Abstract
Oxidative stress, including Ultraviolet (UV) irradiation-induced skin damage, is involved in numerous diseases. This study demonstrates that water extract of Eclipta prostrata L. (WEP) has a potent effect in scavenging 1,1-diphenyl-2-picrylhydrazyl (DPPH), superoxide radicals, and chelating ferrous ion, exhibiting IC50 values of 0.23 mg/mL, 0.48 mg/mL, and 1.25 mg/mL, respectively. The WEP total phenol content was 176.45 mg gallic acid equivalents (GAE)/g sample. Chlorogenic acid, a component of the plant's active ingredients, was determined by HPLC and antioxidative assay. However, no caffeic acid, stigmasterol, or wedelolactone was present in WEP. WEP absorbs both UVA and UVB irradiation, and furthermore, the extract shows a dose-dependent response in the protection of HaCaT human keratinocytes and mouse fibroblasts 3T3 cells against UVB-induced cytotoxicity, which may result from a synergistic effect between chlorogenic acid and other active components present in WEP.
1. Introduction
The generation of free radicals is a feature of cellular function such as in the mitochondrial respiratory chain, in phagocytosis, and in arachidonic acid metabolism [1]. However, excessive production of free radicals impairs cell membrane integrity [2], causes defects in the susceptible proteins required for mRNA translation, and induces DNA damage and gene mutation [3]. Excess reactive oxidative stress (ROS) which is susceptible to redox dysregulation and oxidative stress is associated with many diseases including atherosclerosis [4], cancer [5, 6], diabetic retinopathy [7], and chronic inflammatory disease [8]. Excess reactive oxygen species is also associated with aging processes [9]. Biological systems evolved endogenous defense mechanisms including employing antioxidants and antioxidative enzymes, to help protect against free radical-induced cell damage [10, 11].
Reactive oxygen species [12] including hydrogen peroxide, superoxide anion, and singlet oxygen are significantly induced in the skin under UV irradiation [13]. Exposure to solar UV radiation is a key factor in the initiation of several skin disorders such as wrinkling, scaling, dryness, pigment abnormalities, and skin cancer [14, 15]. The solar UV spectrum can be divided into three segments based on wavelength bands: UVC: 200–290 nm, UVB: 290–320 nm, and UVA: 320–400 nm. Each spectral range has a characteristic penetration of the epidermal and dermal layers of human skin. Potent antioxidative polyphenols from natural products such as catechins and genistein can prevent UV-induced cytotoxicity [16]. These observations support the notion that UV-induced oxidative stress and damage contribute to melanoma pathogenesis and skin aging and could be targeted using antioxidative preventive therapies. The observations of oxidative stress-induced cytotoxicity that can be attenuated by antioxidative compounds such as alpha-ketoglutarate and N-acetyl cysteine also suggest that oxidative stress plays a pivotal role in the progression of many diseases and could serve as a useful target for treatment [17, 18].
Eclipta prostrata L. (Asteraceae) is widely distributed throughout India, China, Taiwan, Thailand, and Brazil. The plant has a folk reputation in Taiwan as a remedy for the treatment of bleeding, hemoptysis and itching, hepatitis, diphtheria, and diarrhea. Stigmasterol, caffeic acid, and wedelolactone have been determined as markers of E. prostrata L. Methanol extracts E. prostrata L. have been used to treat jaundice, leishmaniasis [19], mouse osteoblast differentiation [20], and hepatic stellate cell proliferation [21]. Water extract of E. prostrate L. (WEP) showed a significant reduction in total cholesterol, triglyceride, total protein, and elevation in high-density lipoprotein cholesterol concentrations [22, 23]. The extract is reported to suppress maternal aggression [24]. However, there are no reports of WEP antioxidative activity or the capability of WEP in preventing UV-induced cytotoxicity being investigated or evaluated.
Therefore, the aim of this study is to investigate WEP active ingredients and antioxidative activities. We also review the role of WEP in the prevention of UVB irradiation-induced cytotoxicity.
