Abstract
Background and Objectives
Limiting alcohol consumption may help prevent alcohol-mediated smoking relapse in heavy drinking smokers. This pilot study examined whether combining a nicotine patch with nicotine nasal spray has stronger attenuating effects on alcohol response and consumption than a nicotine patch alone.
Methods
Twenty-two non-alcohol dependent heavy drinking smokers completed the double-blind cross-over, placebo-controlled study (21mg nicotine patch + nicotine or placebo nasal spray). Six hours after 21mg nicotine patch application, subjective and physiological responses to a priming drink (0.3 g/kg) were assessed, followed by two 1-hr alcohol self-administration periods, with possible consumption of up to 4 drinks per period (each 0.15 g/kg). Nasal spray (1 mg [active] or 0 mg [placebo] per dose) was administered 10 min prior to the priming dose and each self-administration period.
Results
Active nasal spray did not increase serum nicotine levels, compared with placebo administration. The number of drinks consumed did not differ by the nasal spray conditions. However, positive subjective responses to the priming drink were lower in the active nasal spray condition than the placebo nasal spray condition. During the self-administration period, urge to drink was also lower in the active spray condition than the placebo condition.
Conclusions and Scientific Significance
Augmenting the nicotine patch with nicotine nasal spray attenuated positive subjective alcohol response and craving and suggests that future studies should investigate whether these findings translate to a clinical setting.
Keywords: Combined nicotine replacement therapy, alcohol self-administration, heavy drinkers, alcohol response, craving
INTRODUCTION
In response to the substantial health risks associated with concurrent alcohol and tobacco use,1–4 efforts to address smoking cessation in individuals experiencing alcohol problems continue to grow. While much clinical research and practice have focused on alcohol dependent smokers, non-alcohol dependent heavy drinkers also have substantially high rates of smoking,5 and how to best promote smoking cessation in this population remains relatively unexplored. It is well established that heavy drinking is associated with poor smoking cessation outcomes,6 with studies identifying alcohol consumption as a precipitant to smoking lapse.7–10 Heavy drinking smokers are vulnerable to smoking relapse partly due to their frequent alcohol consumption, which may serve as a cue to smoke. One way to promote smoking cessation for heavy drinkers may be to limit their alcohol consumption, which in turn may help prevent alcohol-mediated smoking lapse.
Preclinical and clinical evidence suggests an interplay of systems involved in the reinforcing effects of nicotine and alcohol.11 As a result, increasing attention has been paid to understand the effects of pharmacological interventions developed for smoking cessation, such as nicotine replacement therapy (NRT) and nicotinic receptor antagonists, on alcohol use behaviors. Using a self-administration paradigm consisting of a priming dose followed by ad-libitum drinking (i.e., self-administration), Young et al. (2005)12 found that mecamylamine, a nicotinic acetylcholine receptor antagonist, reduced subjective ratings of stimulant-like alcohol effects in response to a priming dose, and reduced alcohol preference over money, only in those who reported high stimulant-like effects of alcohol. Acheson et al. (2006)13 further demonstrated that nicotine patch facilitated alcohol self-administration in male social drinking smokers, but attenuated self-administration in female social drinking smokers. Among nicotine-deprived heavy drinking smokers, nicotine patch therapy, compared to placebo, significantly reduced alcohol craving and subjective effects of alcohol after a priming drink, and reduced the number of drinks consumed during ad-lib drinking.14 Varenicline, a partial nicotinic acetylcholine receptor antagonist, was similarly found effective in attenuating alcohol craving, subjective response to alcohol, and reduced alcohol consumption in heavy drinking smokers who were not nicotine deprived.15 Although previous research provides mixed and complex results, pharmacological interventions for smoking cessation may be effective in attenuating drinking in heavy drinking smokers.
Compelling evidence from randomized smoking cessation clinical trials and meta-analysis studies supports stronger effectiveness of combining a nicotine patch and a fast acting, self-administered form of NRT (e.g., nicotine gum, nasal spray, inhaler) than monotherapy.16–19 It is thought that the increased effectiveness of combined NRT is due to higher plasma nicotine levels, although higher dose of nicotine patch does not appear to lead to better outcomes.19,20 Alternatively, better outcomes with combined NRT may be explained by providing immediate relief of suddenly experienced craving and withdrawal symptoms, in addition to a constant concentration of nicotine to relieve cravings and tobacco withdrawal symptoms through the nicotine patch.21 A randomized clinical trial with alcohol-dependent smokers found that those who received nicotine patch plus nicotine gum treatment had better smoking cessation outcomes than those who received nicotine patch and placebo gum,22 suggesting its superior effectiveness in a relapse prone, heavy drinking population. To the best of our knowledge, however, no study has examined the effectiveness of a combined NRT on alcohol craving and alcohol self-administration in heavy drinking smokers. Given our prior findings that nicotine patch reduced alcohol consumption and cravings,14 it is possible that a combined NRT may have stronger attenuating effects on alcohol craving and consumption than patch alone.
