Abstract
Pancreatic cancer remains a lethal malignancy with poor prognosis owing to therapeutic resistance, frequent recurrence and the absence of treatment strategies that specifically target the tumour and its supporting stroma. Deregulated cell-surface proteins drive neoplastic transformations and are envisioned to mediate crosstalk between the tumour and its microenvironment. Emerging studies have elaborated on the role of mucins in diverse biological functions, including enhanced tumorigenicity, invasiveness, metastasis and drug resistance through their characteristic O-linked and N-linked oligosaccharides (glycans), extended structures and unique domains. Multiple mucin domains differentially interact and regulate different components of the tumour microenvironment. This Review discusses: the expression pattern of various mucins in the pancreas under healthy, inflammatory, and cancerous conditions; the context-dependent attributes of mucins that differ under healthy and pathological conditions; the contribution of the tumour microenvironment in pancreatic cancer development and/or progression; diagnostic and/or prognostic efficacy of mucins; and mucin-based therapeutic strategies. Overall, this information should help to delineate the intricacies of pancreatic cancer by exploring the family of mucins, which, through various mechanisms in both tumour cells and the microenvironment, worsen disease outcome.
Introduction
Cellular transformation to malignancy involves the accumulation of various mutations and epigenetic modifications. Malignant cells recruit additional cellular components to the neighbouring stroma to develop and establish clinical disease. Histologically, pancreatic ductal adenocarcinoma, which accounts for >90% of all pancreatic cancer cases,1 is characterized by a remarkably dense stroma. In stark contrast to a healthy pancreas, which is devoid of or weakly expresses mucins, pancreatic cancer is characterized by aberrant expression of multiple mucins, as well as their secreted and spliced forms. With the emerging role of mucins in tumour development, progression and metastasis, it is becoming apparent that pancreatic cancer exploits the unique properties of mucins (namely, aberrant expression, altered glycosylation, differential localization and adhesive and anti-adhesive traits) to interact with its microenvironment, survive and proliferate in an otherwise inhospitable local environment, and invade and metastasize to distant locations. This Review summarizes emerging data on the role of mucins in the development and progression of pancreatic cancer and its microenvironment. Furthermore, it elaborates on the diagnostic and therapeutic contributions of mucins for patients with pancreatic cancer.
Mucins
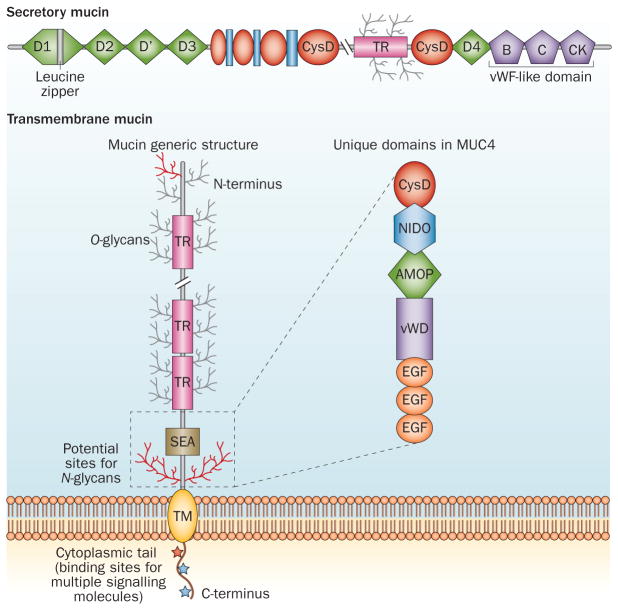
With 21 members in the family, mucins are high-molecular-weight glycoproteins characterized by the presence of a heavily O-glycosylated tandem repeat region (TRR) that is rich in proline, threonine and serine residues (called PTS sequences) (Figure 1).2,3 Mucin genes display high levels of polymorphism owing to variable number of tandem repeats.2,3 The serine/threonine residues in the TRR are extensively glycosylated with O-linked (O-glycans; common) and N-linked (N-glycans; less common) oligosaccharides. The presence of large numbers of glycans on the polypeptide core of mucins results in large, flexible, rod-like molecules that can extend up to 200 nm from the apical cell surface into the lumens of ducts and glands.4 On the basis of their physiological and structural characteristics, mucins are further divided into two subfamilies— the membrane-bound (also called transmembrane mucins, which are characterized by the presence of a hydrophobic plasma membrane-spanning domain) and secreted mucins (Figure 1).3,5 Transmembrane family members include MUC1, MUC3A/B, MUC4, MUC11–13, MUC15–17, MUC20 and MUC21. Secreted mucins are further subdivided into gel-forming (MUC2, MUC5AC, MUC5B, MUC6 and MUC19) and non-gel-forming (MUC7) groups (Table 1). Secreted mucins form the mucus layer on the apical surfaces of healthy epithelial cells that are exposed to the external environment, including the lining of the respiratory and gastrointestinal tracts, and lumen of ducts in specialized organs such as liver, pancreas, gall bladder, kidney, salivary glands, lacrimal glands and eye.6 The gel-forming attribute is a result of the presence of an oligomerizable D domain (Figure 1).7,8 The N-glycans and O-glycans, extended structure and multiple functional domains present in the mucins [namely, epidermal growth factor (EGF), nidogen-like domain (NIDO), sea urchin sperm protein–enterokinase– agrin (SEA), von Willebrand factor D domain (vWD), cysteine knots and cytoplasmic tail], are known to interact with cell surface receptors, signalling mediators and the extracellular matrix (ECM). A comprehensive view of mucin domains and their associated roles is detailed in Box 1.
Figure 1.
Domain structure of transmembrane and secretory mucins. The characteristic domains of transmembrane mucins (represented by MUC1 as well as domains present exclusively in MUC4) and secretory mucins (represented by MUC5AC). See Box 1 for further details on the individual domains. Abbreviations: AMOP, adhesion-associated domain in MUC4 and other proteins; EGF, epidermal growth factor; NIDO, nidogen-like domain, SEA, sea urchin sperm protein– enterokinase–agrin; TM, transmembrane; TR, tandem repeat, vWD, von Willebrand factor D domain; vWF-like, von Willebrand factor like domain.
Table 1.
Mucin expression in fetal and adult pancreas
| Mucin | Fetal pancreas | Adult pancreas | Pancreatitis | PanINs I/II/III | PDAC | Metastatic PDAC | MCN | IPMN | Study |
|---|---|---|---|---|---|---|---|---|---|
| Gel-forming mucins | |||||||||
| MUC2 | − | − | − | − | − | ND | ++ (goblet cells) | ++ (goblet cells) | 9–11,128–130 |
| MUC5B | ± | ± | ++ | ND | +++ | ND | ND | ++ | 131 |
| MUC5AC | − | − | ± | +/++/+++ | +++ | +++ | +++ | ++ | 9,10,128,130 |
| MUC6 | + | + (interlobular and intralobular ducts; centroacinar cells) | ++ to +++ | ++/++/++ | +++ | ± | ND | ++ | 9 |
| Membrane-bound mucins | |||||||||
| MUC1 | ± | + (ductal cells; centroacinar cells) | ++ | ++/++/+++ | +++ | +++ | +++ (invasive cases) | +++ | 9,10 |
| MUC3 | ± | ± (interlobular and intralobular ducts; centroacinar cells) | ± | +/+/+ | ++ | ND | ND | ± | 44,83,128 |
| MUC4 | − | − | − | +/++/+++ | +++ | +++ | +++ | ++ | 9,10 |
| MUC7 | − | − | + | ND | + | +++ | ND | − | 83,128 |
| MUC13 | ND | ± (focal staining) | ND | ND | +++ | ND | ND | ND | 18 |
| MUC16 | ND | − | ± | +/++/++ | +++ | +++ | ND | ND | 17,132 |
| MUC17 | − | ++ (islets) | − | +++/+++/+++ | ++ | ND | ND | ND | 44 |
| MUC20 | + | + | ND | ND | ND | ND | ND | ND | 133 |
| MUC21 | ND | + | ND | ND | ND | ND | ND | ND | 134 |
Abbreviations: −, negative; +, low; ++, moderate; +++, intense; ±, negative or positive depending on study; IPMN, intraductal papillary mucinous neoplasms; MCN, mucinous cystic neoplasm; ND, not determined; PanIN, pancreatic intraepithelial neoplasia; PDAC, pancreatic ductal adenocarcinoma.
Box 1. Important domains and glycan modifications of mucins.
Tandem repeat region (TRR) comprises a proline, threonine and serine rich domain, heavily decorated with O-glycans, which is poorly conserved and repeated multiple times; the sequence and number of repeats varies in each family member148
D/von Willebrand D (vWD) domains are implicated in oligomerization and participate in cell adhesion, migration, homing and oligomerization149,150
Sea urchin sperm protein-enterokinase-agrin (SEA) domains are highly conserved; the extracellular domain undergoes a molecular strain-dependent autocatalytic cleavage during folding in the endoplasmic reticulum or upon application of a mechanical force151,152
Epidermal-growth-factor (EGF) domains act as intramembrane ligands of ERBB2 and are involved in inhibition of apoptosis and stimulating cell migration in colonic epithelial cells153
Adhesion-associated domain in MUC4 and other proteins (AMOP) is exclusive to MUC4; AMOP with eight invariant cysteine residues is predicted to mediate adhesion154
Nidogen-like domain (NIDO) is similar to the nidogen-EGF domain of ancestral nidogen proteins; this unique domain of MUC4 facilitates metastasis of pancreatic cancer cells79
Cytoplasmic tail has multiple phosphorylation sites and protein–protein interaction motifs, which mediate oncogenic signalling5,6,24,62
Mucins in pancreatic cancer
Pancreatic cancer is characterized by altered expression, glycosylation and localization of mucins during the transition from healthy to dysplastic and neoplastic states.
