [Google Scholar]
- 30.Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ. 1996;312(7028):406–10. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. Brit Med J. 1991;303(6809):1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23 (Suppl 8):S1–107. [PubMed] [Google Scholar]
- 33.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. Brit Med J. 2001;323(7325):1331–5. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Brit Med J. 2000;320(7240):967–71. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109(2):194–9. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 36.Singhal A, Farooqi IS, O’Rahilly S, Cole TJ, Fewtrell M, Lucas A. Early nutrition and leptin concentrations in later life. Am J Clin Nutr. 2002;75(6):993–9. doi: 10.1093/ajcn/75.6.993. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. [Accessed August 22, 2008];HIV and Infant Feeding: Framework for Priority Action, Guidelines and Related Tools. 2004 http://www.who.int/child_adolescent_health/documents/pdfs/hiv_if_slide_set.pdf.