Using a double-blind cross-over, placebo-controlled design, we conducted a preliminary study to examine the effects of augmenting nicotine patch with either nicotine or placebo spray on reactivity to a priming drink and subsequent alcohol self-administration behaviors. Based on the literature supporting better smoking cessation outcomes in combined NRT than monotherapy, it was hypothesized that a combined NRT condition versus a patch-alone condition would reduce alcohol craving and positive subjective effects following a low fixed-dose of alcohol and reduce the number of drinks consumed during the subsequent self-administration period.
METHODS
Participants
Twenty-two participants (mean age = 37.1 ± 10.2; 22.7% women) completed the study. Eligible participants were 21–55 years old, able to read and write English, smoked 10–40 cigarettes per day, and drank at least four alcoholic drinks on any day (three drinks for women) at least three days per week in the past 30 days. Exclusion criteria included: current diagnosis of DSM-IV Axis I disorders (including alcohol dependence but not nicotine dependence or alcohol abuse, diagnosed with the Structured Clinical Interview for DSM-IV),23 illicit drug use, currently seeking treatment for alcohol use or smoking behavior, or medical conditions that would contraindicate alcohol use, transdermal nicotine use, or nasal nicotine use. In addition, women who were pregnant or nursing were excluded. The majority of the participants were non-Hispanic White (64.0%) and 36% were non-Hispanic African American. The majority of participants (59.1%) had completed high school.
Participants smoked, on average, 20.80 (SD = 6.80) cigarettes per day, had baseline carbon monoxide (CO) readings of 28.57 ppm (SD = 12.86), and had average Fagerstrom nicotine dependence scores (FTND)24 of 4.0 (SD = 1.45; range 1–10). Participants consumed an average of 38.07 (SD = 25.72) standard drinks per week, drank an average of 4.94 (SD = 1.67) times per week, had average Alcohol Use Disorders Identification Test (AUDIT)25 scores of 7.18 (SD = 3.59; ≥ 8 indicate problematic drinking), and 55% met DSM-IV criteria for current alcohol abuse.
Procedures
Intake assessment
The experimental protocol was approved by the Yale Human Investigation Committee, and the procedures are in compliance with the Declaration of Helsinki for human subjects. Written informed consent was obtained from all participants at the beginning of intake session. All participants underwent a physical exam, which included an electrocardiogram, urine toxicology (for opiates, THC, PCP, cocaine, amphetamine, and methadone), pregnancy tests for women, and basic blood chemistries including liver function tests. Eligible participants were then scheduled for the laboratory sessions.
Laboratory sessions
Each participant individually completed two 14-hour (hr) laboratory sessions (21 mg nicotine patch + active nasal spray vs. 21 mg nicotine patch + placebo nasal spray; see Figure 1 for assessment timeline). The laboratory sessions started at 8:00 AM, and the procedures were similar to our previous alcohol self-administration studies.14,15 The order of the sessions was counterbalanced. Participants received $120 as compensation for their time in the lab at the completion of each laboratory session, and received a $50 bonus for successful completion of both sessions.
Figure 1.
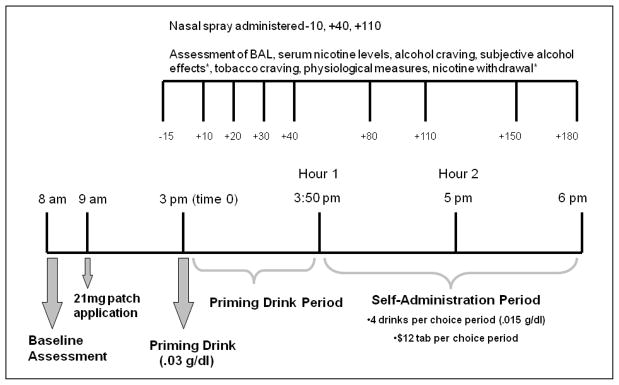
Timeline of laboratory procedures and assessments.*subjective alcohol effects and nicotine withdrawal were not administered at +10min and +30min.
