Abstract
Fatigue induced via a maximal isometric contraction of a single-limb muscle group can evoke a “cross-over” of fatigue that reduces voluntary muscle activation and maximum isometric force in the rested contralateral homologous muscle group. We asked whether a cross-over of fatigue also occurs when fatigue is induced via high-intensity endurance exercise involving a substantial muscle mass. Specifically, we used high-intensity single-leg cycling to induce fatigue and evaluated associated effects on maximum cycling power (Pmax) in the fatigued ipsilateral leg (FATleg) as well as the rested contralateral leg (RESTleg). On separate days, 12 trained cyclists performed right leg Pmax trials before and again 30s, 3, 5, and 10min after a cycling time trial (TT, 10min) performed either with their right or left leg. Fatigue was estimated by comparing exercise-induced changes in Pmax and maximum handgrip isometric force (Fmax). Mean power produced during the right and left leg TT’s did not differ (203±8 vs. 199±8W). Compared to pre-TT, FATleg Pmax was reduced by 22±3% at 30s post-TT and remained reduced by 9±2% at 5min post-TT (both P<0.05). Despite considerable power loss in the FATleg, post-TT RESTleg Pmax (596–603W) did not differ from pre-TT values (596±35W). There were no alterations in handgrip Fmax (529–547N). Our data suggest that any potential cross-over of fatigue, if present at all, was not sufficient to measurably compromise RESTleg Pmax in trained cyclists. These results along with the lack of changes in handgrip Fmax indicate that impairments in maximal voluntary neuromuscular function were specific to working muscles.
Keywords: Muscle fatigue, central fatigue, neuromuscular function, contralateral limb
Introduction
Exercise-induced fatigue is defined as a reversible reduction in the force- and power-generating ability of the neuromuscular system (Fitts and Holloszy 1976; Bigland-Ritchie et al. 1983) and can manifest through central and/or peripheral mechanisms. Specifically, central fatigue results in a failure of the central nervous system to excite and drive motorneurons (Gandevia 2001) whereas peripheral fatigue results in a failure of the muscle to respond to neural excitation (Allen et al. 2008). The development of central fatigue, as estimated by changes in voluntary muscle activation assessed via superimposed motor nerve and/or motor cortex/corticospinal tract stimulations, is traditionally evaluated during a maximal isometric contraction of the exercising muscle group (Merton 1954). Similarly, exercise-induced peripheral muscle fatigue is frequently quantified via the pre- to post-exercise change in force output in response to direct electric/magnetic motor nerve stimulation. Together, assessments of central and peripheral fatigue in the exercised muscle group can provide insight into associated changes in voluntary neuromuscular function.
Some previous authors (Martin and Rattey 2007; Rattey et al. 2006; Todd et al. 2003) have induced fatigue in a single limb muscle group and reported reductions in voluntary muscle activation in the rested contralateral homologous muscle group. For example, Martin and colleagues (Martin and Rattey 2007) reported that sustained maximal isometric knee extension exercise in one leg reduced voluntary muscle activation of rested contralateral knee extensors by 9%. Because direct electrical stimulation of the contralateral knee-extensors revealed no peripheral muscle fatigue, these results suggest a “cross-over” of central fatigue from a fatigued limb muscle group to the rested contralateral homologous muscle group. It is possible that metabo- and mechanosensitive group III/IV muscle afferents originating in the exercising muscle group could have contributed, amongst others, to this cross-over of central fatigue by exerting inhibitory influences on central motor drive also to the rested contralateral muscle group (Amann 2011; Gandevia 2001).
Interestingly, this fairly small cross-over of central fatigue was associated with a significant reduction in maximum voluntary isometric force of the rested contralateral muscle group (Martin and Rattey 2007). However, not all investigations confirm this functional consequence (Rattey et al. 2006; Todd et al. 2003). This discrepancy could be explained by the fact that a small cross-over of central fatigue might not be sufficient to measurably impair the functional capacity of the rested contralateral muscle group when fatigue is induced via a maximal isometric contraction of a single muscle group. Furthermore, it is unknown if a cross-over of fatigue (possibly central in origin) also occurs when fatigue is induced via high-intensity endurance exercise involving a substantial muscle mass and whether it would have a more pronounced functional consequence for the rested contralateral muscle groups as compared to those observed following a maximal isometric contraction of only a single muscle group.
During locomotor exercise, potential cross-over effects of fatigue could be delineated by using a single-leg cycling model. That is, high-intensity single-leg cycling (Abbiss et al. 2011; Bundle et al. 2006) could be used to induce fatigue in the working leg. Subsequent evaluation of maximum cycling power in the fatigued ipsilateral leg and rested contralateral leg would offer a paradigm for examining the functional impact of exercise-induced fatigue as well as potential cross-over effects of fatigue. For example, in this model if maximum power was reduced in the rested contralateral leg, this would indicate a cross-over effect of fatigue. Conversely, if maximum power was maintained in the rested contralateral leg, this would indicate that fatigue was specific to the exercised muscles of the ipsilateral leg.
Our purpose for conducting this study was to examine the effects of voluntary muscle fatigue in one leg on maximum power and determine whether fatigue crossed-over to the rested contralateral leg. Specifically, we induced fatigue via exhaustive high-intensity single-leg cycling and evaluated associated effects on maximum cycling power in the fatigued ipsilateral leg as well as the rested contralateral leg. We hypothesized that exercise-induced fatigue would result in a cross-over effect, impairing maximum cycling power in the rested contralateral leg.
Methods
Participants
Twelve endurance trained male cyclists (age: 26 ± 4 yr; body mass: 78 ± 9 kg; height: 1.83 ± 0.06 m; maximal oxygen consumption (VO2max): 61 ± 7 ml·kg−1 ·min−1) volunteered to participate in this investigation. Participants had regularly trained in cycling for 6 ± 3 yr. At the time of study, participants were training 11 ± 3 h·wk−1 and competing in local road cycling, triathlon, and/or mountain bike racing events. Experimental procedures were reviewed by the University of Utah Institutional Review Board and all participants provided written informed consent prior to testing.
