Abstract
The increased availability of highly palatable foods is a major contributing factor toward the development of compulsive eating in obesity and eating disorders. It has been proposed that compulsive eating may develop as a form of self-medication to alleviate the negative emotional state associated with withdrawal from highly palatable foods. This study was aimed at determining whether withdrawal from chronic, intermittent access to a highly palatable food was responsible for the emergence of depressive-like behavior. For this purpose, a group of male Wistar rats was provided a regular chow diet 7 days a week (Chow/Chow), whereas a second group of rats was provided chow for 5 days a week, followed by a 2-day access to a highly palatable sucrose diet (Chow/Palatable). Following 7 weeks of diet alternation, depressive-like behavior was assessed during withdrawal from the highly palatable diet and following renewed access to it, using the forced swim test, the sucrose consumption test, and the intracranial self-stimulation threshold procedure. It was found that Chow/Palatable rats withdrawn from the highly palatable diet showed increased immobility time in the forced swim test and decreased sucrose intake in the sucrose consumption test compared with the control Chow/Chow rats. Interestingly, the increased immobility in the forced swim test was abolished by renewing access to the highly palatable diet. No changes were observed in the intracranial self-stimulation threshold procedure. These results validate the hypothesis that withdrawal from highly palatable food is responsible for the emergence of depressive-like behavior, and they also show that compulsive eating relieves the withdrawal-induced negative emotional state.
Keywords: anhedonia, brain stimulation reward, depression, eating disorders, food addiction, forced swim test, rat, sucrose
Introduction
The increased availability of energy-dense, highly palatable foods (e.g. foods rich in sugars and/or fats) is believed to be a contributing factor in the emergence of certain forms of obesity and eating disorders (Yach et al., 2006). Overeating of highly palatable foods is typically characterized by episodes of excessive, rapid, and compulsive food consumption within short periods of time (APA, 2000; Corwin, 2006; Avena et al., 2007; Cottone et al., 2012). Because of perceived cultural norms for thinness or health, episodes of overeating are typically followed by dieting and restriction to ‘safe’ foods. Dietary restraint, in turn, sustains cravings for the more appetitive palatable foods and promotes the next binge of ‘forbidden foods’. Therefore, a systematic alternation between foods of different palatability results in a self-perpetuating vicious circle of a binge/restriction pattern of consumption (Polivy and Herman, 1985; Laessle et al., 1989; De Castro, 1995; Mela, 2001).
This cycling pattern of consumption has raised the question of whether a ‘food addiction’ may indeed exist (Corwin and Grigson, 2009; Epstein and Shaham, 2010). Obesity and eating disorders, like drug addiction, have been proposed to be chronic relapsing conditions with alternating periods of abstinence and relapse from highly palatable foods that continue despite negative consequences. Many analogies have been drawn between drug dependence and compulsive eating in obesity and eating disorders, including loss of control over drug/food, inability to terminate one’s drug use/overeating despite the knowledge of adverse consequences, distress, and dysphoria when attempting to abstain from drug/food (Willard, 1991; Geliebter and Aversa, 2003; Steiger et al., 2005; Wolfe et al., 2009).
The shift from positive to negative reinforcement is hypothesized to be responsible for the transition from casual drug use to dependence in drug addiction. (Koob and Kreek, 2007; Koob, 2008). In the addiction stage, craving and compulsive drug use are believed to be sustained by the negative emotional state and dysphoria associated with abstinence (e.g. withdrawal). Similarly, it has been proposed that compulsive eating may result as a form of self-medication to alleviate the negative emotional state associated with withdrawal from highly palatable foods (Cottone et al., 2009a, 2009b; Parylak et al., 2011). Abstinence from highly palatable foods may then be responsible for the emergence of a withdrawal syndrome characterized by dysphoria, anxiety, and anhedonia, which may, in turn, drive relapse and binge eating.
In this context, it has been shown recently that chronic, intermittent access to highly palatable foods results not only in hyperphagia of the highly palatable diet but also in withdrawal-dependent behaviors, which include hypophagia, motivational deficits to obtain the less palatable food, and anxiogenic-like behavior (Cottone et al., 2008, 2009a, 2009b). However, whether the negative emotional state observed upon the removal of a highly palatable diet also includes a depressive-like behavior is still unknown. Therefore, this study aimed at determining whether depressive-like behavior occurs following withdrawal from chronic, intermittent access to a highly palatable diet. To test this hypothesis, we evaluated the emergence of (i) immobility, using a forced swim test, (ii) anhedonic-like behavior, measuring the consumption of a sucrose solution, and (iii) brain reward deficit, measuring the threshold for intracranial self-stimulation (ICSS), in diet cycled rats during both withdrawal from the highly palatable diet and during renewed access to it.
Methods
Subjects
Male Wistar rats, weighing 180–230 g and 41–47 days old at arrival (Charles River, Wilmington, Massachusetts, USA), were double housed in wire-topped, plastic cages (27 × 48 × 20 cm) on a 12 h reverse light cycle (lights off at 9:00 a.m.), in an AAALAC-approved humidity-controlled (60%) and temperature-controlled (22°C) vivarium. Rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012; 65%kcal carbohydrate, 13% fat, 21% protein, metabolizable energy 341 cal/100 g; Harlan, Indianapolis, Indiana, USA) and free access to water at all times unless otherwise specified. The procedures used in this study adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85-23, revised 1996) and the Principles of Laboratory Animal Care and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee (IACUC).