Participants were asked not to consume alcohol 24 hrs prior to coming to the laboratory session, but were free to smoke as they normally would up to 8:00 AM. Baseline assessments consisted of breath alcohol, physiological measures (heart rate and blood pressure), plasma cotine and nicotine levels, urine drug and pregnancy screens. At 9:00 AM, a nicotine patch (21 mg Nicoderm CQ Transdermal System®, GlaxoSmithKline Consumer Healthcare) was applied to the participant’s upper arm to assure a 6-hr absorption window to achieve peak nicotine levels 26 at the time of priming alcohol administration. For the next 6 hrs, participants were able to watch TV and read. Lunch was provided at 12:00 PM to standardize the time and amount of last food intake.
Priming Drink Period
Between 3:00–3:05 PM, participants consumed the priming drink (0.3 g/kg) that consisted of one part 80-proof liquor of the participant’s choosing to three parts mixer chosen from a selection of equicaloric, non-caffeinated, non-carbonated drinks.27 This priming dose was used to prime further drinking behavior.28 Ten minutes (min) prior to the priming drink administration, the first active nicotine (1 mg, two 0.5 mg/spray) or placebo spray (saline + nasal capsaicin to mimic the brief nasal irritation from active nasal spray)29 was administered as a 1 mg delivery of nicotine nasal spray which produces a peak nicotine level of 6 ng/ml within10 min, followed by decay over the next 50 min.30
Self-Administration Period
Participants were exposed to two consecutive, 1-hr ad-lib drinking periods, starting at 50 and 120 min following consumption of the priming drink. Participants were permitted to drink up to four alcoholic drinks (each 0.15 g/kg) or to receive monetary reinforcement for each drink not consumed ($3 per drink, up to a total of $24). To coordinate peak nicotine levels during the course of the self-administration sessions, nasal spray was also administered 10 min prior to the start of each drinking period. Participants were discharged at 10:00 PM, at which time their breath alcohol levels had fallen below 0.02% (NIAAA guidelines).
Measures
Blood alcohol levels (BALs) and nicotine serum levels
Blood samples were taken for BALs and serum nicotine levels throughout the laboratory session (see McKee et al.14 for detailed procedures). The lower limit of quantification for nicotine was set to 4 ng/ml.
Outcome measures
Alcohol craving was assessed with the Alcohol Urge Questionnaire (AUQ).31 Tobacco craving in response to positive (factor 1) or negative (factor 2) reinforcement was assessed with the Tiffany Questionnaire of Smoking Urges – Brief (QSU-Brief).32 The Biphasic Alcohol Effects Scale (BAES)33 was used to measure the stimulant and sedative effects of alcohol. The Alcohol Effects Scale (AES)14 assessed subjective alcohol effects with five items (high, like, rush, feel-good, intoxicated). A mean score of these five items was calculated. AUQ, QSU-Brief, BAES, and AES all used Visual Analogue Scale (VAS) with a range of 1–100. DSM-IV symptoms of nicotine withdrawal were assessed with the 8-item Minnesota Nicotine Withdrawal Scale (MNWS; range = 0–32).34 Systolic and diastolic blood pressure (SBP and DBP, respectively) and heart rate (HR) were also measured.
Side Effects
Adverse events typically associated with nicotine patch were evaluated once 5 hrs following nicotine patch administration. Adverse events associated with nasal spray were evaluated 5 min after the first nasal spray administration, and 40 min following the second two nasal spray administrations. Subjects rated the presence (yes/no) and severity (4-point scale; 1 = minimal to 4 = severe) of each symptom.
Statistical Analysis
Repeated measures of analysis of variances (ANOVA) was used to examine the within-subject effect of nasal spray conditions (0 mg vs. 1 mg) on the number of drinks consumed during each ad-lib drinking period. Repeated measures ANOVA were conducted separately for the priming drink and self-administration periods to examine the within-subject effects of nasal spray conditions and time on BALs, subjective measures (AUQ, QSU-B factor 1 & 2, MNWS, AES, BAES), and physiologic measures. Across analyses, there were no effects of order or gender observed.
RESULTS
Serum Nicotine Levels
A series of paired t-tests (1 mg vs. 0 mg at each assessment point) revealed no significant effect of the nasal spray conditions on nicotine serum levels across all the assessment time points (all p > .05), although the nicotine levels were generally higher in the active spray condition than the placebo spray condition (Table 1).
Table 1.