Experimental Protocol
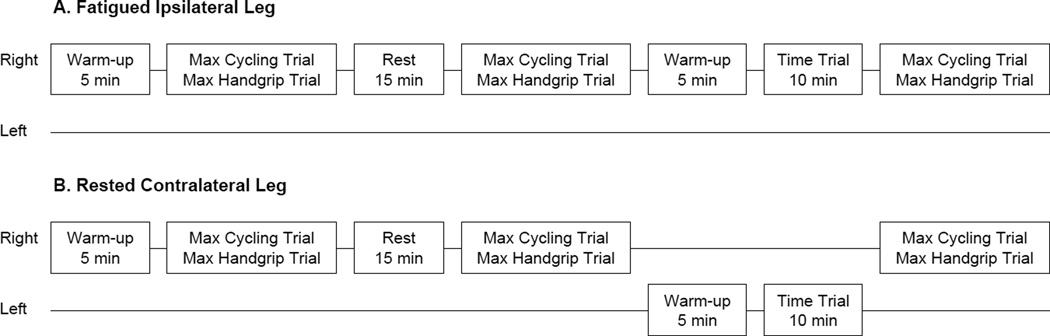
During the first week, participants performed familiarization trials of maximal single-leg cycling and maximal isometric handgrip trials. Participants also performed practice intervals of submaximal single-leg cycling and an incremental double-leg cycling test (Amann et al. 2004) for determination of VO2max. During the second, experimental week, participants reported to the laboratory on two separate days to perform: 1) fatigued ipsilateral leg or 2) rested contralateral leg cycling protocol, which are described below and also illustrated in Figure 1. The fatigued ispilateral leg and rested contralateral leg cycling protocols were performed in a counter-balanced order. Experimental visits were separated by at least 48 h and were completed at the same time of day. Participants were instructed to avoid vigorous exercise 24 h before each experimental visit.
Figure 1.
Schematic illustration of the fatigued ipsilateral (A) and rested contralateral (B) leg protocols. Protocols were administered in a counter balanced fashion. Maximal cycling and isometric handgrip trials were performed at 30 s, 3 min, 5 min, and 10 min post-TT.
For the fatigued ipsilateral leg cycling protocol participants performed a five min single-leg cycling warm-up with their right leg followed by a maximal single-leg cycling trial (~4.5 s) with their right leg. Subsequently, participants rested for 15 min and then again performed a maximal single-leg cycling trial with their right leg. Participants then performed a five min single-leg cycling warm-up with their right leg followed by a 10 min maximal effort single-leg cycling time trial (TT) with their right leg. Within 30 s after the TT, participants performed a maximal single-leg cycling trial with their right leg. Maximal cycling trials were also performed at 3, 5, and 10 min post-TT with the right leg. Immediately following each pre- and post-TT maximal cycling trial, participants performed a maximal isometric handgrip trial with their right arm. For the rested contralateral leg cycling protocol, participants repeated the protocol described above with the only difference being that the single-leg cycling TT was performed with the left leg.
Maximal Single-leg Cycling
Participants performed maximal single-leg cycling trials (~4.5 s) with their right leg on an inertial-load cycle ergometer (Martin et al. 1997). Participants were instructed to remain seated throughout each trial and were given standardized verbal encouragement. The ergometer was fitted with racing handlebars, cranks, and saddle, and fixed to the floor and participants wore cycling shoes that locked onto the pedal (Speedplay Inc., San Diego, CA, USA). A 97 N counterweight was attached to the contralateral ergometer crank to facilitate smooth single-leg cycling (Elmer et al. 2010) and the foot of the non-exercising leg rested on a stabilization platform. Inertial-load method determines maximal power across a range of pedaling rates (e.g., 60–180 rpm) in a single brief trial. These methods have been previously described by Martin and colleagues (1997). Briefly, participants began each trial from rest and accelerated maximally for eight pedal revolutions with resistance provided solely by the moment of inertia of the flywheel. Angular position data were low pass filtered at 8 Hz using a 5th order spline (Woltring 1986) and angular velocity and acceleration were determined from the spline coefficients. Torque averaged over each complete crank revolution was calculated as the rate of change in angular momentum. For each trial, torque-pedaling rate relationship was determined and linear extrapolation was performed to obtain values for maximum torque (i.e., isometric) and maximum pedaling rate. Power averaged over each complete crank revolution was calculated as rate of change in kinetic energy and maximum power was identified as the highest value (averaged over a complete revolution of the crank) during each trial. For each trial, the power-pedaling rate relationship was determined and the optimal pedaling rate that elicited maximum power was also identified.
Single-leg Cycling Time Trial
Participants performed a self-paced maximal effort single-leg cycling TT (10 min) with their right or left leg on a friction-braked cycle ergometer (Monark Exercise AB, Vansbro, Sweden). Before the TT, participants were instructed to cycle as “hard as you can go” in order to produce the greatest amount of average power for the trial and were given standardized verbal encouragement throughout the TT. Participants were also instructed to maintain a similar pedaling rate for each TT (e.g., 90 rpm). A fan was placed near the participants to provide cooling. The ergometer was fitted with racing handlebars, cranks, and saddle, and fixed to the floor and participants wore cycling shoes that locked onto the pedal (Speedplay Inc., San Diego, CA, USA). As described above a counterweight was attached to the contralateral ergometer crank to facilitate smooth single-leg cycling. Mean cycling power was quantified using a power meter (Schoberer Rad Messtechnik, SRM, Jülich, Germany), a system that has previously been shown to accurately quantify power output (Abbiss et al. 2009). During the final 30 s of the TT overall rating of perceived exertion (RPEoverall) and specific leg perceived exertion (RPElegs) were assessed using a Borg 6–20 scale (Borg 1970). Heart rate (Polar CS300, Kempele, Finland) was also assessed during the final 30 s of the TT. Whole blood lactate (ARKRAY Lactate Pro LT-1710, Kyoto, Japan) was collected from the finger at 90 s post-TT.