Free-access palatable diet alternation
The free-access palatable diet alternation was performed as described previously (Cottone et al., 2008, 2009a, 2009b). Briefly, after acclimation, rats were divided into two groups matched for food intake, body weight, and feed efficiency from the previous 3–4 days. One group was then provided with free access to a chow diet (Chow) 7 days a week (Chow/Chow, the control group of this study) and a second group was provided with free access to chow for 5 days a week, followed by 2 days of free access to a highly palatable, chocolate-flavored, high-sucrose diet (Palatable; Chow/Palatable). All the behavioral tests were performed in rats that had been diet-cycled for at least 7 weeks. The ‘chow’ diet was the above-described corn-based chow from Harlan, whereas the palatable diet was a nutritionally complete, chocolate-flavored, high-sucrose (50% kcal), AIN-76A-based diet that is comparable in macronutrient proportions and energy density to the chow diet [chocolate-flavored Formula 5TUL: 66.7%kcal carbohydrate, 12.7% fat, 20.6% protein, metabolizable energy 344 kcal/100 g; TestDiet, Richmond, Indiana, USA; formulated as 45mg precision food pellets to increase its preferredness (Cooper and Francis, 1979; Laboure et al., 2001)]. For brevity, the first 5 days (chow only) and the last 2 days (chow or palatable according to the experimental group) of each week are referred to in all experiments as C and P phases. Diets were never concurrently available. The relative diet preferences, calculated as the percentage of daily intake (kcal) of the first diet in relation to the second diet, were as follows: 5TUL Chocolate Diet (sugary Palatable diet) vs. Harlan LM-485 chow (M±SEM preference 90.7±3.6%), as published previously (Cottone et al., 2009b). Feed efficiency was calculated as mg body weight gained/kcal energy intake (Cottone et al., 2009b).
Forced swim test
The forced swim test was adapted from the test described by Porsolt et al. (1977) and Castagné et al. (2011), using a larger diameter cylinder and deeper water to increase sensitivity, as described previously (Wieland and Lucki, 1990; Detke et al., 1995; Sabino et al., 2009a). Under light, rats (n=19) were individually placed in two clear polypropylene cylinders (38 cm height, 27 cm diameter) that were separated by an opaque screen. The cylinders contained 23–25°C, 24 cm deep water. At this depth, rats are unable to support themselves by standing (Wieland and Lucki, 1990; Detke et al., 1995). The water was changed between subjects. Two swim sessions were conducted: an initial 15-min pretest, followed 24 h later by a 5-min test. Following each swim session, the rats were removed from the cylinders, dried, placed in heated cages for 10 min, and then returned to their home cages. Test sessions were videotaped and later scored manually using a timer; the time spent immobile, swimming, and climbing was determined. Chow/Palatable rats were diet-cycled for 7 weeks as described above. During the 8th week of cycling, Chow/Palatable rats were tested during either the C or the P phase, with Chow/Chow rats being concurrently tested in a between-subject design. The 15-min pretest was performed 1 day after the switches (P→C or C→P), whereas the 5-min test was performed 24 h later. Chow/Chow control rats were tested concurrently in a between-subjects design. The respective diet was freely available until the time of testing. Rats were approximately 4 months old at the time of the forced swim test.
Sucrose consumption test
The sucrose consumption test was adapted from Chen et al. (2012). Rats from the ICSS study (n=15, a subject was removed from the study because of place preference) were exposed to a 0.8% sucrose solution with food, water, and the sweet solution freely available in their home cage for at least 1 week to familiarize them to the sweet beverage. Previous exposure occurred during diet alternation and was used to circumvent the possible avoidance of the novel taste because of neophobia (D’Souza and Markou, 2010). The positions of the sucrose and water bottles were alternated every day to prevent place preference. On the first day of both the P phase and the C phase, rats were allowed to drink the 0.8% sucrose solution provided in their home cage for 1 h during the dark cycle. Sucrose consumption was evaluated in both C and P phases in the same animals using a within-subjects design. Sucrose intake was measured as ml/kg of body weight.
Intracranial self-stimulation
Surgeries for electrode placement
After acclimation, rats (n=16) underwent unilateral implantation of a 0.125mm diameter bipolar stainless-steel electrode (MS303/3-B/SPC, length 10.5mm; Plastics One, Roanoke, Virginia, USA) into the left or the right medial forebrain bundle at the level of the lateral hypothalamus using the following coordinates: AP − 0.5mm from the bregma, ML ±1.7mm, DV − 9.7mm from the skull with the incisor bar set 5.0mm above the interaural line, according to the atlas of Pellegrino (1979). Four stainless-steel jeweler’s screws were fastened to the rat’s skull around the electrode. Dental restorative filled resin (Henry Schein Inc., Melville, New York, USA) and acrylic cement were applied forming a pedestal that firmly anchored the electrode. The surgery involved anesthetizing the rats (isoflurane, 2–3% in oxygen) and securing them in a Kopf Instruments stereotaxic frame (David Kopf Instruments, Tujunga, California, USA; Cottone et al., 2007). Subjects were allowed to recover from surgery for at least 7 days before the initiation of the ICSS training.
Apparatus
ICSS training and testing took place in clear polycarbonate/aluminum modular operant test chambers encased in individual sound-attenuating and ventilated environmental cubicles (66 × 56 × 36 cm) (Med Associates, St Albans, Vermont, USA) (Blasio et al., 2011; Sabino et al., 2011). Each chamber had a grid floor and there was a retractable lever on a side wall (Sabino et al., 2006, 2009b). Subjects were connected to the electrical stimulation circuit by bipolar leads (Plastics One) and gold contact swivel commutators (Plastics One). Constant current square wave stimulators (Med Associates) were used to deliver electrical brain stimulation. All programming functions were controlled by a computer with a 10-ms resolution.