Means and Standard Errors of Serum Nicotine Levels by Nasal Spray Conditions.
| Time | Active Spray | Placebo Spray | t-test |
|---|---|---|---|
| Priming dose | |||
| +0 min | 14.04 (2.93) | 14.78 (2.72) | .29 |
| +10 min | 16.85 (3.72) | 15.72 (2.61) | −.45 |
| +20 min | 14.87 (2.86) | 15.58 (2.60) | .33 |
| +30 min | 15.49 (3.53) | 14.98 (2.56) | −.23 |
| +40 min | 15.10 (3.14) | 14.93 (2.55) | −.09 |
| Self-administration period | |||
| +80 min (Hour 1) | 14.91 (2.92) | 14.57 (2.30) | −.20 |
| +110 min (Hour 1) | 16.69 (3.54) | 15.37 (2.49) | −.68 |
| +150 min (Hour 2) | 15.93 (2.37) | 14.89 (1.90) | −.58 |
| +180 min (Hour 2) | 15.13 (2.74) | 16.34 (2.89) | .49 |
Notes. All times are minutes since the completion of the priming dose administration. Nasal spray was administered at −10 min, +40 min, and +110 min. All t-tests (0 mg. vs. 1 mg) were not significant (p > .05).
Side Effects
There were no significant differences in the number or severity of adverse events by nasal spray conditions (Table 2).
Table 2.
Symptom counts and mean severity ratings of side effects from nicotine patch, active nasal spray, and placebo nasal spray.
| Patch + Active Condition | Patch + Placebo Condition | |||
|---|---|---|---|---|
|
| ||||
| Count | Severity | Count | Severity | |
| Patch | ||||
| Skin irritation | 1 | 2 | - | - |
| Heart Rate > 100 | 1 | - | 1 | - |
| Dizziness | - | - | - | - |
| Nausea | - | - | - | - |
| Abdominal Discomfort | 1 | 1 | - | - |
| Nasal Spray | ||||
| Nasal irritation | 17 | 2.2 | 11 | 1.8 |
| Throat irritation | 13 | 2.0 | 1 | 3.0 |
| Eyes watering | 17 | 1.9 | 10 | 1.7 |
| Heart rate > 100 | 1 | - | 1 | - |
| Dizziness/Light-headed | 4 | 1.5 | 3 | 1.3 |
| Nausea | - | - | - | - |
| Hands or feet cold | 1 | 3.0 | 2 | 2.0 |
Notes. Severity of side effects were rated on a four point scale (1 = minimal, 2 = mild, 3 = moderate, 4 = severe); Across reported side effects, severity ratings did not differ across active and placebo spray conditions.
Priming Drink Period
Blood alcohol levels
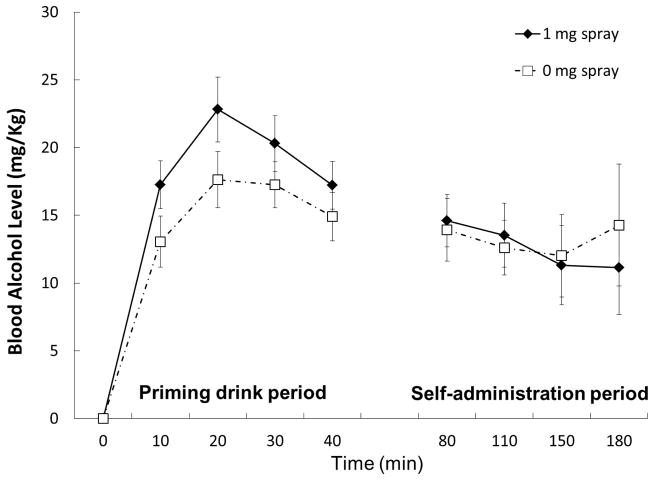
BALs demonstrated a significant time effect, F(4, 68) = 81.15, p < .001, partial η2 = .83. BALs were slightly higher with active nicotine spray with the main effect demonstrating a trend, F(1, 17) = 3.28, p = .09, partial η2 = .16 (Figure 2).
Figure 2.
Means and standard errors of blood alcohol Levels (BALs) across the session by nasal spray condition.