Maximal Isometric Handgrip
Participants performed a maximal isometric hand grip trial (3 s) with their right hand using a hydraulic handgrip dynamometer (Smith & Nephew Rehabilitation, Memphis, TN, USA). Participants were instructed to squeeze the device with maximal effort while maintaining a 90° elbow angle. Standardized verbal encouragement was provided during each trial.
Quantification of Fatigue
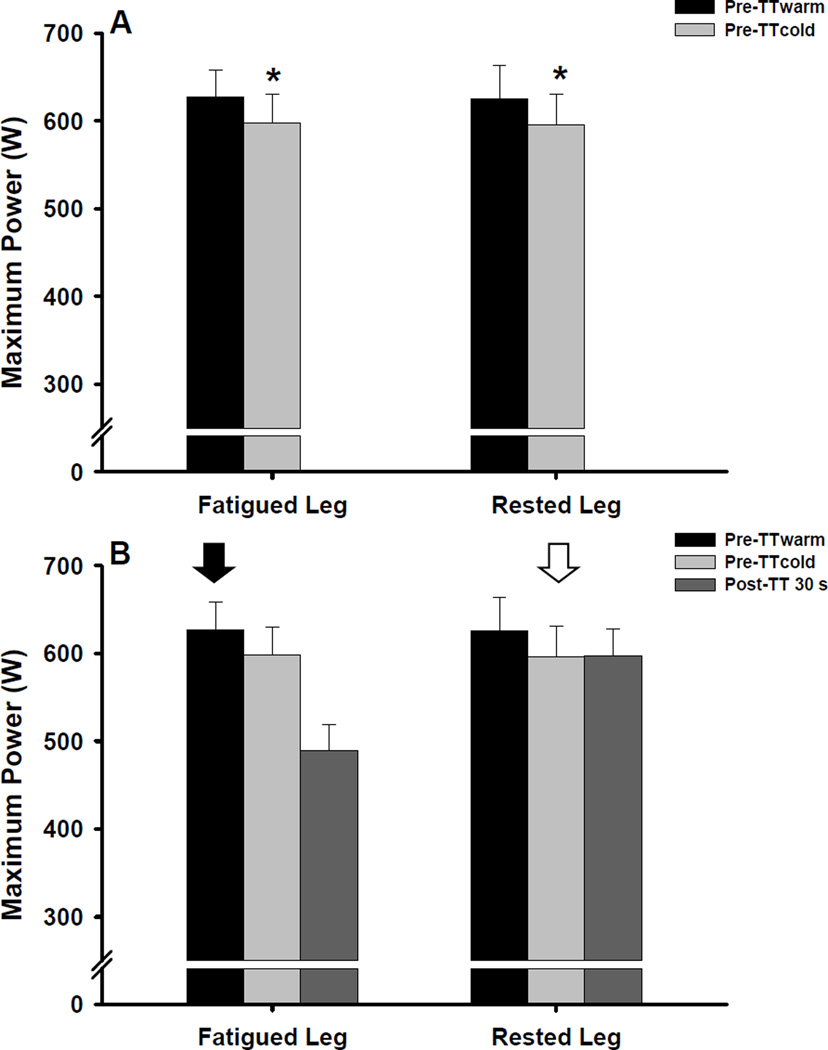
To quantify exercise-induced fatigue, we compared pre- to post-TT changes in maximum cycling power of the fatigued ipsilateral leg. To evaluate potential cross-over effects of fatigue, we compared pre- to post-TT changes in maximum cycling power of the rested contralateral leg. It is important to note that the rested contralateral leg was passive during the TT and was likely not warmed-up prior to the post-TT maximal cycling trials. Thus, potential reductions in maximum cycling power of the rested contralateral leg might be influenced by the warm-up protocol and associated changes in muscle temperature (Sargeant 1987). Therefore, to account for possible warm-up and temperature-related effects, we included a pre-TT maximal cycling trial that was preceded by a 5 min warm-up (pre-TTwarm) and an additional pre-TT maximal cycling trial that was preceded by 15 min of rest (i.e., no warm-up, pre-TTcold). If maximum power was lower when performed without a warm-up, pre-TTcold trial was used for the pre- to post-TT comparison for the rested contralateral leg only. Finally, we also evaluated pre- to post-TT changes in maximum isometric handgrip force to determine if exercised-induced fatigue altered neuromuscular function in rested muscles of the upper limb.
Data Analysis
A two-way repeated measures analysis of variance (ANOVA) was performed to assess differences in maximum cycling power produced by the fatigued ipsilateral and rested contralateral legs between pre-TTwarm and pre-TTcold maximal cycling trials. Separate student’s paired t-tests were used to assess differences in power, heart rate, lactate, RPEbody, and RPElegs between the right (fatigued ispilateral leg cycling protocol) and left (rested contralateral leg cycling protocol) leg TT’s. Separate two-way repeated measures ANOVA procedures were used to compare both absolute and relative pre- to post-TT changes in dependent variables (maximum cycling power, extrapolated maximum isometric torque, optimal pedaling rate, extrapolated maximum pedaling rate, and maximum isometric handgrip force). If the ANOVA procedures revealed significant main effects or significant interactions, then subsequent post hoc procedures (Fisher least significant differences) were performed to determine where those differences occurred. Data are presented as mean ± standard error of the mean (SEM) and initial alpha was set to 0.05.
Results
Warm-up Effects
The repeated measures ANOVA revealed a significant effect of cycling trial (P < 0.001) on pre-TT maximum cycling power values, indicating that power was lower during pre-TTcold compared to pre-TTwarm (Figure 2). Pre-TT maximal cycling power values did not differ between the fatigued ipsilateral and rested contralateral legs (P = 0.83) and the leg × cycling trial interaction was also not significant (P = 0.99). Subsequent post hoc analyses used to assess for simple main effects indicated that maximum cycling power produced by the fatigued ipsilateral and rested contralateral legs was reduced by 5 ± 1% and 4 ± 1%, respectively, during pre-TTcold compared to pre-TTwarm (both P < 0.01, Figure 2). Based on these warm-up effects, pre-TTcold values were used for all subsequent pre- to post-TT comparisons for the rested contralateral leg only. Conversely, pre-TTwarm values were used for the pre- to post-TT comparisons for fatigued ipsilateral leg.