Intracranial self-stimulation threshold procedure
After recovery from surgery, thresholds for rewarding brain stimulation were determined using the rate-independent discrete-trial current intensity procedure designed originally by Kornetsky and colleagues (Marcus and Kornetsky, 1974; Esposito and Kornetsky, 1977; Kornetsky et al., 1979) and described in detail by Markou and Koob (1991, 1992). Rats were trained to lever press on a fixed ratio (FR) 1 schedule of reinforcement to obtain 500-ms trains of electrical stimulation. Each stimulus consisted of a 500-ms train with a pulse width of 0.2 ms and a delay of 0.2 ms between the positive and the negative pulses. All rats were first tested at the 50 Hz frequency, and if the current level at which they responded was below 80 or above 120 μA and unstable, then the frequencies were individually adjusted for each animal to reach the desired range of current and were maintained constant for the entire experimental procedure (Kenny and Markou, 2005). Once stable FR1 operant responding for the electrical stimulus was established, ICSS thresholds were assessed using the following procedure. At the beginning of each trial, rats received a noncontingent stimulus (S1), after which they had the opportunity, during a 7.5 s limited period, to lever press, which resulted in the delivery of a contingent stimulus (S2) that was identical to the previous S1. A 7.5–22.5 s (average 15 s) period of time elapsed between S2 delivery and the delivery of the next S1. If no response occurred, this time period began at the end of the 7.5-s period allotted for response. These time periods were randomized so that animals could not ‘predict’ the next S1 delivery. A‘trial’ consisted of five presentations of S1 at a fixed current intensity (in μA). Three or more responses at that intensity were scored as a plus (+) for that trial, whereas two or fewer responses were scored as a minus (−) for that trial. If the animal scored a (+) for the first trial, the second trial began at an intensity of 5 μA lower than the first. The current intensity continued to decrease by the same fixed intensity until the animal scored a (−) for two consecutive trials. When this occurred, the current intensity at the second trial at which a (−) score was obtained was repeated and the current intensities were then ascended by 5 μA for each trial until the animal scored a (+) for two consecutive trials. Each set of ascending or descending current intensities was defined as a ‘column’, and a total of six alternating descending/ascending columns were performed for each session. The intensity at the midpoint between (+) and (−) was defined as the column threshold. The threshold for each session was calculated as the mean of the last four column thresholds; the first and second column thresholds were, therefore, excluded. An increase in the reward threshold indicated that stimulus intensities that were previously perceived as reinforcing were no longer perceived as rewarding, reflecting a decrease in reward function and suggesting a depressive-like state. Conversely, lowering of the reward threshold reflected increased reward function (Markou and Koob, 1991).
To discourage the subject from responding during the inter-trial interval, any response during this period postponed the onset of the S1 for an additional 22.5 s (a length of time that exceeded or was equal to the original random duration of the inter-trial interval). These ‘punished’ responses were recorded as timeout responses and represented a measure of impulsivity-like disinhibition of responding. Excessive lever responses within 2 s after the initial response had no consequences and were recorded as cluster responses.
Response latency was defined as the time between the delivery of the S1 and the animal’s response on the lever. The average response latency for each test session was defined as the mean response latency of all trials for which the animal responded. After recovery from surgery, rats were trained daily in the ICSS procedure 2 h after diet switch. Following threshold stabilization, rats underwent diet cycling. Given the length of the diet alternation (7 weeks), animals were tested only once a week to avoid the loss of the electrode implant. Rats were provided the opportunity to be retrained daily during the 7th week of diet cycling, and they were finally tested daily during weeks 8, 9, and 10 of the diet-cycling procedure.
Statistical analysis
Immobility, swimming, and climbing time in the forced swim test during the first and the second day of the test was analyzed using one-way analyses of variance (ANOVAs), with diet condition as a between-subjects factor. Two-way ANOVAs with diet condition as a between-subjects factor and time bin as a within-subjects factor were used to analyze the time course of immobility. Sucrose consumption was analyzed using a two-way ANOVA with diet schedule as a between-subjects factor and phase as a within-subjects factor. Planned Bonferroni’s corrected t-tests were used to compare Chow/Chow and Chow/Palatable groups during the two phases, with the level of significance set at P value less than 0.025. The daily ICSS thresholds and latencies to respond were averaged within each phase during weeks 8, 9, and 10. They were analyzed using three-way mixed ANOVAs with diet schedule as a between-subjects factor and week and phase as within-subject factors. The software/graphic packages used were Systat 11.0, SigmaPlot 11.0 (Systat Software Inc., Chicago, Illinois, USA), InStat 3.0 (GraphPad, San Diego, California, USA), Statistica 7.0 (Statsoft Inc., Tulsa, Oklahoma, USA), PASW Statistics 18.0 (SPSS Inc., Chicago, Illinois, USA), and G*Power 3.1 (http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/).
Results
Effects of palatable diet alternation on immobility time in the forced swim test
As shown in Fig. 1a, Chow/Palatable rats withdrawn from chronic, intermittent access to palatable food showed increased immobility time in both the 15-min pretest [F(2,16)=4.37, P<0.05] and the 5-min test [F(2,16)= 3.78, P<0.05], compared with Chow/Chow rats. The increase in the immobility time of palatable food-withdrawn rats was ~97% in the pretest session and ~187% in the test session, compared with the control rats. Interestingly, the immobility time of Chow/Palatable rats, when tested when the palatable diet was offered (P phase), did not differ from that of the control Chow/Chow rats in either the 15-min pretest or the 5-min test. As the forced swim test cannot be repeated on the same animals, a between-subjects design was used. However, because of the small sample size of Chow/Chow subjects available for this study (n=19, effect size=0.4, α probability error=0.05, power=0.4), the Chow/Chow animals tested in the two phases were pooled into a single group, as they were not statistically different. For completeness, the forced swim test immobility data, parsed into C and P phases for all groups, were as follows (mean±SEM): pretest C phase 107.8±16.4 vs. 323.3±33.3, pretest P phase 201.1±33.5 vs. 180.4±61.5; test C phase 23.8±14.7 vs. 101.2±19.1, test P phase 42.9±4.8 vs. 61.0±17.1, Chow/Chow and Chow/Palatable, respectively. Moreover, the two-way ANOVAs performed on the time bins of immobility across the 15min of the pretest or the 5 min of the test showed significant main effects of the Diet Schedule [pretest: F(2,16)=4.37, P<0.05; test: F(2,16)=3.78, P<0.05] and of Time [pretest: F(4,64)=18.55, P<0.001; test: F(4,64)=15.44, P<0.001], but the Time × Diet Schedule interactions were not significant [pretest: F(8,64)=1.06, NS; test: F(8,64)=0.97, NS].
Fig. 1.