Subjective measures
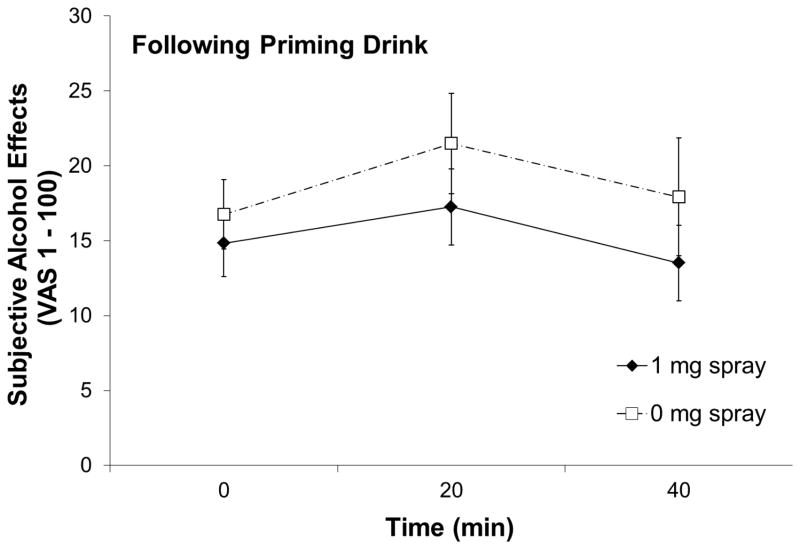
Ratings of AES (mean of high, like, rush, feel-good, and intoxicated) were significantly higher in the placebo spray condition than in the active spray condition, F(1, 20) = 6.59, p = .018, partial η2 = .25 (Figure 3). Two spray conditions were not significantly different in AUQ, F(1, 21) = .46, p = .50, partial η2 = .02, QSU-B (factor 1), F(1, 21) = .01, p = .95, partial η2 = .00, QSU-B (factor 2), F(1, 21) = 1.29, p = .27, partial η2 = .06, BAES-stimulation, F(1, 20) = .07, p = .79, partial η2 = .00, BAES-sedation, F(1, 20) = 1.44, p = .24, partial η2 = .07, or MNWS, F(1, 19) = .48, p = .50, partial η2 = .02.
Figure 3.
Subjective alcohol effects rating by nasal spray condition after the priming dose administration. Subjective alcohol effects were calculated as means of five items (high, like, rush, feel good, and intoxicated).
Physiological measures
SBP, F(4, 21) =3.62, p = .019, partial η2 = .15, and DBP, F(4, 21) = 7.38, p = .002, partial η2 = .26, demonstrated significant quadratic time effects. Both SBP and DBP increased at the start of the priming drink period and then decreased. HR also increased following the priming drink, F(4, 21) = 8.21, p < .001, partial η2 = .28. There were no effects of nasal spray condition on SBP, DBP, or HR.
Self-Administration Period
Drinking behavior
Mean total numbers of drinks consumed over the 2-hr ad-lib drinking period were not significantly different between active and placebo spray conditions (1.59 ± 2.24 and 1.64 ± 2.30, respectively), F(1, 21) = .11, p = .75, partial η2 = .01.
Blood alcohol levels
There was a significant spray condition-by-time interaction on BALs, F(4, 68) = 2.83, p = .041, partial η2 = .14, but no significant main effect of spray condition or time (both p > .05). Figure 2 shows means and standard errors of BALs during the course of the self-administration period for 0 mg vs. 1 mg administration.