Figure 2.
Effect of a warm-up (A) on pre-TT maximum cycling power (mean ± SEM). Pre-TTwarm represents the maximal cycling trial that was preceded by a 5 min cycling warm-up whereas pre-TTcold represents the maximal cycling trial that was preceded by 15 min of rest (i.e., no warm-up). *P < 0.01 vs. pre-TTwarm. Quantification of exercise-induced fatigue and potential cross-over fatigue (B). Arrow indicates pre-TT maximal cycling trial used for the pre- to post-TT comparison for the fatigued ipsilateral (solid arrow) and rested contralateral (empty arrow) leg.
Time Trial Performance
Mean power, heart rate, blood lactate, and RPE assessed during the right (fatigued ispilateral leg cycling protocol) and left (rested contralateral leg cycling protocol) leg TT’s did not differ (all P > 0.05, Table 1).
Table 1.
Physiological responses to the 10 min single-leg cycling time trial (TT)
| Right Leg | Left Leg | |
|---|---|---|
| Power (W)a | 203 ± 8 | 199 ± 8 |
| HR (beats·min−1)b | 177 ± 3 | 175 ± 4 |
| RPEoverallb | 18.3 ± 0.5 | 17.7 ± 0.6 |
| RPElegsb | 19.6 ± 0.1 | 19.3 ± 0.2 |
| Lactate (mmol·L−1)c | 11.2 ± 0.6 | 11.5 ± 0.5 |
Values are reported as Mean ± SEM. Note that the right leg TT was part of the fatigued ipsilateral leg protocol and that the left leg TT was part of the rested contralateral leg protocol.
Averaged over 10 min
Assessed during final 30 s
Assessed at 90 s post-TT
Pre- to-post Time Trial Changes in Neuromuscular Function
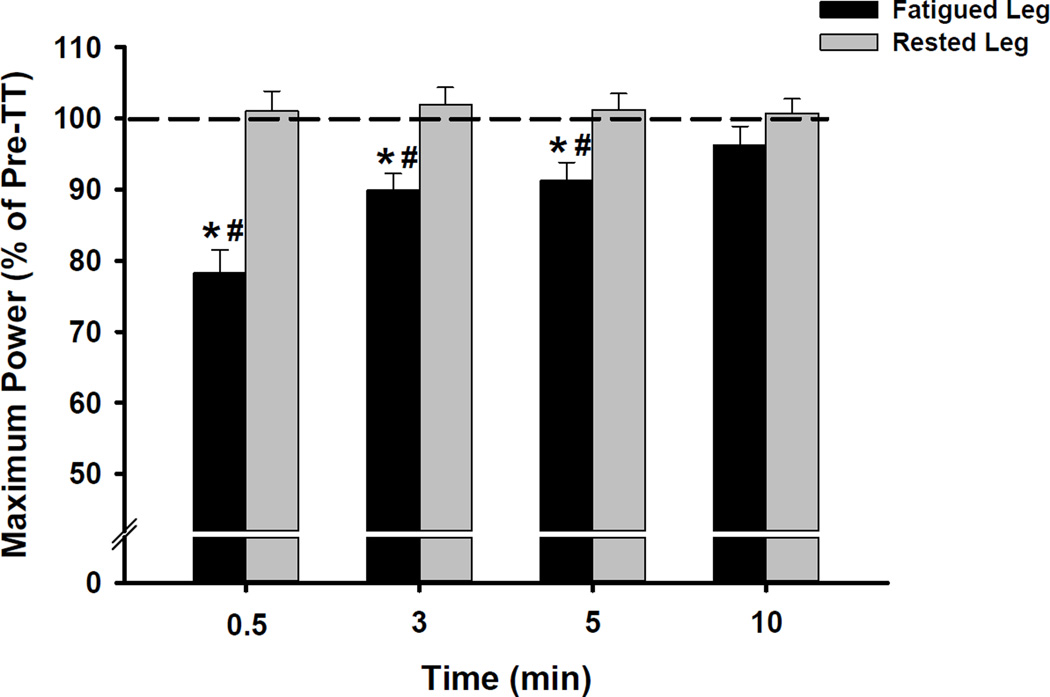
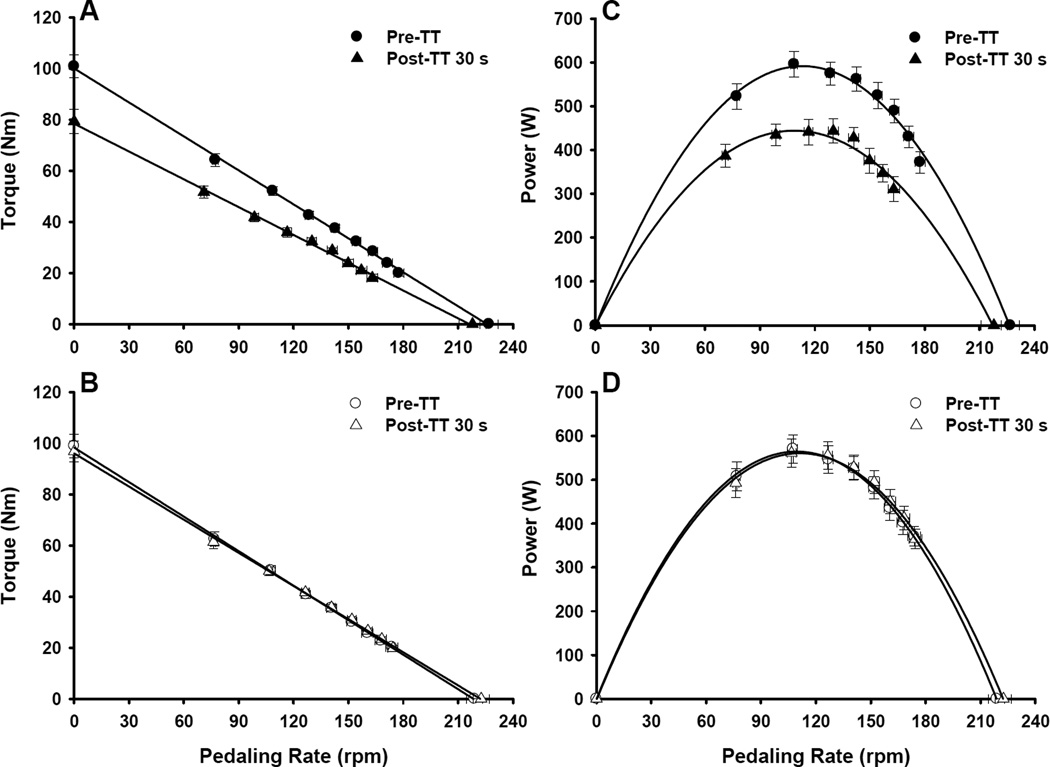
The repeated measures ANOVA revealed significant effects of leg (P < 0.05) and time (P < 0.001) and a significant leg × time interaction (P < 0.001) on maximum cycling power values. Subsequent post hoc analyses indicated that maximum cycling power values differed between the fatigued ipsilateral and rested contralateral legs at 30 s and 3 and 5 min post-TT (all P < 0.05; Figure 3, Table 2). Compared to pre-TT, maximum cycling power produced by the fatigued ipsilateral leg was reduced by 22 ± 3% at 30 s post-TT and remained reduced by 9 ± 2% at 5 min post-TT (both P < 0.05, Figure 3, Table 2). Post-TT maximum cycling power produced by the rested contralateral leg did not differ from pre-TT values (P = 0.89, Figure 3, Table 2). Complete power-pedaling rate relationships are illustrated in Figure 4 for descriptive purposes. There were also significant effects of leg (P < 0.001) and time (P < 0.001) and a leg × time interaction (P < 0.001) on extrapolated maximum