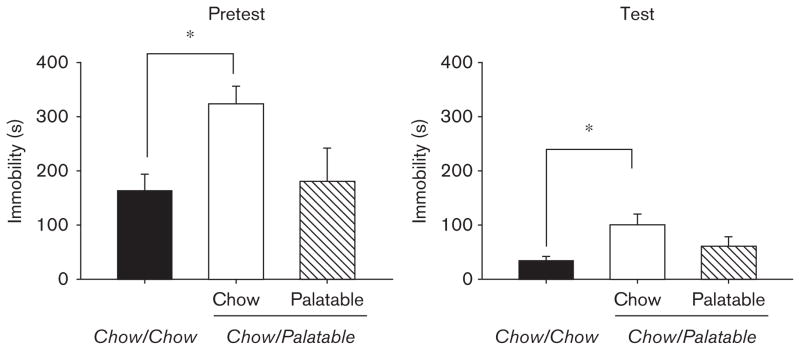
Effects of chronic, intermittent access to a highly palatable diet on immobility, assessed using a forced swim test in Wistar rats (mean±SEM: n =19), in the 15-min pretest (left panel), and the 5-min test (right panel). *Chow/Palatable (C phase) differs from Chow/Chow, P<0.05 (Fisher’s least significant difference test).
Significant effects on swimming time were also observed in both the pretest [F(2,16)=4.50, P<0.05] and the test session [F(2,16)=5.27, P<0.02], with palatable food-withdrawn Chow/Palatable rats swimming ~22 and ~27% less than Chow/Chow rats during the two sessions, respectively (data not shown). Again, the swimming time of Chow/Palatable rats, which were tested during the P phase, did not differ from the control Chow/Chow rats in either session. Climbing time did not differ among groups in either the pretest [F(2,16)=0.52, NS] or the test session [F(2,16)=3.13, NS] (data not shown). There was no difference in body weight among groups at the time of the test [mean±SEM:558±26.8 vs. 519±21.8 vs. 533±11.4; F(2,16)=0.92, NS, Chow/Chow vs. Chow/Palatable in the P phase vs. Chow/Palatable in the C phase, respectively].
Effects of palatable diet alternation on the sucrose consumption test
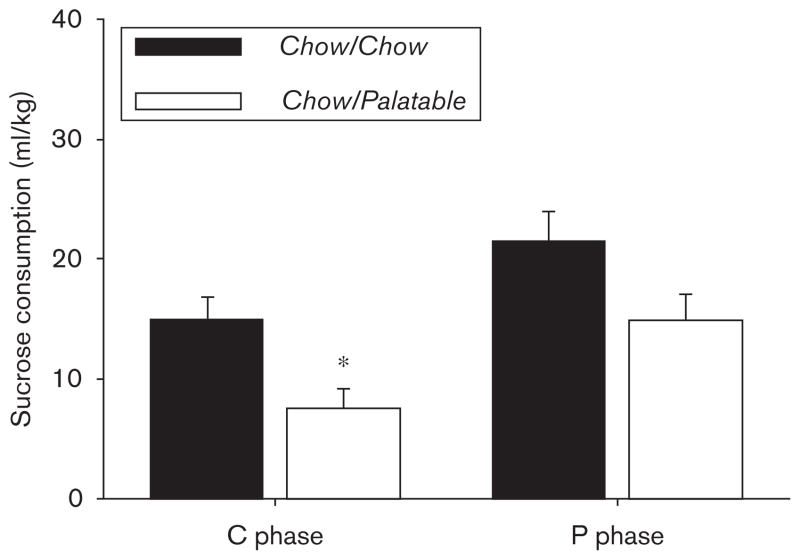
As shown in Fig. 2, rats withdrawn from chronic, intermittent access to the highly palatable diet showed decreased sucrose consumption compared with Chow/Chow rats that were continuously fed the standard chow [Diet Schedule: F(1,13)=6.74, P<0.05; Phase: F(1,13)= 26.681, P<0.001; Diet Schedule × Phase: F(1,13)= 0.084, NS]. Indeed, a Bonferroni corrected t-test showed that during the first day of withdrawal from the chocolate-flavored diet (C phase), Chow/Palatable rats drank significantly less sucrose compared with Chow/Chow rats. The sucrose consumption of the Chow/Palatable rats withdrawn from the highly palatable diet decreased by more than 50% compared with Chow/Chow rats. There was a tendency for sucrose consumption to decrease during the P phase; however, this trend was not statistically significant. There was no significant difference in the absolute body weight between groups at the time of the test (mean±SEM: 575±28.4 vs. 591±29.5; t(15)=0.69, NS, Chow/Chow vs. Chow/Palatable, respectively).
Fig. 2.
Effects of chronic, intermittent access to a highly palatable diet on sucrose consumption in Wistar rats (mean±SEM: n=15). *Chow/Palatable differs from Chow/Chow, P<0.05 (Bonferroni’s corrected t-test).