2. Materials and Methods
2.1. Materials
2,2-Diphenyl-2-picrylhydrazyl (DPPH; Aldrich), FeCl2·4H2O (Fluka), trichloroacetic acid (Sigma), phenazine methosulfate (PMS; Sigma), nicotinamide adenine dinucleotide (NADH; Sigma), nitro blue tetrazolium (NBT; Sigma Aldrich), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma), butylated hydroxytoluene (BHT; Aldrich), stigmasterol (Sigma), caffeic acid (Sigma), wedelolactone (Sigma), and ethylenediaminetetraacetate (EDTA; Sigma) were purchased from Sigma Chemical Co. (St. Louis, MO). Chlorogenic acid was purchased from Acros Organics (Thermo Fisher Scientific Inc.). Ferrozine, ferric chloride (FeCl3), and potassium ferricyanide (K3Fe (CN)6) were purchased from Showa Co., Ltd. (Tokyo, Japan). Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen), fetal bovine serum (FBS, Gibco), and penicillin-streptomycin were purchased from Gibco BRL (Life technology, Paisley, Scotland).
2.2. Sample Preparation
E. prostrata L. was purchased from a traditional Chinese medicine market in Taipei, Taiwan. Fresh aerial parts of the plant were washed, air-dried, weighed, and ground to small pieces. The samples were then immersed in double distilled water (sample to water ratio 1 : 2 by weight) and boiled at 100°C for 20 min. After boiling, samples were filtered through Whatman No.1 paper with vacuum assistance. The water extracts were then freeze-dried and stored at −20°C. Prior to use, samples were dissolved in double distilled water at a concentration of 10 mg/mL to prepare a stock solution.
2.3. WEP RP-HPLC Analysis
The RP-HPLC system included a binary pump and UV detector (Agilent infinity 1200). Analytical RP-HPLC of the WEP extract was performed on 20 μL samples injected into a 5C18-AR-II analytical column (250 × 4.6 mm, 5 μm). The mobile phase was a ternary gradient of solvent A (10 mM KH2PO4, pH 4.0) and solvent B (acetonitrile/methanol/water = 3/1/1). The gradient flow program was 0 min: 100% A, 0% B; 10 min: 90% A, 10% B; 20 min: 80% A, 20% B; 30 min: 60% A, 40% B, at a flow rate of 0.8 mL min−1, and elution was monitored at 320 nm. Gallic acid (1 μg/mL) provided the internal control.
2.4. Total Phenolic Content
Total phenolic content was measured based on the redox reaction between Folin-Ciocalteu with phenolics in the sample. The total phenolic content was determined using a previously described method [25]. Briefly, various concentrations (0.1 mg/mL, 0.3 mg/mL, 1 mg/mL, 3 mg/mL, and 5 mg/mL) of 0.3 mL E. prostrata L. solutions were mixed with 2.4 mL of distilled water and 0.3 mL Folin-Ciocalteu reagent. Double distilled water was used as vehicle control. Sodium carbonate (20%, 0.6 mL) was added to the reaction mixture and allowed to stand for 30 min. The absorbance at 730 nm was measured and compared to a gallic acid calibration curve and expressed as mg gallic acid equivalents (GAE) per gram of sample.
2.5. DPPH Free Radical Scavenging Assay
The principle of DPPH method is based on the reduction of DPPH in the presence of a hydrogen donating antioxidant. The scavenging activity of WEP extracts on DPPH radicals was determined using a previously described method [25]. A total of 50 μL of various concentrations (0.01 mg/mL, 0.1 mg/mL, 0.3 mg/mL, 1 mg/mL, and 3 mg/mL) of WEP or chlorogenic acid were mixed with 150 μL of freshly prepared 1 mM DPPH in ethanol. Double distilled water was used as the vehicle control, and ascorbic acid (0.1 mg/mL, 0.3 mg/mL, 1 mg/mL, and 3 mg/mL) was used as positive control. The mixture was kept in darkness for 30 min. DPPH absorbance was then measured at 517 nm using an ELISA reader (TECAN, Austria). Percent activity was calculated using the following equation:
| (1) |
The IC50 value, which is the sample concentration required for 50% inhibitory activity, was determined by interpolation. Each test was performed in triplicate.