isometric torque values. Subsequent post hoc analyses indicated that extrapolated maximum isometric torque values differed between the legs at 30 s and 3 and 5 min post-TT (all P < 0.05, Table 2). Compared to pre-TT, maximum isometric torque generated by the fatigued ipsilateral leg was reduced by 20 ± 2% at 30 s post-TT and remained reduced by 4 ± 2% at 10 min post-TT (both P < 0.05, Table 2). There were no alterations in maximum isometric torque produced by the rested contralateral leg (P = 0.14, Table 2). Complete torque-pedaling rate relationships are also illustrated in Figure 4.
Figure 3.
Relative pre- to post-TT changes in maximum cycling power (mean ± SEM) for the fatigued ipsilateral and rested contralateral leg. *P < 0.05 vs. pre-TT (dotted line). #P < 0.05 vs. rested contralateral leg.
Table 2.
Pre- to post-TT changes in neuromuscular function assessed during maximal cycling
| Fatigued Ipsilateral Leg | Rested Contralateral Leg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 30 s | 3 min | 5 min | 10 min | Pre | 30 s | 3 min | 5 min | 10 min | |
| Pmax (W)a,b,c | 627 ± 31# | 489 ± 30*,# | 561 ± 29*,# | 570 ± 30*,# | 602 ± 33 | 596 ± 35 | 597 ± 31 | 603 ± 32 | 597 ± 27 | 596 ± 31 |
| Tmax (Nm)a,b,c | 101 ± 5 | 79 ± 5*,# | 89 ± 4*,# | 90 ± 4*,# | 96 ± 4* | 99 ± 5 | 97 ± 4 | 97 ± 4 | 94 ± 4 | 95 ± 4 |
| RPMoptb | 113 ± 9 | 109 ± 4 | 114 ± 3 | 115 ± 3 | 114 ± 3 | 110 ± 2 | 111 ± 2 | 114 ± 2 | 114 ± 2 | 115 ± 2 |
| RPMmaxb | 227 ± 5 | 218 ± 7 | 227 ± 5 | 230 ± 7 | 231 ± 7 | 219 ± 4 | 223 ± 4 | 228 ± 4 | 230 ± 4 | 231 ± 4 |
Values are reported as Mean ± SEM. Pmax, maximum cycling power; Tmax, maximum isometric torque produced at the onset of maximal cycling (extrapolated value); RPMopt, optimal pedaling rate that elicited maximum cycling power; RPMmax, maximum pedaling rate (extrapolated value). Note that pre-TT values for the rested contralateral leg tended to be lower than fatigued ipsilateral values due to the lack of a cycling warm-up.
main effect of leg (P < 0.05)
main effect of time (P < 0.05)
leg × time interaction (P < 0.05)
P < 0.05 vs. pre-TT
P < 0.05 vs. rested contralateral leg
Figure 4.
Maximal cycling torque-pedaling rate (A, B) and power-pedaling rate (C, D) relationships for the fatigued ipsilateral (closed symbols) and rested contralateral leg (open symbols). Maximum isometric torque and maximum power were reduced for the fatigued ipsilateral leg at 30 s post-TT, but did not change for the rested contralateral leg. Data are presented as mean ± SEM.