Aberrant expression
In contrast to a healthy pancreas, the expression profiles of both transmembrane (MUC1, MUC3, MUC4, MUC7, MUC13, MUC16, and MUC17) and secretory mucins (MUC5AC, MUC5B and MUC6) differ widely in pancreatic cancer (Table 1). For example, a healthy pancreas expresses low levels of MUC1 on the luminal surface of centroacinar cells as well as in the intralobular and interlobular ducts.9–11 By contrast, histological studies revealed a multifold increase in its expression as early as the pancreatic intraepithelial neoplasia (PanIN) lesion stage, which further increases during pancreatic cancer progression.9–11 Pancreatic cancer is also accompanied by de novo expression of MUC4, MUC5AC, MUC5B, MUC13, MUC15, MUC16 and MUC17 in PanIN lesions and their expression increases further with disease progression (Table 1).12–18
Aberrant changes in mucin expression are also the characteristic event in early lesions of pancreatic mucinous cystic neoplasms (MCN, mucin-producing epithelial cells associated with an ovarian type of stroma) and intraductal papillary mucinous neoplasms (IPMN, a mucin-producing epithelial neoplasm with a papillary architecture that is mainly present within the main pancreatic duct or one of its branches; Table 1). A meta-analysis of histological studies examining the correlation between mucin expression and progressive malignant lesions of IPMN indicated expression of MUC1, MUC2 and MUC5AC in benign lesions, which increased with malignant development.19 Interestingly, MUC1 was found to have the strongest association with malignant progression of IPMN, whereas the expression of MUC5AC had the weakest association.19,20 Elevated levels of MUC4 have been observed in cystic fluid from high-risk IPMN cases.21
Emerging data from genome-wide sequence studies and RT-PCR analysis of mucin transcripts have indicated the presence of multiple alternatively spliced forms in pancreatic cancer (for example, 24 for MUC4).22,23 To determine the biological role of these alternatively spliced forms in pancreatic cancer (in comparison to their roles in healthy pancreatic tissue or pancreatitis specimens), analyses of their expression, oncogenic signalling and efficacy as diagnostic and prognostic markers need to be explored.
Loss of epithelial cell polarity
The loss of epithelial cell polarity is one of the hallmarks of tumour development. Loss of asymmetric distribution during tumour initiation brings apical mucins into close proximity with basolateral receptor tyrosine– protein kinases (RTKs), including epidermal growth factor receptor (EGFR), ERBB2, ERBB3 and fibroblast growth factor receptor (FGFR). These RTKs are central regulators of signalling cascades involved in cell survival, growth, proliferation and metastasis (Figure 2).24,25
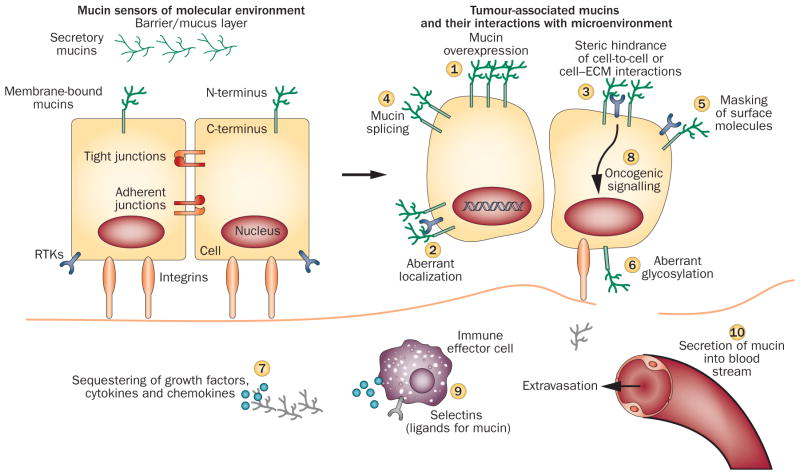
Figure 2.
Mucins in transition from normal to malignant cells. Mucins with their extended structure, hygroscopic nature and gelation abilities act as both sensor and defensive barrier to foreign insults under normal conditions. Cancer cells manipulate mucins at multiple levels to promote tumorigenicity. Events initiated by mucins (circled numbers) mediate the interactions between tumour cells and the surrounding stroma to create conditions favourable for tumour progression.
The interaction of mucins with various receptors is associated with altered trafficking, signalling and poor therapeutic response of anti-RTK antibodies. Indeed, the interaction of MUC1 with EGFR results in increased receptor internalization, recycling and nuclear localization, along with its reduced degradation in breast cancer cells.26 Interestingly, loss or knockdown of MUC1 drastically reduces EGFR expression and mammary tumour progression in transgenic mouse models.27,28 Furthermore, anti-MUC1 antibody (GP1.4) blocks EGFR-mediated signalling, leading to reduced proliferation and migration of pancreatic cancer cells.29 In depolarized breast cancer cells, MUC1 also constitutively associates with ERBB2, which, in turn, targets the MUC1–γ catenin complex to the nucleolus, leading to activation of the Wnt signalling pathway.30
MUC4 is proposed to be a transmembrane ligand for ERBB2, leading to its stabilization at the plasma membrane and enhanced activation.31 Enhanced surface accumulation of both ErbB2 and ErbB3 is mediated by the Muc4–sialomucin complex (a rat homologue of MUC4) by preventing their internalization.32 Additionally, the Muc4–sialomucin complex sterically hinders binding of anti-ErbB2 antibodies to the cell surface leading to a poor therapeutic response.33 Interestingly, transmembrane mucins are characterized by the presence of multiple structural motifs with sequence homology to EGF (referred to as EGF-like), which are thought to mediate heterodimerization of mucins with ERBB receptors (Box 1).5,34,35 The affinities and functional relevance of these interactions for altered oncogenic signalling are being explored.
Altered localization
Nuclear localization of mucins has been associated with large and poorly differentiated tumours, highly metastatic phenotypes and poor prognosis.5,34,36–38 Translocation of the MUC1 cytoplasmic tail to the nucleus in conjunction with β-catenin and EGFR is implicated in the generation of a metastatic gene signature and the epithelial-to-mesenchymal transition (EMT) of tumour cells.39,40 Aberrantly expressed MUC5AC has also been observed to disturb intercellular junctions by interfering with membrane localization of E-cadherin and disturbing E-cadherin-dependent cell–cell interactions.39,41 Furthermore, MUC5AC, being secretory, is speculated to form a protective gel around tumours that might impart a growth advantage to all kinds of neoplasms—benign or malignant—whereas MUC1, by altering the activity of RTKs, facilitates oncogenic signalling, and is suggested to be responsible for the progression of IPMN from benign to malignant lesions. All of these studies emphasize the point that mucins have a substantial role in imparting survival benefits to tumour cells during the changing microenvironment of neoplasia. As pancreatic cancer is characterized by altered expression and localization of multiple mucins, many of which are still unexplored, a systematic analysis of these mucins and their interacting partners will help to delineate the critical junctures responsible for pancreatic cancer aggressiveness.
Altered glycosylation
The extent of mucin glycosylation, mucin type as well as its expression pattern, fluctuate during neoplastic developments in various malignancies (Figure 2).42,43 Mucins in healthy tissues are decorated with core 3 structure, whereas glycosylation on ectodomains of tumour-associated mucins are largely Tn antigen, sialyl Tn and fucosylated core 1 structures (T/TF), which form important tumour-associated antigens (TAAs) (Figure 3).44 The core structure is shortened in tumour-associated mucins resulting in the exposure of internal sugar units and cryptic peptide sequences.50,51 Frequent elevation of TF, fucose and Lewis antigen on MUC1 and MUC5AC were observed in serum from patients with pancreatic cancer when compared with healthy individuals.45 In addition to core structures, more complex Lewis antigens and their sialylated (sialyl Lewisx/a) and sulphated forms are also expressed by tumour cells and shed into circulation. Furthermore, high levels of modified mannose-rich N-glycans were observed on MUC1, MUC16 and MUC5AC from patients with pancreatic cancer; these modified glycans are speculated to be involved in the suppression of cytolytic responses mediated by natural killer cells.45,46 Altered expression and localization of glycosyltransferases are the major contributors to the aberrant glycosylation of these mucins.47,48
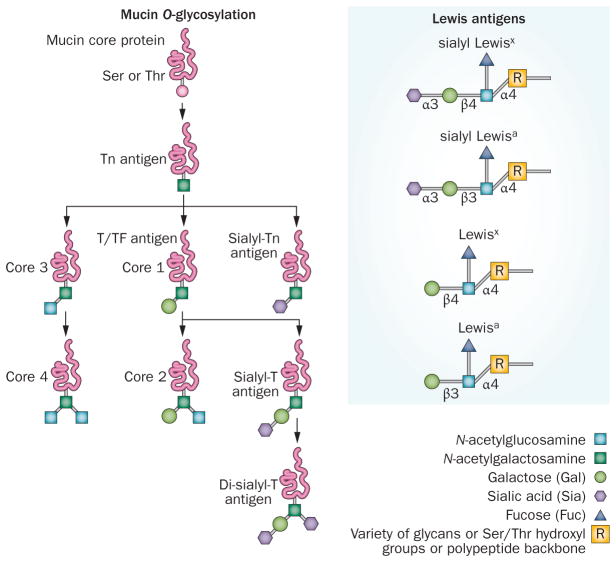
Figure 3.