2.6. Reducing Power Assay
The reducing power of the WEP extracts was determined by using a previously described method [26]. The method is based on the principle that substances react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+). Potassium ferrocyanide then reacts with ferric chloride to form ferric ferrous complex which has an absorption maximum at 700 nm. Briefly, 100 μL of WEP at various concentrations (double distilled water) was mixed with phosphate buffer (100 μL, 2 M, pH 6.6) and K3Fe(CN)6 (100 μL, 1% w/v). Double distilled water was used as a negative control, and BHT (0.1 mg/mL, 0.3 mg/mL, and 1 mg/mL) was used as a positive control. The mixture was incubated at 50°C for 20 min in a water bath. Trichloroacetic acid (10% w/v; 100 μL) was added and the resulting mixture was centrifuged at 1,050 G for 10 min. The supernatant (100 μL) was combined with distilled water (100 μL) and FeCl3 solution (20 μL, 0.1% w/v). The absorbance was then measured at 700 nm using a V630 UV-Vis Spectrophotometer (JASCO Co. LTD, Japan).
2.7. Fe2+-Chelating Capacity Assay
WEP iron-chelating capacity was determined using a method proposed by Dinis et al. [27]. The principle is based on the formation of ferrozine-Fe2+ complex and its disruption in the presence of chelating agents. Briefly, 25 μL aliquots of WEP sample at various concentrations between 0.1 and 1 mg/mL were prepared from stock solution and mixed with 175 μL of methanol, 25 μL of 400 μM FeCl2·4H2O, and 25 μL of 2 mM ferrozine. The mixture stood for 10 min, and the absorbance was then measured at 562 nm using an ELISA reader. EDTA (0.1 mg/mL, 0.3 mg/mL, 1 mg/mL, 3 mg/mL, 5 mg/mL, and 10 mg/mL) was used as a positive control. This test was conducted in triplicate.
2.8. Superoxide Radical Scavenging Assay
WEP superoxide anion-scavenging ability was measured using a previously described method [28]. The phenazine methosulfate nicotinamide adenine dinucleotide (PMS-NADH) system generates superoxide radicals, which reduce NBT to a purple-colored diformazan compound. Briefly, reaction solutions containing various concentrations of WEP (50 μL, 0.1 mg/mL to 1 mg/mL) mixed with PMS (80 μM), NADH (1248 μM), and NBT (200 μM) in phosphate buffer (0.1 M, pH 7.4) were incubated at room temperature for 5 min. Double distilled water was used as a negative control, and quercetin (0.1 mg/mL, 0.3 mg/mL, and 1 mg/mL) was used as a positive control. The color was read at 560 nm against blank samples. The superoxide anion radical scavenging percentage was calculated from the following equation:
| (2) |
2.9. UV Spectrum of WEP
WEP samples with concentrations of 0.01, 0.03, 0.1, 0.2, and 0.3 mg/mL were investigated in the range 200–400 nm using Ultraviolet-visible (UV-Vis) spectroscopy at room temperature.
2.10. Determination of UV-Induced Cytotoxicity-Protective Effect
Human keratinocytes (HaCaT cells) and mouse fibroblast cells (3T3) were cultured at a density of 5 × 104 cells/mL in DMEM medium supplemented with FBS (10% v/v), streptomycin (100 μg/mL), and penicillin (10 U/mL) and kept at 37°C under a 5% CO2 humidified atmosphere. To determine the protective effect of WEP against 30 mJ/cm2 or 60 mJ/cm2 UVB-induced toxicity by UV cross-linking (302 nm, UVP CL-1000), HaCaT cells were seeded in a 96-well plate and treated with various concentrations of WEP (0.1–1 mg/mL) for 24 h. The viabilities of HaCaT and 3T3 cells were determined using MTT assay, a colorimetric assay that measures the reduction of yellow MTT by mitochondrial succinate dehydrogenase to an insoluble, colored (dark purple) formazan product. Briefly, MTT (10 μL, 5 mg/mL) was added to each well and stood for 1 h before removal of the supernatant. The remaining formazone crystals were dissolved in 100 μL DMSO, and the absorbance read at 570 nm with an ELISA reader. Cell viability was expressed as a percentage of surviving cells relative to surviving control cell samples.
2.11. Statistical Analysis
Three samples were prepared for each assay. The results were expressed as mean and standard deviations. Data analysis included one-way ANOVA, followed by Duncan's Multiple Range Test (P < 0.05), and a correlation test using the SigmaStat 3.5 software program.