Repeated measures ANOVA procedures revealed a significant effect of time on optimal pedaling rate values (P < 0.01). Optimal pedaling rate values did not differ between the fatigued ipsilateral and rested contralateral legs (P = 0.82) and the leg × time interaction was also not significant (P = 0.40). Similarly, there was a significant effect of time on extrapolated maximum pedaling rate values (P < 0.001) while the effect of leg (P = 0.88) and the leg × time interaction (P = 0.22) were not significant. Both optimal pedaling rate and maximum pedaling rate increased at 5 and 10 min post-TT (all P < 0.05, Table 2), suggesting that participants improved their ability to perform single-leg cycling at higher pedaling rates over time. Note that, relative pre- to post-TT changes in maximum cycling power, extrapolated maximum isometric torque, optimal pedaling rate, and extrapolated maximum pedaling rate values agreed well with the absolute comparisons. Finally, there were no alterations in maximum isometric handgrip force following either cycling protocol (pooled values: P = 0.10, pre-TT: 534 ± 22, post-TT 30 s: 540 ± 21, post-TT 3 min: 525 ± 19, post-TT 5 min: 529 ± 20, post-TT 10 min: 547 ± 19 N).
Discussion
In this investigation, we used high-intensity single-leg cycling (i.e., 10 min TT) to induce fatigue and subsequently evaluated maximum cycling power of the rested contralateral leg as well as the fatigued ipsilateral leg. Our main finding was that maximum cycling power in the rested contralateral leg was maintained despite considerable power loss in the fatigued ipsilateral leg. These results suggest that any potential cross-over of fatigue (central and/or peripheral in origin), if present at all, was not sufficient to measurably compromise maximum power of the rested contralateral leg. Additionally, maximum isometric handgrip force was unaffected by leg fatigue. Taken together, our results suggest that, following high-intensity endurance exercise involving a substantial muscle mass, compromises in maximal voluntary neuromuscular function are limited to those muscles involved in the fatiguing locomotor task.
Warm-up Effects and Quantification of Fatigue
An important part of our experimental design was that we included two different pre-TT maximal cycling trials in order to determine the effect of a brief warm-up on maximum cycling power. This was necessary as the rested contralateral leg was passive during the TT and thus not likely warmed-up prior to the post-TT maximal cycling trial. As expected, pre-TT maximum cycling power was reduced in the absence of a warm-up. Based on this finding, we used the pre-TT maximal cycling trial that was preceded by 15 min of rest (i.e., no warm-up, pre-TTcold) for the pre- to post-TT comparison for the rested contralateral leg. Therefore, any potential changes in maximum cycling power of the rested contralateral leg would be due to a cross-over of fatigue rather than changes in muscle temperature (Sargeant 1987). Alternatively, if we had compared the post-TT maximal cycling data to pre-TTwarm, the confounding effect of warm-up/temperature would have lead us to conclude, incorrectly, that significant fatigue had crossed over to the rested contralateral leg. No adjustment was made for the fatigued ipsilateral leg as this leg was active during the TT and likely sufficiently warmed-up at the time the post-TT maximal cycling trial was conducted. Accordingly, the pre-TT maximal cycling trial that was preceded by a 5 min cycling warm-up (pre-TTwarm) was used for the pre- to post-TT comparison for the fatigued ipsilateral leg.
Exercise-Induced Fatigue
During the TT, participants were able to produce substantial power with one leg (~200 W) which resulted in increases in heart rate, blood lactate, and RPE generally similar to those reported during high-intensity double-leg cycling (Amann et al. 2008; Amann et al. 2009; Elmer et al. 2012; Marcora and Staiano 2010a). Previous authors (Abbiss et al. 2011; Bundle et al. 2006) have demonstrated that high-intensity single-leg cycling permits higher individual leg power outputs compared to double-leg cycling. Thus, not only did our exercise modality place considerable stress on the whole-body but it also likely facilitated extraordinarily high changes in the intramuscular metabolic milieu within the working locomotor muscles (Klausen et al. 1982). After the TT, maximum power in the fatigued ipsilateral leg was reduced by 22% and, despite some recovery, remained reduced at 5 min post-TT. These results generally support previous findings (Beelen and Sargeant 1991; Marcora and Staiano 2010a; Elmer et al. 2012) of a ~30% reduction in maximum double-leg cycling power and indicate that high-intensity single-leg cycling was effective for inducing fatigue in the ipsilateral leg. The observed exercise-induced reduction in maximum power in the fatigued ipsilateral leg presumably manifested through a combination of central and peripheral mechanisms (Amann 2011), however, additional measurements are needed to confirm this.
Interestingly, reductions in maximum cycling power in the fatigued ipsilateral leg were not associated with significant reductions in optimal or maximum pedaling rate. This suggests that reductions in maximum power were largely due to reductions in torque-generating capacity (i.e., muscular force). Indeed, maximum isometric torque (as estimated by linear extrapolation) was reduced by a similar magnitude as maximum power (20% vs. 22%). Our results contrast those of Buttelli and colleagues (Buttelli et al. 1997) who reported reductions in maximum pedaling rate without changes in maximum isometric torque following high-intensity constant power cycling. These contrasting results may be related to muscle mass involved with the cycling task (single vs. double-leg) and/or differences between our inertial-load ergometer (Martin et al. 1997) and the ergometer (Seck et al. 1995) used by Buttelli and colleagues (1997). Nonetheless, the results from the present study also demonstrate that power-pedaling rate relationships maintain their parabolic shape following high-intensity single-leg cycling which may provide some insight into a recent debate on whether power-pedaling rate relationships apply to fatigued states (MacIntosh and Fletcher 2011, 2012; Marcora and Staiano 2010b, 2011).
Potential Cross-over of Fatigue
In contrast to the reductions in maximum cycling power in the fatigued leg, maximum cycling power was unaffected in the rested contralateral leg. In fact, pre- to post-TT torque-pedaling rate and power-pedaling rate relationships were nearly identical (Figure 4). This similarity is quite impressive given that participants were working close to maximal effort and producing substantial power with the fatigued ipsilateral leg during the TT. Thus, despite substantial fatigue in the ipsilateral leg, a cross-over of fatigue was either not present or not large enough to measurably impair maximum power of the rested contralateral leg. These results imply that the output from spinal motor neurons was sufficient to enable participants to generate the same baseline maximum cycling power in the rested contralateral leg. With this in mind, central motor drive to the rested contralateral leg was likely unaffected by fatigue in the ipsilateral leg. However, a limitation of the current study is that changes in central motor drive were not quantified. Therefore, future work including electromyographic (EMG) measurements is needed to provide additional insight into this phenomenon.