Effects of palatable diet alternation on the intracranial self-stimulation threshold
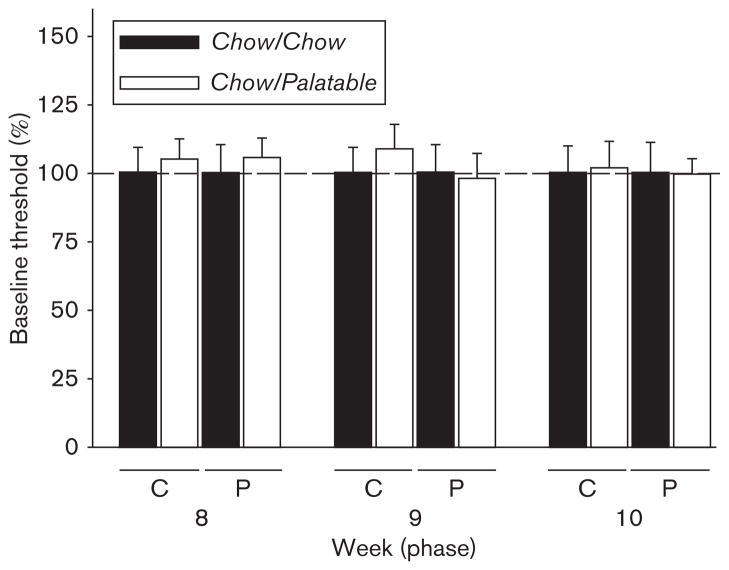
The ICSS threshold of the Chow/Chow and Chow/Palatable groups was analyzed during the withdrawal phase (C) and the renewing access phase (P) for three consecutive weeks (8, 9, and 10). As indicated by the three-way ANOVA and shown in Fig. 3, intermittent access to the highly palatable diet had no significant effect on the ICSS threshold [Diet Schedule: F(1,14)=0.05, NS; Diet Schedule × Phase: F(1,14)=1.58, NS; Diet Schedule × Week: F(2,28)=0.29, NS; Diet Schedule × Phase × Week: F(2,28)=0.24, NS]. In the same time period, highly palatable diet alternation did not affect the latency to respond [Diet Schedule: F(1,14)=0.54, NS; Diet Schedule × Phase: F(1,14)=2.39, NS; Diet Schedule × Week: F(2,28)=2.61, NS; Diet Schedule × Phase × Week: F(2,28)=0.30, NS] (Table 1). There was no significant difference in the absolute body weight among groups at the time of the test [mean±SEM: 527.89±15.15 vs. 507.0±19.74; t(14)=0.40, NS, Chow/Chow vs. Chow/Palatable, respectively].
Fig. 3.
Effects of chronic, intermittent access to a highly palatable diet on brain reward function assessed measuring intracranial self-stimulation thresholds (percentage change from control Chow/Chow) in Wistar rats (mean±SEM: n=16).
Table 1.
Effects of chronic, intermittent access to a highly palatable diet on latencies to respond assessed using an intracranial self-stimulation procedure in Wistar rats (mean±SEM: n=16)
| Week | Chow/Chow
|
Chow/Palatable
|
||
|---|---|---|---|---|
| C phase | P phase | C phase | P phase | |
| 8 | 3.15±0.17 | 3.07±0.19 | 3.13±0.18 | 3.13±0.19 |
| 9 | 3.13±0.20 | 3.04±0.20 | 3.35±0.24 | 3.47±0.25 |
| 10 | 3.08±0.19 | 2.99±0.19 | 3.35±0.29 | 3.32±0.28 |
Discussion
The results of the present study show that withdrawal from chronic, intermittent access to highly palatable food is responsible for the emergence of an increased immobility in the forced swim test. Moreover, cycled Chow/Palatable rats showed anhedonic-like behavior as indicated by a decrease in the consumption of a familiar 0.8% sucrose solution. Interestingly, the intermittent, extended access to a highly palatable food did not increase the reward threshold in the ICSS paradigm, which would be interpreted as a brain reward system dysfunction.
Upon removal of the highly palatable diet, cycled rats showed an increase in immobility in the forced swim test. Equally important, the immobility time in Chow/Palatable rats reverted to a control level following renewed access to the sugary diet. The paradoxical therapeutic value of the highly palatable food observed in the forced swim test is consistent with the protective effects of a high-fat diet against the depressive-like behavioral phenotype induced by early life stress or chronic stress (Maniam and Morris, 2010a, 2010b, 2010c; Finger et al., 2011). Indeed, a palatable high-fat diet has been shown to ameliorate the heightened immobility induced by maternal separation and nonhandling (Maniam and Morris, 2010a, 2010b, 2010c). Moreover, mice fed a high-fat diet were protected against the depressive-like effects induced by unpredictable chronic psychosocial stress (Finger et al., 2011). The alternative interpretation that the increased immobility time in Chow/Palatable rats could be the result of an improved floating ability because of an increased body weight can be ruled out as the two groups did not differ significantly in body weight (Cottone et al., 2008, 2009a). Further studies will be required to ascertain how many weeks of cycling are required to develop depressive-like and/or anxiety-like behaviors following withdrawal from intermittent access to highly palatable food, as well as how long the maladaptive behaviors persist following the switch to the less preferred regular chow diet.
The forced swim test is known to have good predictive validity as it detects clinically used antidepressants reliably (Borsini and Meli, 1988). However, describing the immobility in the forced swim test as a depression-related measure is still highly controversial. Over the years, there have been many explanations and theories regarding the meaning of the immobility response in the forced swim test. Immobility in the forced swim test is widely interpreted as a passive behavior and a behavioral correlate of negative mood (Cryan and Mombereau, 2004; Slattery and Cryan, 2012). The immobility in the forced swim test has been interpreted as an inability or reluctance to maintain effort, rather than as a generalized hypoactivity (Willner, 1990); this reluctance correlates with the clinical findings that depressed patients show pronounced psychomotor impairments in tests that require sustained expenditure of effort, therefore conferring some construct validity to this test (Weingartner and Silberman, 1982). Although caution should be exercised to avoid overextrapolation of the behavioral readout in the forced swim test, it is also noteworthy that greater immobility in the forced swim test is induced by many factors including genetic predisposition (West and Weiss, 1998), the effects of stress (Solberg et al., 1999; Alonso et al., 2000; Tannenbaum et al., 2002), changes in food intake (Alcaro et al., 2002), and acute drug withdrawal (Cryan et al., 2003). Many of these factors also influence or are altered by the course of major depression in humans. Therefore, the forced swim test seems to measure a behavioral dimension that is relevant to depression and presents itself as an attractive model for assessing depression-related factors in animals.
We showed that rats with intermittent access to highly palatable food show a decreased consumption of a sucrose solution. Sucrose is a natural reinforcer; therefore, a reduced consumption or preference for a sucrose solution has been proposed to reflect a decreased sensitivity to rewards and, more generally, anhedonia (Papp et al., 1991; Muscat and Willner, 1992; D’Souza and Markou, 2010). A relevant point of discussion is related to the counterintuitive effect on sucrose consumption observed when rats were withdrawn from the sugary, palatable diet. One may expect that rats abstained from the sugary diet would increase, rather than decrease, their intake of sucrose solution because of a sucrose deprivation effect. However, the solution used to assess anhedonia in this study had a very low percentage of sucrose (0.8%), as is typical for this type of study (Rygula et al., 2005; D’Souza and Markou, 2010; Chen et al., 2012), but in clear opposition to the highly palatable diet, which had a very high percentage of sucrose (~50%). Therefore, the two tastants were clearly not equally rewarding.