3. Results
3.1. RP-HPLC Analysis of WEP
Our determination of compounds present in WEP showed that chlorogenic acid is a major component, present at 1.75 mg/g sample (Figure 1). Wedelolactone, caffeic acid, and stigmasterol, which have been reported as major components of methanol/ethanol extracts of E. prostrata L. [12, 21], were not present in WEP. This result indicated that active ingredients of WEP are different from those of methanol/ethanol extracts of E. prostrata L., and this may account for their different biological activities [12, 21].
Figure 1.
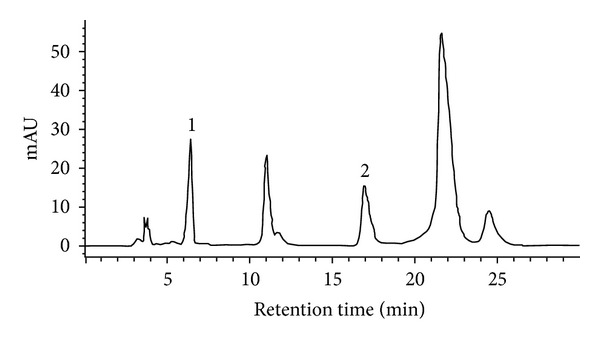
RP-HPLC analyses of water extracts from E. prostrata L. Peak 1 is gallic acid used as an international standard. Peak 2 represents chlorogenic acid.
3.2. Total Phenolic Content
We measured the total phenolic content of our WEP samples as 175.45 ± 11.56 mg GAE/g (Table 1). Total phenolic content may positively correlate with antioxidative capacity [29]. Therefore, based on our current results, the potent antioxidative effects of WEP may provide profound benefits in combating chronic degenerative disorders, or UV-induced cytotoxicity caused by oxidative stress [19, 30].
Table 1.
Total phenolic contents and phenolic compounds of WEP samples.
| Sample | Total phenols (mg GAE/g sample) |
Chlorogenic acid (mg/mL) |
Caffeic acid (mg/mL) |
|---|---|---|---|
| WEP | 176.45 ± 11.56 | 1.75 ± 0.01 | ND |
ND: not determined.
3.3. DPPH Radical Scavenging Activity
WEP's DPPH radical scavenging activity increased sigmoidally with increasing sample concentrations between 0.01 and 3 mg/mL sample (Figure 2), indicating that WEP's DPPH radical scavenging activity reached a saturation point at concentrations of 3 mg/mL. However, the saturation concentration of chlorogenic acid was 0.1 mg/mL (Figure 1). Chlorogenic acid demonstrated more potent effects than WEP did in scavenging DPPH radicals; the chlorogenic acid and WEP IC50 values were 0.050 ± 0.002 mg/mL and 0.75 ± 0.11 mg/mL, respectively [31]. This may explain why DPPH radical scavenging activity observed in our study correlated well with chlorogenic acid content in WEP.
Figure 2.
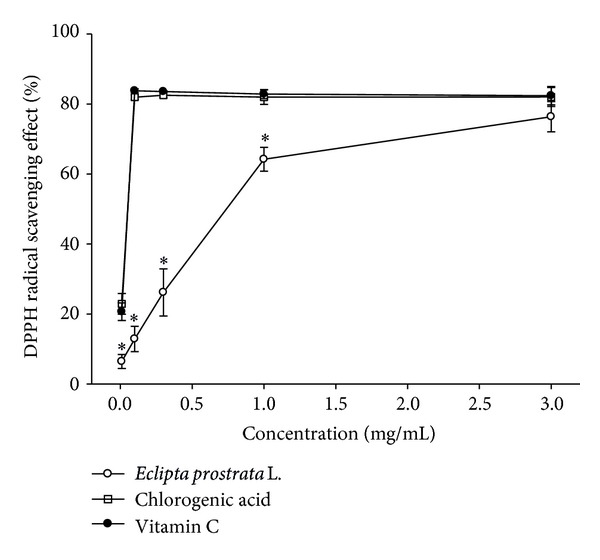
DPPH radical scavenging activity of WEP and chlorogenic acid. Values are means ± SD (n = 3). The samples with different lower case letters indicate a significant difference from the vitamin C standard (P < 0.05). Vitamin C (0.1 mg/mL, 0.3 mg/mL, 1 mg/mL, and 3 mg/mL) is used as a positive control.