Subjective measures
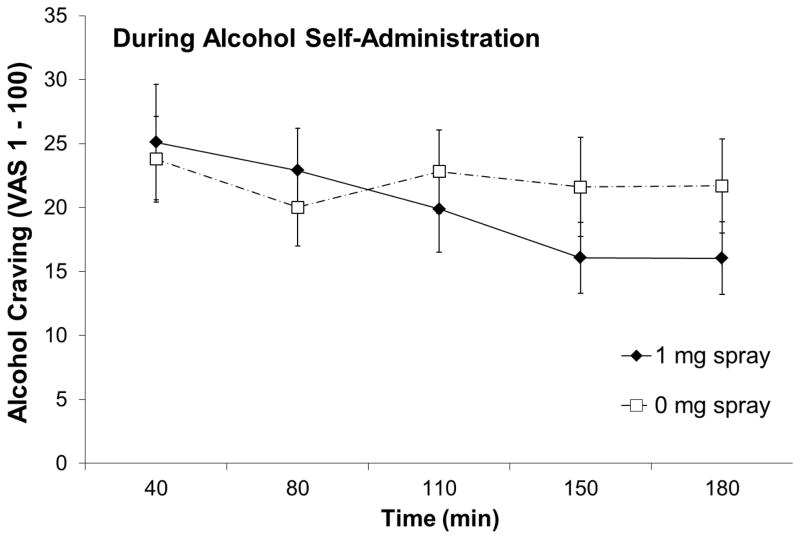
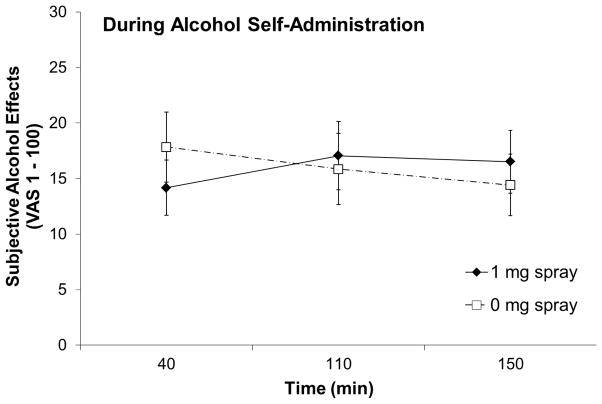
There were significant spray condition-by-time interactions for AUQ, F(4, 84) = 3.20, p = .042, partial η2 = .13 (Figure 4) and AES, F(2, 42) = 5.30, p = .009, partial η2 = .20 (Figure 5), but not for QSU-B (factor 1), F(4, 84) = .69, p = .60, partial η2 = .03, QSU-B (factor 2), F(4, 84) = .77, p = .50, partial η2 = .04, BAES-stimulation, F(2, 42) = 1.30, p = .28, partial η2 = .06, BAES-sedation, F(2, 42) = 0.19, p = .95, partial η2 = .01, or MNWS, F(2, 42) = 1.57, p = .23, partial η2 = .07.
Figure 4.
Alcohol craving score (i.e., mean AUQ score) by nasal spray condition during the alcohol self-administration period.
Figure 5.
Subjective alcohol effects rating by nasal spray condition during the alcohol self-administration period. Subjective alcohol effects were calculated as means of five items (high, like, rush, feel good, and intoxicated).
Physiological measures
SBP, F(1, 20) = 7.47, p = .002, partial η2 = .27 and DBP, F(1, 20) =3.97, p = .026, partial η2 = .16, demonstrated significant quadratic time effects. Blood pressure decreased at the end of the first drinking period, and then increased at the end of the second drinking period. HR did not vary across the self-administration period.
DISCUSSION
This pilot study was the first to examine whether 21mg nicotine patch combined with nicotine nasal spray versus placebo spray would have greater attenuating effects on alcohol consumption, craving, and subjective effects associated with alcohol use in heavy drinking smokers, using a drinking paradigm known to be sensitive to medication effects.15 Contrary to our prediction, the active nasal spray administration did not result in fewer drinks consumed, compared to the placebo nasal spray condition. This may partly be attributable to the lack of augmentation in serum nicotine levels in the combined NRT condition. Alcohol self-administration occurred during peak absorption from the nicotine patch, which has been shown to be sufficient to minimize drinking.14 However, at peak absorption, prolonged nicotine exposure results in the desensitization of nicotine receptors.35 Combining nicotine patch with active nasal spray might have not further reduced alcohol consumption because nicotinic receptor desensitization was maximized at the time of alcohol administration. While serum nicotine levels suggested adequate nicotine replacement for our sample of pack-a-day smokers, it would be possible to further increase nicotine levels to probe effects on alcohol consumption. However, maximizing nicotine levels in this way may reduce drinking by increasing nausea levels. In the current study we had no experience of nausea.
This study also experienced a floor effect with regards to alcohol consumption. Regardless of the nasal spray conditions, our sample consumed fewer drinks than in our previous study that examined the effect of nicotine patch on alcohol use behaviors.14 Using the same self-administration paradigm, participants in McKee et al.14 consumed, on average, 3.6 drinks in the nicotine patch condition and 4.9 drinks in the placebo patch condition, while the current sample consumed on average 1.6 drinks in both active and placebo spray conditions. Compared with McKee et al. 14 our sample was older (26.7 vs. 37.1 years old), and reported fewer drinking problems (mean AUDIT scores 10.63 vs. 7.18) and milder nicotine dependence symptoms (mean FTND scores 5.16 vs. 4.94), which might have contributed to less ad-lib drinking in this study. Alternatively, as indicated by the low levels of alcohol craving following the priming drink, it is possible that blocking introceptive cues typically associated with drinking (i.e., olfactory cues) due to nasal irritation from both active and placebo nasal sprays may have resulted in the low levels of alcohol consumption seen in the current study. Blocking visual and olfactory stimuli associated with alcohol consumption has been shown to reduce positive subjective reactivity and consumption in social drinkers.36 Results such as these suggest that manipulating introceptive cues associated with drinking, possibly with pharmacological targets, may be effective in reducing drinking. Additionally, studying other forms of fast acting NRT medication, such as nicotine gum or inhaler, may clarify the effectiveness of combined NRT on alcohol use behaviors in heavy drinking smokers.