The sucrose consumption of the Chow/Chow and Chow/Palatable groups tended to differ as a function of the phase, as indicated by a strong trend (P=0.08) of the interaction between the Diet Schedule and the Phase factors. Post-hoc comparisons showed that the groups differed only in the C phase, but not in the P phase, suggesting that the renewed access to the highly palatable diet may relieve the anhedonic-like behavior, analogous to what was observed in the forced swim test. These results are in agreement with the reported ability of comfort foods, such as high-fat diets, to reverse the anhedonia induced by maternal separation, measured as a decrease in the preference for a sucrose solution. However, it is important to note that, as only a nonsignificant interaction between the two factors was found, it could also be argued that the general decrease in consumption of 0.8% sucrose observed in the Chow/Palatable group might be dependent on a sensory adaptation, hedonic habituation, or negative hedonic contrast because of chronic exposure to the 50% sucrose diet.
The results of this study confirm the hypothesis that chronic intermittent access to highly palatable foods is responsible for the emergence of a negative emotional affect and that renewing access to it is able to relieve the withdrawal-induced negative affective (Cottone et al., 2008, 2009a, 2009b; Parylak et al., 2011), analogous to what is hypothesized for the development of drug dependence (Koob and Kreek, 2007; Koob, 2008). Withdrawal from drugs of abuse has been extensively shown to be accompanied by depressive-like behavior measured as increased behavioral despair in the forced swim test, decreased sucrose consumption, or decreased brain reward function in the ICSS. Indeed, increased immobility in the forced swim test has been shown during withdrawal from nicotine (Picciotto et al., 2002; Mannucci et al., 2006; Renoir et al., 2012), ethanol (Getachew et al., 2010; Walker et al., 2010; Williams et al., 2012), cocaine (Overstreet et al., 2000; Filip et al., 2006; Perrine et al., 2008), amphetamine (Cryan et al., 2003), MDMA (McGregor et al., 2003; Renoir et al., 2008), opiates (Anraku et al., 2001; Chartoff et al., 2012), and phencyclidine (PCP) (Noda et al., 1995). Moreover, a large body of evidence exists showing that chronic treatment with drugs of abuse including amphetamine (Barr and Phillips, 1999; Der-Avakian and Markou, 2010), nicotine (Ribeiro-Carvalho et al., 2011), and cannabinoids (Rubino et al., 2008; Bambico et al., 2010) can produce anhedonia during withdrawal, as measured by a reduction in sucrose/saccharin consumption. In addition, withdrawal from drugs of abuse results in a spontaneous increase in reward thresholds for ICSS, an effect shared by amphetamine (Paterson et al., 2000), cocaine (Markou and Koob, 1991), alcohol (Schulteis et al., 1995), THC (Gardner and Vorel, 1998), and nicotine (Epping-Jordan et al., 1998). Elevations in the ICSS threshold have also been observed when withdrawal is pharmacologically precipitated in opiate and nicotine dependence (Schulteis et al., 1994; Epping-Jordan et al., 1998; Kenny and Markou, 2006). Precipitated withdrawal is a procedure in which an antagonist is used to block the ongoing activity of a reinforcing drug at receptor targets. This procedure brings the timing of withdrawal under experimental control and is an effective tool for studying dependence processes when spontaneous withdrawal is difficult to measure or to obtain.
Surprisingly, in this study, intermittent access to a highly palatable diet did not influence the ICSS threshold. The effects of access to sweet or palatable tastants on the brain reward function have not been studied extensively, and existing findings are contrasting. Sukhotina et al. (2003) showed that deprivation from a nondrug reinforcer, saccharin –a non-caloric sweetener– is not associated with depressive-like behavior and can lower the ICSS threshold. In contrast, Johnson and Kenny (2010) showed recently that 18–23 h/day access to a cafeteria diet, which results in the development of obesity, can increase the reward threshold. Therefore, the lack of effect on the ICSS threshold in our study could be explained by many different factors, including the tastants used, the duration of access to the diet, and the development – or not – of obesity. In addition, an alternative explanation for the lack of any spontaneous alteration in the ICSS threshold in Chow/Palatable rats is that withdrawal may need to be pharmacologically precipitated to detect a deficit in the brain reward function. Furthermore, it is possible that diet-cycled rats showed alterations in the brain reward threshold at a time of day different from the one chosen in the present study. Therefore, specific training conditions could also potentially account for the lack of effect in the ICSS paradigm. Future studies will be required to validate these hypotheses. The discrepancy between the negative results obtained in the ICSS experiment and the positive results observed in the sucrose intake and forced swim test is an interesting point of discussion. Although the tests used in this study all assess depressive-like behavior, they measure markedly different behavioral outcomes: the forced swim test measures immobility in a putative life-threatening situation; the sucrose consumption test measures a subject’s motivation for a rewarding stimulus; and ICSS, through the direct stimulation of neurons of the medial forebrain bundle, measures the minimum intensity of current that reinforces behavior. Given the profound diversity of the paradigms used, it is likely that the three tests rely on different neurobiological substrates and that different neurotransmitters are involved. Therefore, a uniformity of the outcomes in the different tests may not necessarily be the only possible expected result. For instance, in another study, analogous to what was observed here, chronic mild stress was able to reduce the intake of a sucrose solution, but did not modify ICSS performance in PVG hooded rats (Nielsen et al., 2000).