Antigenic and differently expressed O-glycans of pancreatic cancer. Tn and sialyl-Tn antigen are the simplest O-glycans formed by the addition of N-acetylgalactosamine to the –OH group of serine/threonine residues. These antigens are expressed in >80% of human carcinomas and are involved in mediating invasion and metastasis of tumour cells.123,124 Thomsen-Friedenreich (TF)/T antigen/core 1 are formed by the addition of galactose to Tn antigen through β1-3 linkage; these glycans are expressed in >90% of human malignancies. MUC1-associated TF and galectin-3 interactions promote metastasis by facilitating adhesion to endothelial cells.125 Core 3 glycans are synthesized by the addition of N-acetylglucosamine to Tn antigen; further addition of β-1,6-N-acetylglucosamine to the core 3 forms core 4 glycans. These glycans are tumour suppressive and are predominantly expressed in healthy cells.126,127 Lewis antigen: Fucosylated carbohydrate antigen sialyl Lewisa (or CA19-9, prognostic marker for PC) and sialyl Lewisx (or NCC-ST-439, prognostic marker for breast cancer) are overexpressed during neoplastic developments, whereas disialyl Lewisa and sialyl-6-sulpho-Lewisx are expressed in healthy cells. By acting as ligands for E-selectin, sialyl Lewisx mediates the adhesion of tumour cells to vascular endothelial cells and facilitates their metastasis.92
The modified forms of glycosylation on mucins (that is, sialylated and fucosylated structures) are recognized by the lectin domain of selectins. The selectins are a family of vascular adhesion receptors (comprised of L-selectin, E-selectin and P-selectin) on the surface of leukocytes, endothelial cells and platelets.49 As the initial part of cell adhesion consists of leukocyte tethering and rolling on activated platelets or endothelial cells, the selectins need to support rapid and reversible interaction with their carbohydrate ligands under hydrodynamic flow. The sialyl Lewisx and sialyl Lewisa antigens are the terminal tetrasaccharides that comprise the core carbohydrate structure recognized by selectins. The presence of sialyl Lewisx structures on carcinoma mucins is frequently associated with advanced cancer and metastatic potential.50,51 Although the precise role of unique glycans on individual mucins is unknown, blot rolling and cell-free, flow-based adhesion assays clearly revealed that sialofucosylated MUC16 expressed by pancreatic cancer cells acts as a functional ligand for selectin having high binding activity for E-selectin and L-selectin, but low P-selectin binding activity under in vitro conditions.52 Overall, these studies suggest that mucin O-glycans and N-glycans are exploited by tumour cells for invasion and migration, as well as intravasation and extravasation. Henceforth, identification of tumour-specific glycan epitopes and associated glycosyltransferases will help to improve targeting and diagnosis of pancreatic cancer.
Adhesion and anti-adhesion
Mucins regulate the detachment of cells from the primary tumour mass,53–55 facilitate lymphatic and venous cellular invasion,13,56 enhance cellular survival in blood57 and are major mediators of adhesion at metastatic sites.51,58,59 The anti-adhesion function is generally mediated by the extended structures of mucins that mask the smaller surface adhesion molecules and sterically hinder the cell–cell and/or cell–substratum interactions and negatively charged sialylated residues, which create a repulsive barrier around the cell (Figure 2). The adhesive property of mucins is attributed to receptor–ligand interactions. The carbohydrate structure on mucins acts as a ligand for receptors of opposing cells that supplants their repulsive barrier. Furthermore, cells can regulate their adhesive properties using multiple mechanisms such as varying mucin expression under different microenvironmental conditions, expressing a specific splice variant or glycosyltransferase as well as shedding a mucin extracellular domain.60 MUC1 has been proposed to act both as an anti-adhesive and adhesive molecule.61 Thus, interactions initiated by mucins seem to establish a bridge between tumour cells and the surrounding stroma to produce conditions favourable for cancer cell growth.
Oncogenic signalling
Oncogenic signalling by the cytoplasmic tails of mucins is gaining attention as an important signal transducer between a tumour and the surrounding environment owing to the presence of multiple phosphorylation sites and other protein–protein interaction motifs.5,62 These roles of mucins have been comprehensively reviewed elsewhere.6,24
Interaction with the TME
Approximately 80% of the pancreatic tumour mass is formed by dense stroma comprising a cellular component (fibroblasts, myofibroblasts, pancreatic stellate cells, vascular and lymphatic endothelial cells, immune cells and endocrine cells), an associated acellular extracellular matrix (ECM, formed by collagen, fibronectin, laminin, proteoglycans, and glycosaminoglycans), and a liquid milieu of cytokines, growth factors and proteases.63 All of these components are interconnected and communicate with tumour cells to develop a highly complex and aggressive disease. The cancer cell secretes proinflammatory soluble factors and increases expression of proteins that support and interact with the growing desmoplasia. The interface between mucins and the tumour microenvironment (TME) is chiefly involved in mediating immune evasion, oncogenic signalling, angiogenesis and metastasis (Figure 4).
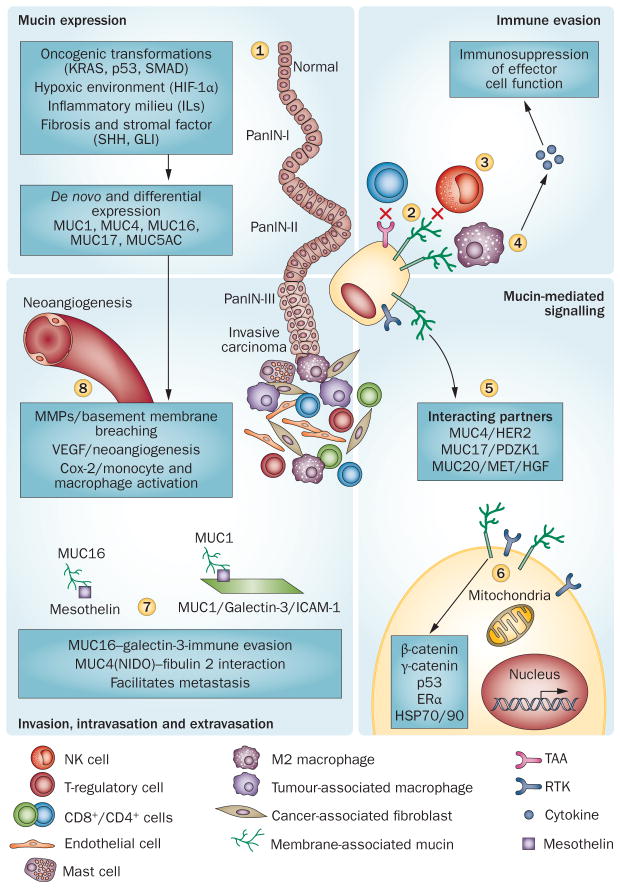
Figure 4.
Tumour cells crosstalk with stromal cells via mucins. Neoplastic developments elicit a proinflammatory and hypoxic environment leading to a desmoplastic and fibrotic reaction and enhanced mucin expression (1).41,76,118–122 Aberrantly upregulated mucins sterically hide the tumour-associated antigens from invading macrophages, neutrophils and cytotoxic T cells (2), and indirectly protect tumour cells from the cytotoxic effects of activated immune effector cells (3). TAG72 and CA125 modulate M2 macrophages to an immunosuppressive phenotype (4). Aberrantly localized mucins interact and impart stability to various RTKs and aggravate oncogenic signalling (5). MUC1 cytoplasmic tail, through interactions with a variety of proteins, mediates oncogenic signalling (6); its interaction with galectins promotes tumour cell survival during metastasis by avoiding killing by NK cells and helps in extravasation through interaction with ICAM1 (7). Similarly, MUC4 and MUC16 interactions facilitate metastasis (7). Overexpression of mucins and the hypoxic environment in pancreatic tumour cells leads to the production of various factors, that in turn, remodel the extracellular matrix and induce neoangiogenesis (8). Abbreviations: MMP, matrix metalloproteinase; PanIN, pancreatic intraepithelial neoplasias; RTK, receptor tyrosine–protein kinase.