Following the consumption of the priming drink, the subjective effects of alcohol were significantly lower in the active spray condition compared to the placebo condition. This finding is consistent with our prior findings that nicotine attenuates positive subjective effects associated with alcohol,14 and suggests that combined NRT can further attenuate these effects. However, although statistically remained at a trend level, BALs were greater following the priming drink in the active spray condition compared to the placebo condition. Thus, the subjective effects of alcohol may be disassociated with the BALs. This is consistent with previous findings documenting large between-individual variability in subjective response to alcohol even when individuals showed similar BALs.37 Alcohol craving was not altered by nicotine spray during the priming drink period, but was reduced during the self-administration period. However, during the self-administration period, subjective alcohol effects increased over time in the active spray condition and decreased in the placebo spray condition, but mean values were different between conditions. Our results provide a rather complex picture for the effects of a combined NRT on subjective drinking-related measures. Combined NRT may further decrease subjective response to a priming drink when compared to nicotine patch administration, but may not have enhanced effectiveness when there is a choice of consuming more alcohol. Further, combined NRT may not have additional attenuating effects on urge to drink after consuming the priming drink, but may be more effective in reducing alcohol craving when there is a choice to consume additional alcohol. Similar to smoking cessation, fast acting NRT medications may also help to quickly relieve sudden increases in craving.18,21
There are some study limitations. First, the study sample might have been small to detect subtle effects of the nicotine nasal spray that was administered during peak absorption period of the nicotine patch, although the sample size was similar to that of other laboratory studies examining the effect of nicotine replacement and other smoking cessation medications on alcohol responses.13–15 Based on reported effect sizes, we believe that we had sufficient power to examine our primary outcomes. Acute nicotine administration may have differential effects on alcohol consumption in men and women.13 However, the sample size precluded us from examining gender differences, and thus future studies should also investigate gender differences. The present study specifically focused on heavy drinking daily smokers, and may not generalize to lighter drinkers or lighter smokers. A different pattern of results may have been attained with treatment seeking sample. Our assessment of peripheral serum nicotine levels was not sensitive enough to detect differences between active and placebo spray conditions, and it is possible that the pharmacokinetics of nicotine nasal spray may differ from when it is used as a monotherapy versus when it is combined with transdermal patch. Further research is needed to understand the dose-response relationship between combined NRT and alcohol use behaviors. Results from clinical trials have indicated that combined NRT is most effective when the fast acting NRT medication is used as needed to relieve breakthrough of cravings and withdrawal symptoms.18,21 The present study administered the nasal spray at fixed time points, rather than administering the nasal spray as needed by the participants according to levels of alcohol and/or nicotine cravings. It is possible that ad-lib administration of nicotine nasal spray may be more effective to reduce alcohol consumption.
In conclusion, this preliminary study suggests that combined patch and nasal spray NRT may have some efficacy for reducing alcohol use behaviors in heavy drinking smokers. Although nicotine patch combined with nasal spray did not decrease alcohol consumption in the current sample, the combination more strongly attenuated positive subjective responses to alcohol, compared to the patch plus placebo spray condition. In particular, urge to drink decreased during the course of the self-administration period in the nasal spray condition compared with the placebo nasal spray condition when alcohol was available for consumption. The observed low subjective cue reactivity during the ad-lib drinking period may be particularly important for heavy drinking smokers in natural drinking settings, where there are no artificial limits placed on drinking behavior. The complexity of these preliminary findings should warrant further research in both clinical and laboratory settings to clarify the effectiveness of the combined NRT on alcohol use among heavy drinking smokers.
Acknowledgments
This study was supported by the NIAAA grants R01AA015596, T32-AA015496, R01AA017976, a CTSA grant UL1RR024139, and a NIDA grant K12DA031050.