The results of this study further validate the hypothesis that chronic, intermittent access to highly palatable food is responsible for the emergence of a negative emotional state, which in turn may trigger compulsive eating. Indeed, extensive preclinical and clinical literature highlights the strong relationship existing between emotionality and overeating (Adam and Epel, 2007; Dallman, 2010), and the key role played by the corticotropin-releasing factor (CRF) system (Ghitza et al., 2006; Teegarden and Bale, 2007; Cottone et al., 2009a; Warne, 2009; Shalev et al., 2010). In the specific context of the animal model we used here, we have shown previously that in rats exposed to intermittent access to a highly palatable diet, both compulsive eating and the withdrawal-dependent behavioral adaptations (i.e. hypophagia of the less preferred diet, the anxiety-like behavior, and the motivational deficit to obtain the less palatable food) were blocked by the selective CRF 1 receptor antagonist (Cottone et al., 2009a). In addition, withdrawal from the highly palatable diet was associated with an increased expression of CRF in the central nucleus of the amygdala, independent of any HPA axis activation, as indicated by the lack of either differential corticosterone release or CRF expression in the paraventricular nucleus of the hypothalamus between control and palatable food cycling subjects (Cottone et al., 2009a). Therefore, although not directly tested in the present paper, it can be speculated that the depressive-like behaviors resulting from chronic intermittent access to palatable food may be mediated by neuroadaptations in the extrahypothalamic CRF system. Indeed, the CRF system mediates the behavioral, autonomic, and endocrine response to stress, and has been proposed to play a key role in a variety of pathophysiological conditions involving abnormal responses to stress, such as depression (Lloyd and Nemeroff, 2011). A large body of evidence, resulting from observations in both laboratory animals and humans, has pointed to the relevance of an overactive CRF/CRF1 receptor system in depression. Importantly, the anxiety-related and depression-related phenotypes that result from chronic exposure to stress in animals have been shown to be dependent on an overactive CRF1 receptor system in limbic forebrain regions, including the amygdala, independent of actions of CRF on HPA axis activity (Nestler et al., 2002; Zorrilla and Koob, 2010).
Conclusion
We have shown previously that rats withdrawn from palatable food show decreased intake of the otherwise acceptable chow diet, decreased motivational effort to obtain the chow diet, and pronounced anxiety-like behavior (Cottone et al., 2009a). We now extend these findings by showing that chronic intermittent access to a sugary diet also induces heightened immobility and anhedonia, commonly interpreted as depressive-like behavior (D’Souza and Markou, 2010). The immobility was withdrawal dependent, as this maladaptive behavior was reverted by renewing access to the highly palatable diet. These results are in agreement with the hypothesis that withdrawal from chronic, intermittent access to highly palatable food induces a negative affective state (Cottone et al., 2009a, 2009b). Therefore, compulsive eating may serve to self-medicate the withdrawal-dependent negative emotional state, similar to what has been postulated for the development of drug addiction (Koob and Kreek, 2007; Koob, 2008).
Acknowledgments
The authors thank Stephen St Cyr for technical assistance, and Duncan Momaney and Tamara Zeric for editorial assistance. This publication was made possible by Grant Numbers DA023680, DA030425, MH091945, MH093650A1, and AA016731 from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), by the Peter Paul Career Development Professorship (P.C.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Cabib S, Ventura R, Puglisi-Allegra S. Genotype- and experience-dependent susceptibility to depressive-like responses in the forced-swimming test. Psychopharmacology (Berl) 2002;164:138–143. doi: 10.1007/s00213-002-1161-8. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Damas C, Navarro E. Behavioral despair in mice after prenatal stress. J Physiol Biochem. 2000;56:77–82. doi: 10.1007/BF03179902. [DOI] [PubMed] [Google Scholar]
- Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl) 2001;157:217–220. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2007;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobio Dis. 2010;37:641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to D-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Blasio A, Narayan AR, Kaminski BJ, Steardo L, Sabino V, Cottone P. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: effects of 5-HT(2A/C) and 5-HT (1A) receptor agonists. Psychopharmacology (Berl) 2011;219:377–386. doi: 10.1007/s00213-011-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. In: Enna SJ, Williams M, editors. Current Protocols in Neuroscience. Unit 8.10A. Chapter 8. New York: Wiley; 2011. pp. 8.10A.1–8.10A.14. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Rada PV, Butzler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Francis RL. Effects of acute or chronic administration of chlordiazepoxide on feeding parameters using two food textures in the rat. J Pharm Pharmacol. 1979;31:743–746. doi: 10.1111/j.2042-7158.1979.tb13649.x. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Bingeing rats: a model of intermittent excessive behavior? Appetite. 2006;46:11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Grigson PS. Symposium overview – food addiction: fact or fiction? J Nutr. 2009;139:617–619. doi: 10.3945/jn.108.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible vs. resistant rats: central effects of urocortin 2. J Physiol. 2007;583:487–504. doi: 10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol. 2008;295:R1066–R1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, et al. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA. 2009a;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009b;34:38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, et al. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.89. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. Curr Top Behav Neurosci. 2010;3:119–178. doi: 10.1007/7854_2009_20. [DOI] [PubMed] [Google Scholar]
- De Castro JM. The relationship of cognitive restraint to the spontaneous food and fluid intake of free-living humans. Physiol Behav. 1995;57:287–295. doi: 10.1016/0031-9384(94)00229-x. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21:359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Shaham Y. Cheesecake-eating rats and the question of food addiction. Nat Neurosci. 2010;13:529–531. doi: 10.1038/nn0510-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R, Kornetsky C. Morphine lowering of self-stimulation thresholds: lack of tolerance with long-term administration. Science. 1977;195:189–191. doi: 10.1126/science.831268. [DOI] [PubMed] [Google Scholar]