In support of our results, previous authors (Rattey et al. 2006; Todd et al. 2003) have reported that fatigue induced via a maximal isometric contraction of a single limb muscle group does not impair maximum isometric force in the rested contralateral muscle group. Conversely, other authors (Martin and Rattey 2007) have reported that sustained isometric knee extensor exercise reduced maximum isometric force in rested contralateral knee extensors by 13%. Regardless, even though there is some variation in these findings, our results along with the majority of previous studies (Rattey et al. 2006; Todd et al. 2003) indicate that fatigue in a single limb muscle group(s) does not impair maximum voluntary function in the rested homologous contralateral muscle group(s).
Our results relating to the absence of a cross-over of fatigue were unanticipated and four alternative explanations are worth mentioning. First, as might be concluded based on the work by Amann and colleagues (Amann 2011; Amann et al. 2008; Amann et al. 2009), during the single-leg TT increased firing of group III/IV muscle afferents from the fatigued ipsilateral leg could potentially have exerted inhibitory influences on central motor drive to the contralateral leg with the associated consequence of a compromised post-TT maximum cycling power (i.e., a potential cross-over of fatigue). However, for a brief period, participants may have been able to overcome this limitation (Rattey et al. 2006; Todd et al. 2003) and produce the same baseline maximum cycling power in the rested leg. To further test this theory, it might be very illuminating to also evaluate the extent to which exercise-induced fatigue in the ipsilateral leg impacts high-intensity endurance performance in the rested contralateral leg. Second, it is salient to note that participants in this study were endurance trained cyclists and were tested in the middle of the racing season. Competitive cycling is inherently a non-steady-state activity performed with intermittent high cycling powers (Quod et al. 2010). Further, chronic endurance training and associated increased brain mitochondrial biogenesis could possibly attenuate the development of central fatigue (Steiner et al. 2011). Therefore, cyclists in this study may have been uniquely prepared to perform high-intensity single-leg cycling and overcome a potential cross-over of fatigue to produce maximum cycling power with the rested contralateral leg. Third, maximum cycling power in the rested contralateral leg may have been initially reduced but could have recovered prior to the post-TT assessment, as there was a short 30 s delay due to ergometer constraints. Finally, it could be argued that central fatigue might not have developed during the 10 min TT and thus could not have crossed over to the rested contralateral leg.
Limb Specificity of Fatigue
In this investigation, we also evaluated pre- to post-TT changes in maximum isometric handgrip force in an attempt to determine if a cross-over of fatigue manifested with a “global” impairment in maximum voluntary neuromuscular function. Specifically, central fatigue could result from exercise-induced alterations in cerebral neurotransmission (Davis and Bailey 1997) with the consequence of reduced neuromuscular function in rested muscles not involved in the fatiguing task. Additionally, humoral factors associated with substantial peripheral fatigue in the ipsilateral leg could have lead to peripheral fatigue in other rested muscles. Although such factors were not directly assessed, the lack of changes in maximum isometric handgrip force as well as maximum power in the rested contralateral leg demonstrate that voluntary neuromuscular function was compromised only in exercised muscles of the fatigued ipsilateral leg. These data also suggest that high-intensity single-leg cycling in the ipsilateral leg did not affect participants’ motivation to perform subsequent maximal voluntary isometric and dynamic contractions in other previously rested limbs/muscles. Finally, the lack of changes in maximum isometric handgrip force is consistent with previous reports that indicate that maximum isometric handgrip force is maintained after high-intensity cycling (Decorte et al. 2012) and prolonged running (Millet et al. 2003; Place et al. 2004; Ross et al. 2010; Ross et al. 2007).
Summary
This is the first investigation to evaluate a potential homologous cross-over of fatigue following high-intensity endurance exercise involving a substantial muscle mass. Even with considerable fatigue in the ipsilateral leg, participants remained capable of generating the same baseline maximum cycling power with the rested contralateral leg. Maximum isometric handgrip force was also unaffected by fatigue. Collectively, these results suggest that fatigue induced via high-intensity single-leg cycling does not impair maximal voluntary neuromuscular function in previously rested muscles/limbs of trained cyclists.
Acknowledgement
The authors would like to sincerely thank the participants who took part in this study for their enthusiastic efforts in performing the cycling trials. We also wish to extend our sincere appreciation to Christopher Call, Jackie Bohn, and Kyle Wehmanen for their assistance with the data collection and to Barry Shultz for providing administrative support. The authors thank Geoffery Power and Francois Billaut for providing insightful comments during the preparation of the manuscript.
Footnotes
The authors report no conflict of interest.