Stromal cells
The protective barrier formed by mucins at the epithelial surfaces acts as a front line of defence against pathogens; however, increasing evidence illustrates their participation in altering the immune response to infection and other pathological conditions. Transmembrane mucins extending beyond the glycocalyx (~50 nm) mask TAAs, protecting tumours cells from cytotoxic components of cell-mediated immunity (Figure 4).53 In addition to this avoidance of immune recognition, activation, and destruction by steric hindrance, modified core 2 glycans on carcinoma-associated MUC1 have been implicated in attenuating the interactions of the NK cell receptor (NKG2D) with the tumour-associated ligand MICA (major histocompatibility complex class I-related chain A) through galectin-3 (Figure 4).64 Galectin-3, a member of the β-galactoside-binding protein family, is differentially expressed in tumours and serum in various malignancies including pancreatic cancer.65 Its overexpression is implicated in neo-angiogenesis, tumour cell adhesion and immune escape.66 Circulating galectin-3 binds to the NKG2D-binding site of MICA through modified core 2 O-glycans of MUC1 and, in turn, inhibits NKG2D–MICA interactions, resulting in the inactivation of natural killer cells and blocking of TNF-mediated apoptosis of tumour cells.64,67,68 As a result, cancer cells remain in circulation for a much longer period of time, promoting metastasis. Furthermore, tandem repeats of sialylated T antigens present on MUC1 act as counter receptors for myelin-associated glycoprotein (a membrane-bound protein expressed on oligodendrocytes and Schwann cells that binds myelin to neurons) and contribute to the adhesion between pancreatic tumour cells and Schwann cells, which facilitates perineural invasion.69
Mucins expressed on immune effector T cells influence their activation and proliferation in response to an antigen. Activated CD4+ T cells induce MUC1 expression that, in turn, enhances their proliferation and cytokine production in association with CD3 through the calcium-dependent NF-AT pathway.70 Overexpression of the Tn and sialyl Tn antigen on tumour-associated mucins correlates with COX-2 overexpression and low infiltration of CD8+ cytotoxic T cells.71
Tumour-associated macrophages are among the major players in directing the response of the immune system against a tumour. Studies have now suggested that tumour-associated macrophages are of the M2 type owing to their development in the T-helper 2 cellular environment that is rich in anti-inflammatory cytokines, including IL-10, IL-4, and IL-13.72 MUC1 expressed by tumour cells interacts with tumour-associated macrophages through sialoadhesin.73 The engagement of M2 macrophages with tumoral mucins, including TAG72 (tumour associated glycoprotein 72 overexpressed in pancreatic cancer) and CA125 (carbohydrate epitope located on the protein core of MUC16), resulted in their differentiation to an immunosuppressive phenotype with an increased production of IL-10, a decrease of the T-helper-1-attracting chemokine CCL3 and absence of the proinflammatory cytokine IL-12 (Figure 4).74 Mucins also promote endothelial cell proliferation and tube formation in response to hypoxia, another characteristic feature of pancreatic cancer.75 HIF1-α upregulates MUC1 expression and arbitrates MUC1 cytoplasmic tail migration to the nucleus in association with β-catenin and p53, resulting in the upregulation of proangiogenic factors such as vascular endothelial growth factor-A and platelet-derived growth factor A and B.76
Extracellular matrix
The mucin ectodomain can potentially sense and interact with the ECM. Various domains of mucins (Box 1) have independently been shown to interact with the ECM and considering these studies, it has been suggested that the vWD domain of mucins might act as a major site for tumour cell interaction with the ECM. Furthermore, MUC1 overexpression in pancreatic cancer cell lines has been shown to enhance invasiveness by decreasing the binding of cancer cells to type I and IV collagen and laminin.77 Glycosylation, especially by sialic acid, increases the adhesiveness of pancreatic cancer cells to the ECM.78 Studies from our group also indicate the presence of an interaction between the NIDO domain of MUC4 with nidogen-interacting protein fibulin 2 (a component of the basement membrane). 79 Deletion of the NIDO domain from MUC4 drastically hampered the interaction of tumour cells with fibulin 2, thus establishing the direct effect of mucin domains on breaching the integrity of the basement membrane and the strong involvement of mucins in metastatic events (Figure 4). The interaction between the MUC4–NIDO domain and galectin-3 from endothelial cells is responsible for the adhesion of the cancer cell to endothelial cell surfaces during the intravasation process.51,79
Integrin clustering, which is affected by MUC1, detects the physical properties of the ECM and is involved in regulating the migration and metastatic potential of cancer cells. Integrin clustering is also influenced by the thickness of the glycocalyx (determined by mucins) and a relatively thinner glycocalyx leads to poor integrin clustering, even in the presence of a high affinity ligand.80 In 2009, when examining the physiomechanical characteristics of a tumour, Levental et al.81 observed that tumours with a stiffer ECM progress faster and possess higher invasive potential than those with a more flexible ECM.51,79,81 The tripartite relationship between mucin (glycocalyx), integrin and collagen might be responsible for stiffness and facilitates metastasis. Mucin expression in pancreatic cancer is directly implicated in RTK oligomerization, as well as sequestration of the ligands secreted in extracellular matrices to potentiate the proliferative signalling pathways. Overall, mucins are key mediators for the alliance between tumour cells and their microenvironment. Future studies to delineate these molecular interactions are urgently required to promote the effective targeting of stroma along with the tumour cell.
Diagnostic markers
Scarcity of a promising diagnostic marker is a major cause for pancreatic cancer lethality. Extensive efforts are currently being exerted to identify a potential marker that can help to detect the disease early for improved therapeutic efficacy and overall survival. The presence and/or secretion of a biomarker early during cancer development, along with absolute sensitivity and specificity of the identified markers, are crucial barriers for definitive diagnosis and curative therapy.
EUS–FNA
Endoscopic ultrasonography-guided, fine-needle aspiration (EUS–FNA) is emerging as a safe and valuable modality for preoperative diagnosis of malignant cases and staging of pancreatic cancer. Marked overexpression of MUC1 (77.5%), MUC2 (10.0%) and MUC5AC (80.0%) has been documented in pancreatic cancer by EUS–FNA. On the other hand, benign pancreatic diseases have only 25.0%, 31.3% and 43.8% positive cases for MUC1, MUC2 and MUC5AC, respectively.82 Similarly, Carrara et al.83 observed MUC7 expression in 73% of malignant cases. With 91% positive reactivity on FNA from carcinoma cases, MUC4 was found to be 100% specific in differentiating pancreatic cancer cases from carcinoma-negative cases.84 Furthermore, MUC4 and MUC16 were found to be 100% specific in distinguishing malignant cases from benign with sensitivities of 63% and 67%, respectively, in atypical FNAs.85 Future studies are ongoing to assess the diagnostic efficacy of mucins in EUS–FNA for clinical applications.
In addition to EUS-based imaging, circulating tumour cells emerged as potential markers for prognosis, prediction of response to therapy, or monitoring a clinical course in patients in various malignancies. Tewes et al.86 observed that 86% of circulating tumour cells in cases of breast cancer are positive for MUC1 expression. Analysis of mucin expression in circulating tumour cells, along with other biomarkers, has great potential in providing superior prognostic information with regard to risk assessment for recurrence and predictive judgement of therapeutic regimens.
Post-translational modifications
As carbohydrate alterations are post-translational modifications on the protein backbone, gauging the expression of a specific mucin gene and/or protein provides only a limited window for estimating their diagnostic and/or prognostic relevance. The mucin backbone also has multiple post-translational modifications and among these modifications, CA19-9 was identified as an antigen for the N19-9 antibody. Although the antibody was generated against colon tumour antigens, N19-9 detected carbohydrate antigen (CA19-9), which was also frequently present in the serum of patients with pancreatic and biliary tract cancer.87 After its FDA approval, CA19-9 remains the most commonly used tumour antigen for follow-up of the therapeutic outcome in patients with pancreatic cancer. Unfortunately, various concerns, including its expression in benign conditions (such as pancreatitis, cirrhosis and acute cholangitis), its absence in 5% of the white population (who have the sialyl Lewis a−/b− genotype), very low positive predictive value (0.5–0.9%) in asymptomatic individuals88 and highly variable sensitivity (68–91%) and specificity (70–90%) limits its potential as a marker for diagnostic screening.89,90 Additional attempts to improve the sensitivity and specificity of the CA19-9 assay by detecting the epitope individually on a specific carrier protein (that is, MUC1, MUC4 and MUC5AC) failed to improve diagnostic screening for pancreatic cancer.51,91 These failures have increased the quest for identifying a novel diagnostic marker as well as ways to improve the sensitivity and specificity of CA19-9-based diagnostic screening. A plethora of carbohydrate-based epitopes have been explored to improve the diagnosis and prognosis (Table 2); however, the majority had a diagnostic and/or prognostic efficacy equivalent to or poorer than CA19-9.
Table 2.
Mucins as diagnostic and therapeutic targets
| Target | Nature, pattern and mode of action | Diagnostic use | Comment | Study |
|---|---|---|---|---|
| CA19-9 | mAb against sialyl Lewisa present on apical surface and supra-nuclear cytoplasm of healthy ductal epithelial cells Located on plasma membrane, cytoplasm of tumour cells and on stroma surrounding the cells | Sensitivity (70–90%) Specificity (68–91%) in serum |
Clinical prognostic marker, but nonspecific as elevated in benign diseases Absent in Lewis a−/b− patients Variable and poor specificity | 87,89,135 |
| CA50 | Monosialoganglioside and a sialylated glycoprotein epitope reactive mAb against epithelial cells of the bile duct | Sensitivity (78–84%) Specificity (70–85%) in serum |
Elevated during jaundice and cholestasis with positivity rates similar to CA19-9 | 135 |
| CA242 | Undefined epitopic structure with antigenic distribution on the membrane of epithelial cells, and in the intraluminal mucus | Sensitivity (57–82%) Specificity (76–93%) in serum |
Elevated during cholestasis and jaundice Poor release into circulation Lower sensitivity and specificity than CA19-9 | 135,136 |
| CA195 | Sialylated Lewis and Lewis glycolipid antigenic epitope | Sensitivity (76–82%) Specificity (73–85%) in serum |
Lower sensitivity and specificity than CA19-9 | 137 |
| CA125/MUC16 | Peptide expressed on MUC16 and reactive with apical surface of epithelial cells | Sensitivity (45–57%) Specificity (76–78%) in serum |
Elevated during cirrhosis, hepatitis, pancreatitis, and jaundice Lower sensitivity and specificity than CA19-9 CA19-9 measurement on MUC16 carrier increases specificity Specificity 100% for FNAs | 17,85,91,135 |
| DUPAN 2 | Sialyl Lewis C antigen with distribution in the apical surface of the epithelial cells | Sensitivity (48–64%) Specificity (85–94%) in serum |
Poor sensitivity | 135 |
| SPan-1 | Reactive with Lewis a−b− epitopic structure Located in the plasma membrane, cytoplasm and stroma surrounding the cells | Sensitivity (82–94%) Specificity (50–85%) in serum |
Similar diagnostic efficacy to CA19-9 Predictor of gemcitabine treatment failure | 135,137 |
| CAM17.1 (mAb reactive against colorectal carcinoma cell membranes) | Sialylated protein with distribution on apical surfaces of epithelial cells | Sensitivity (67%) Specificity (90%) in serum |
Few studies with limited patient group CA19-9 in parallel serum set (76% sensitivity and 78% specificity) | 135,137,138 |
| MUC4 | Transmembrane mucin with de novo expression during PanIN I with cytoplasmic and apical expression | Sensitivity (78–90%) Specificity (100%) in FNAs |
Low levels in serum | 83,85 |
| MUC5AC | Gel-forming mucin with de novo expression during PanIN I | Sensitivity (85%) Specificity (100%) in serum |
Potential diagnostic marker in combination with CA19-9 (our unpublished data) |
139 |
| PAM4 | Reactive mucins and epitope absent in normal pancreas but expression observed in PanIN-I–III and pancreatic cancer | Sensitivity (74%) Specificity (85%) in serum |
Potential diagnostic marker in combination with CA19-9 | 140 |
Abbreviations: FNA, fine-needle aspiration; mAb, monoclonal antibody; PanIN, pancreatic intra-epithelial neoplasia.