References
- 1.Grucza RA, Bierut LJ. Co-occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Research & Health. 2006;29(3):172–178. [PMC free article] [PubMed] [Google Scholar]
- 2.Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996 Apr 10;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 3.Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Research and Health. 2006;29(3):193–198. [PMC free article] [PubMed] [Google Scholar]
- 4.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. Journal of Hepatology. 2005 Feb;42(2):218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 5.McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status as a clinical indicator for alcohol misuse in US adults. Archives of Internal Medicine. 2007 Apr 9;167(7):716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahler CW, Borland R, Hyland A, McKee SA, Thompson ME, Cummings KM. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug and Alcohol Dependence. 2009 Mar 1;100(3):214–220. doi: 10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006 Dec;189(2):201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwaltney CJ, Shiffman S, Sayette MA. Situational correlates of abstinence self-efficacy. Journal of Abnormal Psychology. 2005 Nov;114(4):649–660. doi: 10.1037/0021-843X.114.4.649. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman S, Balabanis MH, Gwaltney CJ, et al. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007 Dec 1;91(2–3):159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiffman S, Gwaltney CJ. Does heightened affect make smoking cues more salient? Journal of Abnormal Psychology. 2008 Aug;117(3):618–624. doi: 10.1037/0021-843X.117.3.618. [DOI] [PubMed] [Google Scholar]
- 11.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature Neuroscience. 2005 Nov;8(11):1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 12.Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcoholism: Clinical and Experimental Research. 2005 Jan;29(1):58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- 13.Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology (Berl) 2006 May;186(1):54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- 14.McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl) 2008 Feb;196(2):189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKee SA, Harrison EL, O’Malley SS, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological Psychiatry. 2009 Jul 15;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. Journal of Consulting and Clinical Psychology. 2011 Feb;79(1):34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine. 2009 Dec 14;169(22):2148–2155. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry. 2009 Nov;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(1):CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Fiore MC, Jaén CR, Baker TB, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. American Journal of Preventive Medicine. 2008 Aug;35(2):158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney CT, Fant RV, Fagerstrom KO, McGovern JF, Henningfield JE. Combination nicotine replacement therapy for smoking cessation: rationale, efficacy and tolerability. CNS Drugs. 2001;15(6):453–467. doi: 10.2165/00023210-200115060-00004. [DOI] [PubMed] [Google Scholar]
- 22.Cooney NL, Cooney JL, Perry BL, et al. Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction. 2009 Sep;104(9):1588–1596. doi: 10.1111/j.1360-0443.2009.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV. Washington, D. C: American Psychiatric Press; 1995. Patient Edition. [Google Scholar]
- 24.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 25.Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 26.Gupta SK, Benowitz NL, Jacob P, 3rd, Rolf CN, Gorsline J. Bioavailability and absorption kinetics of nicotine following application of a transdermal system. British Journal of Clinical Pharmacology. 1993 Sep;36(3):221–227. doi: 10.1111/j.1365-2125.1993.tb04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow KE, Batt RD, editors. Human Metabolism of Alcohol. Vol. 1. Boca Raton, FL: CRC; 1989. pp. 41–56. [Google Scholar]
- 28.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002 Feb;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 29.Perkins KA, Lerman C, Keenan J, Fonte C, Coddington S. Rate of nicotine onset from nicotine replacement therapy and acute responses in smokers. Nicotine & Tobacco Research. 2004 Jun;6(3):501–507. doi: 10.1080/14622200410001696547. [DOI] [PubMed] [Google Scholar]
- 30.Schneider NG, Lunell E, Olmstead RE, Fagerstrom KO. Clinical pharmacokinetics of nasal nicotine delivery. A review and comparison to other nicotine systems. Clinical Pharmacokinetics. 1996 Jul;31(1):65–80. doi: 10.2165/00003088-199631010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995 Jun;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 32.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001 Feb;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 33.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993 Feb;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 34.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986 Mar;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 35.Paradiso KG, Steinbach JH. Nicotine is highly effective at producing desensitization of rat alpha4beta2 neuronal nicotinic receptors. Journal of Physiology. 2003 Dec 15;553(Pt 3):857–871. doi: 10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins KA, Ciccocioppo M, Jacobs L, Doyle T, Caggiula A. The subjective and reinforcing effects of visual and olfactory stimuli in alcohol drinking. Experimental and Clinical Psychopharmacology. 2003;11(4):269–275. doi: 10.1037/1064-1297.11.4.269. [DOI] [PubMed] [Google Scholar]
- 37.Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcoholism: Clinical and Experimental Research. 1998 Dec;22(9):1903–1911. [PubMed] [Google Scholar]