- Filip M, Faron-Gorecka A, Kusmider M, Golda A, Frankowska M, Dziedzicka-Wasylewska M. Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain Res. 2006;1071:218–225. doi: 10.1016/j.brainres.2005.11.099. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eat Behav. 2003;3:341–347. doi: 10.1016/s1471-0153(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol Biochem Behav. 2010;96:395–401. doi: 10.1016/j.pbb.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Laboure H, Saux S, Nicolaidis S. Effects of food texture change on metabolic parameters: short- and long-term feeding patterns and body weight. Am J Physiol Regul Integr Comp Physiol. 2001;280:R780–R789. doi: 10.1152/ajpregu.2001.280.3.R780. [DOI] [PubMed] [Google Scholar]
- Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. Behavioral and biological correlates of dietary restraint in normal life. Appetite. 1989;12:83–94. doi: 10.1016/0195-6663(89)90098-6. [DOI] [PubMed] [Google Scholar]
- Lloyd RB, Nemeroff CB. The role of corticotropin-releasing hormone in the pathophysiology of depression: therapeutic implications. Curr Top Med Chem. 2011;11:609–617. doi: 10.2174/1568026611109060609. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Long-term postpartum anxiety and depression-like behavior in mother rats subjected to maternal separation are ameliorated by palatable high fat diet. Behav Brain Res. 2010a;208:72–79. doi: 10.1016/j.bbr.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010b;35:717–728. doi: 10.1016/j.psyneuen.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: role of hippocampus. Psychoneuroendocrinology. 2010c;35:1553–1564. doi: 10.1016/j.psyneuen.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Mannucci C, Tedesco M, Bellomo M, Caputi AP, Calapai G. Long-term effects of nicotine on the forced swimming test in mice: an experimental model for the study of depression caused by smoke. Neurochem Int. 2006;49:481–486. doi: 10.1016/j.neuint.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Marcus R, Kornetsky C. Negative and positive intracranial reinforcement thresholds: effects of morphine. Psychopharmacologia. 1974;38:1–13. [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Gurtman CG, Morley KC, Clemens KJ, Blokland A, Li KM, et al. Increased anxiety and ‘depressive’ symptoms months after MDMA (ecstasy) in rats: drug-induced hyperthermia does not predict long-term outcomes. Psychopharmacology (Berl) 2003;168:465–474. doi: 10.1007/s00213-003-1452-8. [DOI] [PubMed] [Google Scholar]
- Mela DJ. Determinants of food choice: relationships with obesity and weight control. Obes Res. 2001;9 (Suppl 4):249S–255S. doi: 10.1038/oby.2001.127. [DOI] [PubMed] [Google Scholar]
- Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev. 1992;16:507–517. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Arnt J, Sanchez C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: interstrain and interindividual differences. Behav Brain Res. 2000;107:21–33. doi: 10.1016/s0166-4328(99)00110-2. [DOI] [PubMed] [Google Scholar]
- Noda Y, Yamada K, Furukawa H, Nabeshima T. Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol. 1995;116:2531–2537. doi: 10.1111/j.1476-5381.1995.tb15106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Moy SS, Lubin DA, Gause LR, Lieberman JA, Johns JM. Enduring effects of prenatal cocaine administration on emotional behavior in rats. Physiol Behav. 2000;70:149–156. doi: 10.1016/s0031-9384(00)00245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol Behav. 2011;104:149–156. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Pellegrino LPA. A stereotaxic atlas of the rat brain. New York: Plenum; 1979. [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Dieting and binging. A causal analysis. Am Psychol. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Renoir T, Paizanis E, El Yacoubi M, Saurini F, Hanoun N, Melfort M, et al. Differential long-term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTT knock-out vs. wild-type mice. Int J Neuropsychopharmacol. 2008;11:1149–1162. doi: 10.1017/S1461145708009048. [DOI] [PubMed] [Google Scholar]
- Renoir T, Pang TY, Lanfumey L. Drug withdrawal-induced depression: serotonergic and plasticity changes in animal models. Neurosci Biobehav Rev. 2012;36:696–726. doi: 10.1016/j.neubiorev.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A, Lima CS, Nunes-Freitas AL, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Exposure to nicotine and ethanol in adolescent mice: effects on depressive-like behavior during exposure and withdrawal. Behav Brain Res. 2011;221:282–289. doi: 10.1016/j.bbr.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, et al. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009a;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, et al. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009b;34:1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, et al. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol. 1999;276:R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- Steiger H, Gauvin L, Engelberg MJ, Ying Kin NM, Israel M, Wonderlich SA, et al. Mood- and restraint-based antecedents to binge episodes in bulimia nervosa: possible influences of the serotonin system. Psychol Med. 2005;35:1553–1562. doi: 10.1017/S0033291705005817. [DOI] [PubMed] [Google Scholar]
- Sukhotina IA, Malyshkin AA, Markou A, Bespalov AY. Lack of depression-like effects of saccharin deprivation in rats: forced swim test, differential reinforcement of low rates and intracranial self-stimulation procedures. Behav Neurosci. 2003;117:970–977. doi: 10.1037/0735-7044.117.5.970. [DOI] [PubMed] [Google Scholar]
- Tannenbaum B, Tannenbaum GS, Sudom K, Anisman H. Neurochemical and behavioral alterations elicited by a chronic intermittent stressor regimen: implications for allostatic load. Brain Res. 2002;953:82–92. doi: 10.1016/s0006-8993(02)03273-0. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300:137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Weingartner H, Silberman E. Models of cognitive impairment: cognitive changes in depression. Psychopharmacol Bull. 1982;18:27–42. [PubMed] [Google Scholar]
- West CH, Weiss JM. Effects of antidepressant drugs on rats bred for low activity in the swim test. Pharmacol Biochem Behav. 1998;61:67–79. doi: 10.1016/s0091-3057(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Wieland S, Lucki I. Antidepressant-like activity of 5-HT1A agonists measured with the forced swim test. Psychopharmacology (Berl) 1990;101:497–504. doi: 10.1007/BF02244228. [DOI] [PubMed] [Google Scholar]
- Willard MD. Obesity: types and treatments. Am Fam Physician. 1991;43:2099–2108. [PubMed] [Google Scholar]
- Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, et al. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2691-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Animal models of depression: an overview. Pharmacol Ther. 1990;45:425–455. doi: 10.1016/0163-7258(90)90076-e. [DOI] [PubMed] [Google Scholar]
- Wolfe BE, Baker CW, Smith AT, Kelly-Weeder S. Validity and utility of the current definition of binge eating. Int J Eat Disord. 2009;42:674–686. doi: 10.1002/eat.20728. [DOI] [PubMed] [Google Scholar]
- Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]