Autoantibodies
Detection of autoantibodies in high-risk groups for various malignancies, including smokers and patients with chronic obstructive pulmonary disease, has indicated that these markers of immune response are detectable before radiographic detection in 26.5% of patients with lung cancer.92–95 Mucins (as major TAAs) are prime candidates for exploiting the diagnostic potential of autoantibodies. However, in the past, the absence of specific antibodies against modified carbohydrate-based tumour antigens, along with the absence of arrays displaying a library of glycopeptides and glycoproteins derived from human mucins, hampered the progress to develop carbohydrate-based diagnostic markers. In 2011, Paederson et al.96 used a glycopeptide array displaying a comprehensive library of glycopeptides and glycoproteins derived from a panel of human mucins (MUC1, MUC2, MUC4, MUC5AC, MUC6 and MUC7) for screening autoantibodies in patients with colorectal cancer. This array analysed the patient set with a sensitivity of 79% and a specificity of 92%. Future use of these arrays will both improve the specific antibody screening against carbohydrate-based antigens and illustrate the potential role of autoantibodies as a diagnostic marker for pancreatic cancer.
Therapeutic target(s)
Mucins participate in immune evasion, invasion and metastatic spread by sensing their microenvironment, and affect oncogenic signalling, including cell survival, growth, proliferation and resistance to chemotherapeutics. 2,6,97 Moreover, MUC1, MUC4, MUC5AC and MUC16 have been linked with the progression, poor prognosis and chemoresistance of human pancreatic cancer. Owing to these attributes, mucins have been explored as candidates for pancreatic cancer vaccines and therapeutics (Figure 5; Table 3).
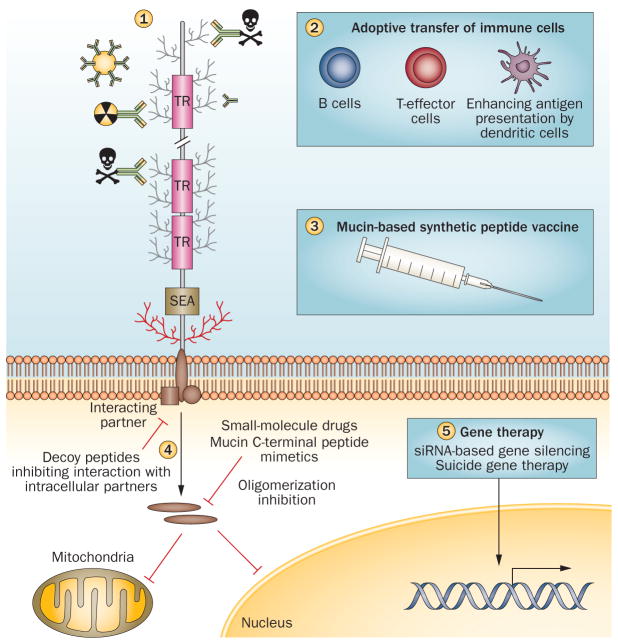
Figure 5.
Mucins as therapeutic targets. Mucin-based immunotherapies being used in clinical and pre-clinical studies include antibody-targeted therapies (antibodies conjugated to radionuclides, immunotoxins and antibody-labelled gold nanoparticles) (1); cell-based therapies comprising adoptive transfer of CTLs, antigen pulsed dendritic cells, or a combination of both dendritic cells and CTLs] (2); recombinant peptide vaccines that enhance the mucin-antigen presentation by dendritic cells stimulating the immune response and tumour cell killing (3); and small-size inhibitory peptides that block mucin cytoplasmic tail interaction with other signalling molecules or mucin mimetic inhibiting cytoplasmic tail oligomerization, thus preventing its translocation to the mitochondria or nucleus (4). Mucin silencing by RNA interference and mucin promoter driven suicide gene therapy are other approaches to develop mucin-based targeted therapy (suicide gene therapy) (5). Abbreviations: CTL, cytotoxic T lymphocyte; TAA, tumour-associated antigen.
Table 3.
Mucins as potential target(s) for pancreatic cancer therapy
| Targeted antigen | Therapeutic regimen | Immune response | Comment | Study |
|---|---|---|---|---|
| MUC1 vaccine (105 amino acid conserved tandem repeat domain) | VNTR peptide with BCG adjuvant (phase I/II); SB-AS2 adjuvant (phase I); and incomplete Freund’s adjuvant (phase I) | Both cellular and humoral immune response | Suppressed immune system Few studies with a limited number of patients | 105–107 |
| MUC1-pulsed dendritic cells | Adoptive transfer of dendritic cells transfected with MUC1 cDNA (phase II); MUC1 peptide (phase I/II) | Vaccine well tolerated; MUC1-reactive CTL response | No increase in anti-MUC1 antibody levels with high, nonspecific T-cell activation | 141,142 |
| MUC1-pulsed dendritic cells or CTLs | MUC1-peptide-pulsed dendritic cells in combination with activated CTLs (phase II) | Low tumour:healthy tissue ratio and high liver accumulation | Well tolerated with mean survival of 9.8 months and stable disease in five patients | 111 |
| PAM4 antibody targeted radiotherapy | 131I-PAM4 mAb 99mTc-PAM4 mAb 90Y-PAM4 mAb and its humanized PAM4 (clivatuzumab tetraxetan) | Efficient targeting of tumour with no secondary reaction toward the formulation | In case of clivatuzumab tetraxetan, 12 of 21 patients with pancreatic cancer were targeted; 3 patients had a partial response144 | 143–146 |
| Inhibitors of oligomerization (GO-201) | Small molecule drugs inhibiting MUC1-CT oligomerization by binding to CQC motif blocks MUC1 functioning | Regressed growth of xenograft tumours | Promising drug candidate | 116,147 |
Abbreviations: BCG, Bacillus Calmette–Guérin; CT, cytoplasmic tail; CTL, cytotoxic T lymphocyte; mAb, monoclonal antibody; PanIN, pancreatic intra-epithelial neoplasia; VNTR, variable number of tandem repeats.
Peptide vaccine
MUC1 has gathered great interest owing to its aberrant expression in various epithelial malignancies, early discovery, cloning and, most importantly, its presence in the cell-mediated and humoral immune response. Moreover, the presence of low-titre anti-MUC1 antibodies and MUC1-specific cytotoxic T-lymphocytes (CTLs) in the tumour-draining lymph nodes of patients with breast, ovarian and pancreatic adenocarcinorna prompted preclinical and clinical studies on MUC1 as a vaccine target.98,99 Interestingly, MUC1 provided immune protection in wild-type mice against MUC1-positive tumour cells; however, transgenic mice (which expressed human MUC1) failed to limit or regress tumour growth owing to the development of immune tolerance.100 The adoptive transfer of CD4+ T cells from wild-type mice provided immune protection to these MUC1 transgenic animals in the absence of a strong autoimmune response.101,102 Vaccine studies using the MUC1–TRR peptide (comprising five copies of the TRR) in conjunction with LEIF adjuvant (Leishmania-derived protein that is known to stimulate human peripheral blood mononuclear cells and antigen-presenting cells, to produce a T-helper-1 type cytokine profile) elicited a T-helper 1 immune response along with the production of IFN-γ in chimpanzees.103 These findings led to the clinical use of MUC1 vaccine formulations for stimulating effective immunity against the tumour antigen.104 Many vaccine formulations of MUC1 have been tested in patients with both resectable and advanced stage pancreatic cancer. Initially, a synthetic mucin MUC1 peptide of 105 amino acid (having five repetitions of the entire conserved TRR observed in MUC1) was delivered with Bacillus Calmette–Guérin or SB-AS2 (adjuvants which elicit a strong T-helper cell and CTL response) or incomplete Freund’s adjuvant (elicits T-helper-2-dominated antibody response).105–107 Furthermore, Kaufman et al.108 used poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. The majority of these vaccine formulations were well tolerated with no grade II–IV level toxicities. Peptide vaccine formulation led to a partial increase in mucin-specific CTLs with no isotype switching of antibody response.105–107
Adoptive immunotherapy
In addition to peptide vaccines, adoptive immunotherapy has been used clinically with MUC1-peptideloaded dendritic cells alone, CTLs alone stimulated by MUC1-expressing human pancreatic cancer cells,109,110 and combination of both these regimes (that is, dendritic cells pulsed with MUC1 peptide [MUC1-DC] and, CTL sensitized with a pancreatic cancer cell line expressing MUC1 [MUC1-CTL]).111 Adoptive immunotherapy with MUC1-activated CTLs in patients with unresectable pancreatic cancer restricted postsurgical hepatic recurrence of pancreatic cancer; however, the treatment failed to prevent local recurrence or peritoneal metastasis.109 Furthermore, no improvement in overall survival was observed in these cases. As adoptive immunotherapy with MUC1-activated CTLs was ineffective for preventing local progression of pancreatic cancer, the research group evaluated the efficacy of this approach using a combination of MUC1-pulsed DCs and autologous-expanded MUC1-specific T cells in 20 patients with unresectable or recurrent pancreatic cancer.111 This combined strategy was found to be more effective for patients with pancreatic cancer. One year survival was >20% for patients with unresectable or recurrent pancreatic cancer and a complete response was seen in one of the two patients who had multiple lung metastases at the time of diagnosis. Five patients had stable disease and the mean survival time was found to be 9.8 months.111 These studies indicate the efficacy of MUC1-based adoptive immunotherapy for pancreatic cancer and warrant future randomized studies with a larger patient set.