References
- Abbiss CR, Karagounis LG, Laursen PB, Peiffer JJ, Martin DT, Hawley JA, Fatehee NN, Martin JC. Single-leg cycle training is superior to double-leg cycling in improving the oxidative potential and metabolic profile of trained skeletal muscle. J Appl Physiol. 2011;110:1248–1255. doi: 10.1152/japplphysiol.01247.2010. [DOI] [PubMed] [Google Scholar]
- Abbiss CR, Quod MJ, Levin G, Martin DT, Laursen PB. Accuracy of the Velotron ergometer and SRM power meter. Int J Sports Med. 2009;30:107–112. doi: 10.1055/s-0028-1103285. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Amann M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc. 2011;43:2039–2045. doi: 10.1249/MSS.0b013e31821f59ab. [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol. 2008;105:1714–1724. doi: 10.1152/japplphysiol.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc. 2004;36:613–622. doi: 10.1249/01.mss.0000122076.21804.10. [DOI] [PubMed] [Google Scholar]
- Beelen A, Sargeant AJ. Effect of fatigue on maximal power output at different contraction velocities in humans. J Appl Physiol. 1991;71:2332–2337. doi: 10.1152/jappl.1991.71.6.2332. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983;50:313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Bundle MW, Ernst CL, Bellizzi MJ, Wright S, Weyand PG. A metabolic basis for impaired muscle force production and neuromuscular compensation during sprint cycling. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1457–R1464. doi: 10.1152/ajpregu.00108.2006. [DOI] [PubMed] [Google Scholar]
- Buttelli O, Vandewalle H, Jouanin JC, Seck D, Monod H. Effects of aerobic exercise on the torque-velocity relationship in cycling. Eur J Appl Physiol Occup Physiol. 1997;75:499–503. doi: 10.1007/s004210050195. [DOI] [PubMed] [Google Scholar]
- Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Decorte N, Lafaix PA, Millet GY, Wuyam B, Verges S. Central and peripheral fatigue kinetics during exhaustive constant-load cycling. Scand J Med Sci Sports. 2012;22:381–391. doi: 10.1111/j.1600-0838.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- Elmer SJ, Marshall CS, Wehmanen K, Amann M, McDaniel J, Martin JC. Effects of locomotor muscle fatigue on joint-specific power production during cycling. Med Sci Sports Exerc. 2012 doi: 10.1249/MSS.0b013e31824fb8bd. [DOI] [PubMed] [Google Scholar]
- Elmer SJ, McDaniel J, Martin JC. Alterations in neuromuscular function and perceptual responses following acute eccentric cycling exercise. Eur J Appl Physiol. 2010;110:1225–1233. doi: 10.1007/s00421-010-1619-z. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Holloszy JO. Lactate and contractile force in frog muscle during development of fatigue and recovery. Am J Physiol. 1976;231:430–433. doi: 10.1152/ajplegacy.1976.231.2.430. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Klausen K, Secher NH, Clausen JP, Hartling O, Trap-Jensen J. Central and regional circulatory adaptations to one-leg training. J Appl Physiol. 1982;52:976–983. doi: 10.1152/jappl.1982.52.4.976. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR, Fletcher JR. The parabolic power-velocity relationship does apply to fatigued states. Eur J Appl Physiol. 2011;111:319–320. doi: 10.1007/s00421-010-1610-8. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR, Fletcher JR. Reply to: Reply to: The parabolic power-velocity relationship does apply to fatigued states. Eur J Appl Physiol. 2012;112:1195–1196. doi: 10.1007/s00421-011-2043-8. [DOI] [PubMed] [Google Scholar]
- Marcora SM, Staiano W. The limit to exercise tolerance in humans: mind over muscle? Eur J Appl Physiol. 2010a;109:763–770. doi: 10.1007/s00421-010-1418-6. [DOI] [PubMed] [Google Scholar]
- Marcora SM, Staiano W. The parabolic power-velocity relationship does not apply to fatigued states. Eur J Appl Physiol. 2010b;109:787–788. doi: 10.1007/s00421-010-1495-6. [DOI] [PubMed] [Google Scholar]
- Marcora SM, Staiano W. Reply to: The parabolic power-velocity relationship does apply to fatigued states. Eur J Appl Physiol. 2011;111:731–732. doi: 10.1007/s00421-010-1689-y. [DOI] [PubMed] [Google Scholar]
- Martin JC, Wagner BM, Coyle EF. Inertial-load method determines maximal cycling power in a single exercise bout. Med Sci Sports Exerc. 1997;29:1505–1512. doi: 10.1097/00005768-199711000-00018. [DOI] [PubMed] [Google Scholar]
- Martin PG, Rattey J. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch. 2007;454:957–969. doi: 10.1007/s00424-007-0243-1. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet GY, Martin V, Lattier G, Ballay Y. Mechanisms contributing to knee extensor strength loss after prolonged running exercise. J Appl Physiol. 2003;94:193–198. doi: 10.1152/japplphysiol.00600.2002. [DOI] [PubMed] [Google Scholar]
- Place N, Lepers R, Deley G, Millet GY. Time course of neuromuscular alterations during a prolonged running exercise. Med Sci Sports Exerc. 2004;36:1347–1356. doi: 10.1249/01.mss.0000135786.22996.77. [DOI] [PubMed] [Google Scholar]
- Quod MJ, Martin DT, Martin JC, Laursen PB. The power profile predicts road cycling MMP. Int J Sports Med. 2010;31:397–401. doi: 10.1055/s-0030-1247528. [DOI] [PubMed] [Google Scholar]
- Rattey J, Martin PG, Kay D, Cannon J, Marino FE. Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Pflugers Arch. 2006;452:199–207. doi: 10.1007/s00424-005-0027-4. [DOI] [PubMed] [Google Scholar]
- Ross EZ, Goodall S, Stevens A, Harris I. Time course of neuromuscular changes during running in well-trained subjects. Med Sci Sports Exerc. 2010;42:1184–1190. doi: 10.1249/MSS.0b013e3181c91f4e. [DOI] [PubMed] [Google Scholar]
- Ross EZ, Middleton N, Shave R, George K, Nowicky A. Corticomotor excitability contributes to neuromuscular fatigue following marathon running in man. Exp Physiol. 2007;92:417–426. doi: 10.1113/expphysiol.2006.035972. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ. Effect of muscle temperature on leg extension force and short-term power output in humans. Eur J Appl Physiol Occup Physiol. 1987;56:693–698. doi: 10.1007/BF00424812. [DOI] [PubMed] [Google Scholar]
- Seck D, Vandewalle H, Decrops N, Monod H. Maximal power and torque-velocity relationship on a cycle ergometer during the acceleration phase of a single all-out exercise. Eur J Appl Physiol Occup Physiol. 1995;70:161–168. doi: 10.1007/BF00361544. [DOI] [PubMed] [Google Scholar]
- Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise Training Increases Mitochondrial Biogenesis in the Brain. J Appl Physiol. 2011;111:1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res. 2003;150:308–313. doi: 10.1007/s00221-003-1379-7. [DOI] [PubMed] [Google Scholar]
- Woltring HJ. A FORTRAN package for generalized, cross validatory spline smoothing and differentiation. Adv Eng Software. 1986;8:104–113. [Google Scholar]