Limited studies have been carried out to test the immunomodulatory efficacy of other mucins. Wu et al.112 mapped the HLA-A*0201-restrictive CTL epitopes of MUC4, and, among various peptides, CTL corresponding to P01204 peptide produced protective immunity against MUC4 positive tumours. Furthermore, an increased number of IFN-γ-producing T cells after treatment with the P01204 peptide emphasizes the importance of the identified peptide for producing anti-tumour CTLs.112
Mucin antibodies
In addition to a T-cell response, anti-mucin antibodies conjugated to toxins or radionuclides have been tested as therapeutic regimens for various malignancies. Among them, Pankomab (Glycotope GmbH, Berlin, Germany) anti-MUC1 antibody is highly tumour specific (breast, gastric, colorectal, liver, cervical and thyroid) and exhibits strong antibody-dependent cell cytotoxicity. Owing to its rapid internalization upon toxin coupling, it is able to effectively induce toxin-mediated antigen-specific tumour cell killing.113 Development of this drug has been moved to phase II trials (NCT01222624) after successful completion of phase I trials, which were done for dose escalation of the antibody in patients with advanced, MUC1-expressing solid malignancies. In addition, the anti-CA125 antibody B43.13, known as oregovomab, emerged as a potential therapeutic agent for patients with advanced stage ovarian cancer as it forms immune complexes with circulating CA125 and generates a broad cellular and humoral immune response against CA125 when compared with the free molecule.113 However, it failed in phase III trials with no improvement in overall patient survival when compared with those given placebo and with no substantial difference in the quality of life.114
Other approaches
In addition to antibody-based and vaccine-based approaches, decoy peptides that attenuate MUC1 cytoplasmic tail interaction with β-catenin are being used in preclinical studies for functional targeting of MUC1.115 The major issue in their usage involves nonspecific targeting of other receptors. Furthermore, small-molecule drugs (GO-201) that inhibit MUC1-cytoplasmic tail oligomerization by binding to the CQC (membrane proximal cysteine–glutamine–cysteine) motif and thus causing functional blockage have shown promising results in various tumour cell lines.116 Also, the MUC1-conjugated aptamer specifically targeted drug-loaded quantum dots to tumour cells.117 Although mucins are attractive targets for therapy, their expression in various normal tissues and large pools of circulating N-terminal ectodomains might limit the efficacy of the targeting agent (mucin-specific antibodies, peptides or aptamers).
Conclusions
Pancreatic cancer is a lethal malignancy characterized by a dense desmoplastic reaction and altered expression of multiple mucins. Aberrant mucin expression involves the complex synchrony of malignant cells and their microenvironment. Oncogenic mutations, epigenetic changes, inflammatory factors and the hypoxic milieu act as major mediators for the observed aberration in mucin expression.41,76,118–122 Mucins, in turn, facilitate tumour growth, proliferation, oncogenic signalling, EMT and metastasis. Moreover, they directly interact with, as well as dictate, the formation and composition of the TME (they recruit and/or interact with stromal cells, ECM and milieu of growth factors and cytokines). This Review discusses the versatile role of mucins in tumour hierarchy. Limited but interesting studies have unravelled both a direct and indirect role of mucins in the composition and behaviour of the TME; however, many areas are still obscure, including: the pathobiological significance of mucins (expression of multiple members of the family), their spliced, polymorphic and mutated forms; types of glycan modification and responsible glycosyltransferases; and contributions from individual domains and their interaction with TME in autochthonous, genetically engineered mouse models of tumours and patient samples. Once these key interactions are elucidated, blocking these processes using inhibitors will be helpful in designing improved therapeutics and early diagnostic and/or prognostic markers. Overall, evolving research in the field of pancreatic cancer with special reference to the role of mucins could lead to the development of therapeutic regimens that combine targeting of the tumour cells and its microenvironment.
Key points.
Mucins, by virtue of their extended ectodomain, variety of domains and varied degree of glycosylation, act as multifaceted glycoproteins that have evolved convergently to protect the exposed surfaces of organisms
Pancreatic cancer is characterized by the aberrant expression of both transmembrane and secretory mucins
De novo expression of MUC4, MUC5AC, and MUC16 is observed in pancreatic cancer as early as pancreatic intraepithelial neoplasia and expression increases gradually with disease progression and subsequent metastasis
Altered attributes of mucins are used by tumour cells to facilitate their growth, proliferation, interaction with the extracellular matrix or stromal cells and detachment from the primary tumour for invasion and metastasis
CA19-9, the FDA-approved prognostic marker for pancreatic cancer, is a carbohydrate antigen (sialyl Lewisa) present on the surface of MUC1, MUC16 and MUC5AC; MUC1-based therapies are in preclinical and clinical trials
Review criteria.
Relevant literature on mucin interactions with the tumour microenvironment were identified by searching the PubMed database for articles published until January 2013. The search terms used in combination with “mucins” were “pancreatic cancer”, “expression”, “localization”, ”glycosylation”, “receptor tyrosine kinases”, “hypoxia”, “extracellular matrix”, “collagen”, “fibronectin”, “AMOP”, “vWD”, “EGF”, “nidogen”, “adhesion”, “anti-adhesion”, “tumour microenvironment”, “macrophages”, “endothelial cells”, “stromal cell”, “diagnostic marker”, “prognostic marker”, “therapy”, “vaccine”, “gene therapy”, “CA19-9”, “carbohydrate antigen”, “circulating tumour cells”, “O-glycans” and “N-glycans”. All papers identified were full-text articles published in peer-reviewed journals. Owing to the journal policy of limitation of citations, only important references are listed.
Acknowledgments
The authors on this work are supported, in part, by grants from the NIH (TMEN U54 CA163120, EDRN UO1 CA111294, SPORE P50 CA127297, RO1 CA131944, RO1 CA133774, RO1 CA78590 and RO3 CA167342).
Footnotes
Author contributions
S. Kaur, S. Kumar, N. Momi and S. K. Batra contributed to all aspects of producing this article. A. R. Sasson substantially contributed to the discussion of content and reviewed and/or edited the article before submission.
Competing interests
The authors declare no competing interests.
Contributor Information
Sukhwinder Kaur, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Centre, 985870 Nebraska Medical Centre, Omaha, NE 68198-5870, USA.
Sushil Kumar, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Centre, 985870 Nebraska Medical Centre, Omaha, NE 68198-5870, USA.
Navneet Momi, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Centre, 985870 Nebraska Medical Centre, Omaha, NE 68198-5870, USA.
Aaron R. Sasson, Department of Surgery, University of Nebraska Medical Centre, 985870 Nebraska Medical Centre, Omaha, NE 68198-5870, USA
Surinder K. Batra, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Centre, 985870 Nebraska Medical Centre, Omaha, NE 68198-5870, USA
References
- 1.Cancer Facts and Statistics. American Cancer Society; 2013. online http://www.cancer.org/research/cancerfactsstatistics/ [Google Scholar]
- 2.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–1326. [PubMed] [Google Scholar]
- 5.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–D1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- 6.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senapati S, Sharma P, Bafna S, Roy HK, Batra SK. The MUC gene family: their role in the diagnosis and prognosis of gastric cancer. Histol Histopathol. 2008;23:1541–1552. doi: 10.14670/HH-23.1541. [DOI] [PubMed] [Google Scholar]
- 8.Singh AP, et al. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008;9:1076–1085. doi: 10.1016/S1470-2045(08)70277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata K, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–254. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 10.Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:108–124. doi: 10.1007/s00534-009-0174-7. [DOI] [PubMed] [Google Scholar]
- 11.Yonezawa S, et al. MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int. 1998;48:319–322. doi: 10.1111/j.1440-1827.1998.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 12.Swartz MJ, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 13.Saitou M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takikita M, et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009;69:2950–2955. doi: 10.1158/0008-5472.CAN-08-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamasaki H, et al. Expression and localization of MUC1, MUC2, MUC5AC and small intestinal mucin antigen in pancreatic tumors. Int J Oncol. 2004;24:107–113. [PubMed] [Google Scholar]
- 16.Moniaux N, Junker WM, Singh AP, Jones AM, Batra SK. Characterization of human mucin MUC17. Complete coding sequence and organization. J Biol Chem. 2006;281:23676–23685. doi: 10.1074/jbc.M600302200. [DOI] [PubMed] [Google Scholar]
- 17.Haridas D, et al. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS ONE. 2011;6:e26839. doi: 10.1371/journal.pone.0026839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan SC, et al. MUC13 mucin augments pancreatic tumorigenesis. Mol Cancer Ther. 2012;11:24–33. doi: 10.1158/1535-7163.MCT-11-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nissim S, Idos GE, Wu B. Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas. 2012;41:1195–1205. doi: 10.1097/MPA.0b013e3182580fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimamoto T, et al. MUC1 is a useful molecular marker for malignant intraductal papillary mucinous neoplasms in pancreatic juice obtained from endoscopic retrograde pancreatography. Pancreas. 2010;39:879–883. doi: 10.1097/MPA.0b013e3181d6ba04. [DOI] [PubMed] [Google Scholar]
- 21.Maker AV, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhury A, et al. Alternate splicing at the 3'-end of the human pancreatic tumor-associated mucin MUC4 cDNA. Teratog Carcinog Mutagen. 2001;21:83–96. doi: 10.1002/1520-6866(2001)21:1<83::aid-tcm8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Escande F, et al. Genomic organization of MUC4 mucin gene. Towards the characterization of splice variants. Eur J Biochem. 2002;269:3637–3644. doi: 10.1046/j.1432-1033.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 26.Pochampalli MR, el Bejjani RM, Schroeder JA. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693–1701. doi: 10.1038/sj.onc.1209976. [DOI] [PubMed] [Google Scholar]
- 27.Pochampalli MR, Bitler BG, Schroeder JA. Transforming growth factor α dependent cancer progression is modulated by Muc1. Cancer Res. 2007;67:6591–6598. doi: 10.1158/0008-5472.CAN-06-4518. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Wang L, Nunes DP, Troxler RF, Offner GD. Suppression of MUC1 synthesis downregulates expression of the epidermal growth factor receptor. Cancer Biol Ther. 2005;4:968–973. doi: 10.4161/cbt.4.9.1913. [DOI] [PubMed] [Google Scholar]
- 29.Hisatsune A, et al. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem Biophys Res Commun. 2011;405:377–381. doi: 10.1016/j.bbrc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, et al. Heregulin targets γ-catenin to the nucleolus by a mechanism dependent on the DF3/MUC1 oncoprotein. Mol Cancer Res. 2003;1:765–775. [PubMed] [Google Scholar]
- 31.Chaturvedi P, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funes M, Miller JK, Lai C, Carraway KL, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281:19310–19319. doi: 10.1074/jbc.M603225200. [DOI] [PubMed] [Google Scholar]
- 33.Price-Schiavi SA, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 34.Chaturvedi P, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 35.Jonckheere N, et al. The mucin MUC4 and its membrane partner ErbB2 regulate biological properties of human CAPAN-2 pancreatic cancer cells via different signalling pathways. PLoS ONE. 2012;7:e32232. doi: 10.1371/journal.pone.0032232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta BK, et al. Increased expression and aberrant localization of mucin 13 in metastatic colon cancer. J Histochem Cytochem. 2012;60:822–831. doi: 10.1369/0022155412460678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, et al. Expression of KL-6/MUC1 in pancreatic ductal carcinoma and its potential relationship with β-catenin in tumor progression. Life Sci. 2011;88:1063–1069. doi: 10.1016/j.lfs.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Singh PK, et al. Phosphorylation of MUC1 by Met modulates interaction with p53 and MMP1 expression. J Biol Chem. 2008;283:26985–26995. doi: 10.1074/jbc.M805036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau SK, et al. EGFR-mediated carcinoma cell metastasis mediated by integrin αvβ5 depends on activation of c-Src and cleavage of MUC1. PLoS ONE. 2012;7:e36753. doi: 10.1371/journal.pone.0036753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitler BG, Goverdhan A, Schroeder JA. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123:1716–1723. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inaguma S, Kasai K, Ikeda H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5ACmediated attenuation of E-cadherin. Oncogene. 2011;30:714–723. doi: 10.1038/onc.2010.459. [DOI] [PubMed] [Google Scholar]
- 42.Kondo A, et al. From glycomics to functional glycomics of sugar chains: Identification of target proteins with functional changes using gene targeting mice and knock down cells of FUT8 as examples. Biochim Biophys Acta. 2006;1764:1881–1889. doi: 10.1016/j.bbapap.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Remmers N, et al. Aberrant expression of mucin core proteins and O-linked glycans associated with progression of pancreatic cancer. Clin Cancer Res. 2013;19:1981–1993. doi: 10.1158/1078-0432.CCR-12-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park HU, et al. Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Lex antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48–e54. doi: 10.1097/00006676-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Yue T, et al. The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol Cell Proteomics. 2009;8:1697–1707. doi: 10.1074/mcp.M900135-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kui WN, et al. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem. 2003;278:28619–28634. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 47.Tu L, Banfield DK. Localization of Golgi-resident glycosyltransferases. Cell Mol Life Sci. 2010;67:29–41. doi: 10.1007/s00018-009-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu CJ, et al. Sialyl Lewis antigens: association with MUC5AC protein and correlation with postoperative recurrence of non-small cell lung cancer. Lung Cancer. 2005;47:59–67. doi: 10.1016/j.lungcan.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Geng Y, Marshall JR, King MR. Glycomechanics of the metastatic cascade: tumor cell-endothelial cell interactions in the circulation. Ann Biomed Eng. 2012;40:790–805. doi: 10.1007/s10439-011-0463-6. [DOI] [PubMed] [Google Scholar]
- 52.Chen SH, Dallas MR, Balzer EM, Konstantopoulos K. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J. 2012;26:1349–1359. doi: 10.1096/fj.11-195669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- 54.Komatsu M, Tatum L, Altman NH, Carothers Carraway CA, Carraway KL. Potentiation of metastasis by cell surface sialomucin complex (rat MUC4), a multifunctional anti-adhesive glycoprotein. Int J Cancer. 2000;87:480–486. doi: 10.1002/1097-0215(20000815)87:4<480::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 55.Besmer DM, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011;71:4432–4442. doi: 10.1158/0008-5472.CAN-10-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohlgraf KG, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011–5020. [PubMed] [Google Scholar]
- 57.Tinder TL, et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181:3116–3125. doi: 10.4049/jimmunol.181.5.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Q, et al. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanders WJ, Katsumoto TR, Bertozzi CR, Rosen SD, Kiessling LL. L-selectin-carbohydrate interactions: relevant modifications of the Lewis x trisaccharide. Biochemistry. 1996;35:14862–14867. doi: 10.1021/bi9613640. [DOI] [PubMed] [Google Scholar]
- 60.Cadron I, et al. The impact of enzastaurin (LY317615.HCl) on CA125 biosynthesis and shedding in ovarian cancer cells. Gynecol Oncol. 2010;118:64–68. doi: 10.1016/j.ygyno.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Agrawal B, Gendler SJ, Longenecker BM. The biological role of mucins in cellular interactions and immune regulation: prospects for cancer immunotherapy. Mol Med Today. 1998;4:397–403. doi: 10.1016/s1357-4310(98)01322-7. [DOI] [PubMed] [Google Scholar]
- 62.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 63.Kleeff J, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 64.Tsuboi S, et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011;30:3173–3185. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senapati S, et al. Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin Cancer Res. 2011;17:267–274. doi: 10.1158/1078-0432.CCR-10-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaida MM, et al. Expression of galectin-3 in pancreatic ductal adenocarcinoma. Pathol Oncol Res. 2012;18:299–307. doi: 10.1007/s12253-011-9444-1. [DOI] [PubMed] [Google Scholar]
- 67.Okamoto T, et al. Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity. Mol Med Report. 2013;7:359–364. doi: 10.3892/mmr.2012.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki Y, et al. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int J Oncol. 2012;40:1831–1838. doi: 10.3892/ijo.2012.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swanson BJ, et al. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 70.Konowalchuk JD, Agrawal B. MUC1 is a novel costimulatory molecule of human T cells and functions in an AP-1-dependent manner. Hum Immunol. 2012;73:448–455. doi: 10.1016/j.humimm.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Ohno S, et al. Expression of Tn and sialyl-Tn antigens in endometrial cancer: its relationship with tumor-produced cyclooxygenase-2, tumor-infiltrated lymphocytes and patient prognosis. Anticancer Res. 2006;26:4047–4053. [PubMed] [Google Scholar]
- 72.Feig C, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nath D, et al. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology. 1999;98:213–219. doi: 10.1046/j.1365-2567.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allavena P, et al. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010;2010:547179. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitamoto S, et al. MUC1 enhances hypoxia-driven angiogenesis through the regulation of multiple proangiogenic factors. Oncogene. doi: 10.1038/onc.2012.478. http://dx.doi.org/10.1038/onc.2012.478. [DOI] [PubMed]
- 76.Chaika NV, et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 α to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci USA. 2012;109:13787–13792. doi: 10.1073/pnas.1203339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsutsumida H, et al. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12:2976–2987. doi: 10.1158/1078-0432.CCR-05-1197. [DOI] [PubMed] [Google Scholar]
- 78.Sawada T, et al. Biphasic effect of cell surface sialic acids on pancreatic cancer cell adhesiveness. Biochem Biophys Res Commun. 1993;195:1096–1103. doi: 10.1006/bbrc.1993.2157. [DOI] [PubMed] [Google Scholar]
- 79.Senapati S, Gnanapragassam VS, Moniaux N, Momi N, Batra SK. Role of MUC4-NIDO domain in the MUC4-mediated metastasis of pancreatic cancer cells. Oncogene. 2011;31:3346–5610. doi: 10.1038/onc.2011.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput Biol. 2009;5:e1000604. doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, et al. Diagnostic value of mucins (MUC1, MUC2 and MUC5AC) expression profile in endoscopic ultrasound-guided fine-needle aspiration specimens of the pancreas. Int J Cancer. 2007;121:2716–2722. doi: 10.1002/ijc.22997. [DOI] [PubMed] [Google Scholar]
- 83.Carrara S, et al. Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol. 2011;106:1359–1363. doi: 10.1038/ajg.2011.22. [DOI] [PubMed] [Google Scholar]
- 84.Jhala N, et al. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 85.Horn A, et al. Immunocytochemistry for MUC4 and MUC16 is a useful adjunct in the diagnosis of pancreatic adenocarcinoma on fine-needle aspiration cytology. Arch Pathol Lab Med. 2013;137:546–551. doi: 10.5858/arpa.2011-0229-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tewes M, et al. Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat. 2009;115:581–590. doi: 10.1007/s10549-008-0143-x. [DOI] [PubMed] [Google Scholar]
- 87.Kaur S, Baine MJ, Jain M, Sasson AR, Batra SK. Early diagnosis of pancreatic cancer: challenges and new developments. Biomark Med. 2012;6:597–612. doi: 10.2217/bmm.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goggins M. Molecular markers of early pancreatic cancer. J Clin Oncol. 2005;23:4524–4531. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- 90.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19–9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Yue T, et al. Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19–9 antigen on specific protein carriers. PLoS ONE. 2011;6:e29180. doi: 10.1371/journal.pone.0029180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shiozaki K, Yamaguchi K, Takahashi K, Moriya S, Miyagi T. Regulation of sialyl Lewis antigen expression in colon cancer cells by sialidase NEU4. J Biol Chem. 2011;286:21052–21061. doi: 10.1074/jbc.M111.231191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu J, et al. Occurrence of autoantibodies to annexin I, 14-3-3 τ and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol. 2008;26:5060–5066. doi: 10.1200/JCO.2008.16.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong L, et al. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2006;1:513–519. [PubMed] [Google Scholar]
- 95.Pedersen JW, et al. Early detection of cancer in the general population: a blinded case–control study of p53 autoantibodies in colorectal cancer. Br J Cancer. 2013;108:107–114. doi: 10.1038/bjc.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pedersen JW, et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int J Cancer. 2011;128:1860–1871. doi: 10.1002/ijc.25778. [DOI] [PubMed] [Google Scholar]
- 97.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karanikas V, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ioannides CG, et al. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993;151:3693–3703. [PubMed] [Google Scholar]
- 100.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- 101.Turner MS, Cohen PA, Finn OJ. Lack of effective MUC1 tumor antigen-specific immunity in MUC1-transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild-type Th cells. J Immunol. 2007;178:2787–2793. doi: 10.4049/jimmunol.178.5.2787. [DOI] [PubMed] [Google Scholar]
- 102.Tempero RM, et al. CD4+ lymphocytes provide MUC1-specific tumor immunity in vivo that is undetectable in vitro and is absent in MUC1 transgenic mice. J Immunol. 1998;161:5500–5506. [PubMed] [Google Scholar]
- 103.Barratt-Boyes SM, Vlad A, Finn OJ. Immunization of chimpanzees with tumor antigen MUC1 mucin tandem repeat peptide elicits both helper and cytotoxic T-cell responses. Clin Cancer Res. 1999;5:1918–1924. [PubMed] [Google Scholar]
- 104.Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166:6555–6563. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 105.Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 106.Ramanathan RK, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamamoto K, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25:3575–3579. [PubMed] [Google Scholar]
- 108.Kaufman HL, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep. 2008;20:155–163. [PubMed] [Google Scholar]
- 110.Kawaoka T, Takashima M, Yamamoto K, Ueno T, Oka M. Adoptive immunotherapy using MUC1--specific CTLs for unresectable pancreatic cancer [Japanese] Nihon Rinsho. 2006;64 (Suppl 1):279–282. [PubMed] [Google Scholar]
- 111.Kondo H, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28:379–387. [PubMed] [Google Scholar]
- 112.Wu J, et al. Identification of an HLA-A*0201-restrictive CTL epitope from MUC4 for applicable vaccine therapy. Immunopharmacol Immunotoxicol. 2009;31:468–476. doi: 10.1080/08923970902795203. [DOI] [PubMed] [Google Scholar]
- 113.Fan XN, Karsten U, Goletz S, Cao Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TAMUC1) with various human carcinomas. Pathol Res Pract. 2010;206:585–589. doi: 10.1016/j.prp.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 114.Berek J, et al. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27:418–425. doi: 10.1200/JCO.2008.17.8400. [DOI] [PubMed] [Google Scholar]
- 115.Bitler BG, et al. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res. 2009;15:100–109. doi: 10.1158/1078-0432.CCR-08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raina D, et al. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011;153:16–22. doi: 10.1016/j.jconrel.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 118.Andrianifahanana M, et al. MUC4-expressing pancreatic adenocarcinomas show elevated levels of both T1 and T2 cytokines: potential pathobiologic implications. Am J Gastroenterol. 2006;101:2319–2329. doi: 10.1111/j.1572-0241.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 119.Ren J, et al. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res. 2006;4:873–883. doi: 10.1158/1541-7786.MCR-06-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choudhury A, et al. Retinoic acid-dependent transforming growth factor-β 2-mediated induction of MUC4 mucin expression in human pancreatic tumor cells follows retinoic acid receptor-α signaling pathway. J Biol Chem. 2000;275:33929–33936. doi: 10.1074/jbc.M005115200. [DOI] [PubMed] [Google Scholar]
- 121.Strobel O, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology. 2010;138:1166–1177. doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Colomb F, et al. TNF regulates sialyl-Lewisx and 6-sulfo-sialyl-Lewisx expression in human lung through up-regulation of ST3GAL4 transcript isoform BX. Biochimie. 2012;94:2045–2053. doi: 10.1016/j.biochi.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 123.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 124.Nanashima A, et al. High serum concentrations of sialyl Tn antigen in carcinomas of the biliary tract and pancreas. J Hepatobiliary Pancreat Surg. 1999;6:391–395. doi: 10.1007/s005340050137. [DOI] [PubMed] [Google Scholar]
- 125.Yu LG, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 126.Tsuboi S, Hatakeyama S, Ohyama C, Fukuda M. Two opposing roles of O-glycans in tumor metastasis. Trends Mol Med. 2012;18:224–232. doi: 10.1016/j.molmed.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee SH, et al. Core3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of α2β1 integrin complex. J Biol Chem. 2009;284:17157–17169. doi: 10.1074/jbc.M109.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Terris B, et al. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632–637. doi: 10.1002/path.1146. [DOI] [PubMed] [Google Scholar]
- 129.Yonezawa S, et al. MUC2 gene expression is found in noninvasive tumors but not in invasive tumors of the pancreas and liver: its close relationship with prognosis of the patients. Hum Pathol. 1997;28:344–352. doi: 10.1016/s0046-8177(97)90134-9. [DOI] [PubMed] [Google Scholar]
- 130.Yonezawa S, et al. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45–54. doi: 10.1046/j.1440-1827.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- 131.Andrianifahanana M, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 132.Shimizu A, et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012;103:739–746. doi: 10.1111/j.1349-7006.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Higuchi T, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 134.Itoh Y, et al. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 135.Rückert F, Pilarsky C, Grützmann R. Serum tumor markers in pancreatic cancer—recent discoveries. Cancer. 2010;2:1107. doi: 10.3390/cancers2021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ni XG, et al. The clinical value of serum CEA, CA19–9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 137.Tsutsumi K, et al. Monitoring of CA19–9 and SPan-1 can facilitate the earlier confirmation of progressing pancreatic cancer during chemotherapy. Pancreatology. 2012;12:409–416. doi: 10.1016/j.pan.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 138.Bunger S, Laubert T, Roblick UJ, Habermann JK. Serum biomarkers for improved diagnostic of pancreatic cancer: a current overview. J Cancer Res Clin Oncol. 2011;137:375–389. doi: 10.1007/s00432-010-0965-x. [DOI] [PubMed] [Google Scholar]
- 139.Yonezawa S, Higashi M, Yamada N, Goto M. Precursor lesions of pancreatic cancer. Gut Liver. 2008;2:137–154. doi: 10.5009/gnl.2008.2.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gold DV, et al. PAM4 enzyme immunoassay alone and in combination with CA 19–9 for the detection of pancreatic adenocarcinoma. Cancer. 2013;119:522–528. doi: 10.1002/cncr.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lepisto AJ, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955–964. [PMC free article] [PubMed] [Google Scholar]
- 142.Pecher G, Haring A, Kaiser L, Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother. 2002;51:669–673. doi: 10.1007/s00262-002-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gulec SA, et al. Treatment of advanced pancreatic carcinoma with 90Y-clivatuzumab tetraxetan: a phase I single-dose escalation trial. Clin Cancer Res. 2011;17:4091–4100. doi: 10.1158/1078-0432.CCR-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cardillo TM, Ying Z, Gold DV. Therapeutic advantage of 90yttrium versus 131iodine-labeled PAM4 antibody in experimental pancreatic cancer. Clin Cancer Res. 2001;7:3186–3192. [PubMed] [Google Scholar]
- 145.Gold DV, Cardillo T, Vardi Y, Blumenthal R. Radioimmunotherapy of experimental pancreatic cancer with 131I-labeled monoclonal antibody PAM4. Int J Cancer. 1997;71:660–667. doi: 10.1002/(sici)1097-0215(19970516)71:4<660::aid-ijc24>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 146.Gold DV, Cardillo T, Goldenberg DM, Sharkey RM. Localization of pancreatic cancer with radiolabeled monoclonal antibody PAM4. Crit Rev Oncol Hematol. 2001;39:147–154. doi: 10.1016/s1040-8428(01)00114-7. [DOI] [PubMed] [Google Scholar]
- 147.Joshi MD, et al. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol Cancer Ther. 2009;8:3056–3065. doi: 10.1158/1535-7163.MCT-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brockhausen I, Schachter H, Stanley P. Essentials of Glycobiology. 2. Ch 9. Cold Spring Harbor; 2009. [Google Scholar]
- 149.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Duraisamy S, Ramasamy S, Kharbanda S, Kufe D. Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 AND MUC16. Gene. 2006;373:28–34. doi: 10.1016/j.gene.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 152.Pelaseyed T, et al. Unfolding dynamics of the mucin SEA domain probed by force spectroscopy suggest that it acts as a cell protective device. FEBS J. 2013;280:1491–1501. doi: 10.1111/febs.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ho SB, et al. Activity of recombinant cysteine-rich domain proteins derived from the membrane-bound MUC17/Muc3 family mucins. Biochim Biophys Acta. 2010;1800:629–638. doi: 10.1016/j.bbagen.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ciccarelli FD, Doerks T, Bork P. AMOP, a protein module alternatively spliced in cancer cells. Trends Biochem Sci. 2002;27:113–115. doi: 10.1016/s0968-0004(01)02049-7. [DOI] [PubMed] [Google Scholar]