3.4. Reducing Power
Figure 3 shows a plot of WEP-reducing power as a function of sample concentration. The reduction capacities of chlorogenic acid and BHT were significantly greater than those of WEP (P < 0.05) (Figure 3). However, at a concentration of 1 mg/mL, no significant differences in reducing power were observed between WEP, chlorogenic acid, and BHT (Figure 3). Thus, at 1 mg/mL concentration, WEP has a similar efficacy to that of chlorogenic acid and BHT.
Figure 3.
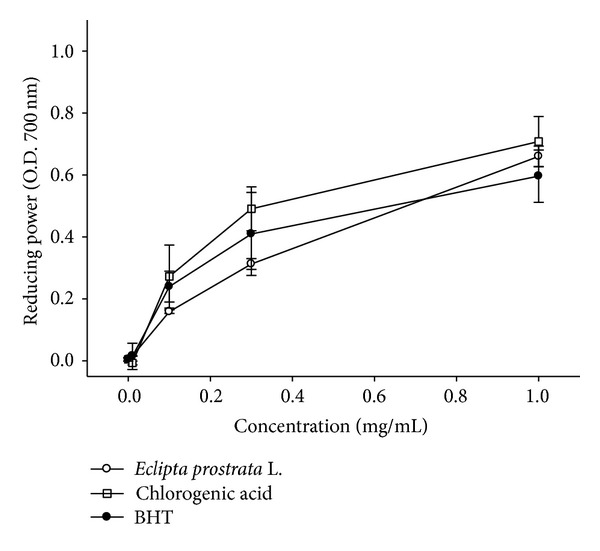
Reducing power of WEP and chlorogenic acid. Values are means ± SD (n = 3). Samples with different lower case letters indicate a significant difference from BHT (P < 0.05). BHT (0.1 mg/mL, 0.3 mg/mL, and 1 mg/mL) is used as a positive control.
3.5. Iron-Chelating Capacity
The iron-chelating capacity of WEP samples was measured by assessing their ability to compete with ferrozine in chelating ferrous ion [32]. This test measures activity by the decrease in absorbance of the red Fe2+/ferrozine complex. Figure 4 presents iron-chelating capacity as a function of WEP concentration. The IC50 chelating capacity of WEP was 3.20 ± 0.27 mg/mL and reached the saturation point at a concentration of 5 mg/mL. The IC50 chelating capacity of chlorogenic acid was 4.5 ± 0.6 mg/mL and reached saturation at 10 mg/mL (Figure 4). This result demonstrates that chlorogenic acid is not a potent iron-chelating agent and makes little or no contribution to WEP iron-chelating activity. The IC50 of the EDTA control was 0.110 ± 0.005 mg/mL.
Figure 4.
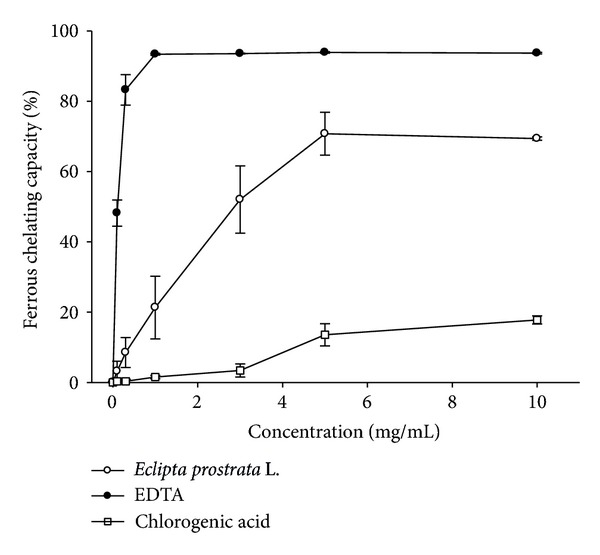
Iron-chelating activity of WEP and chlorogenic acid. Values are means ± SD (n = 3). Samples with different lower case letters indicate a significant difference from EDTA (P < 0.05). EDTA (0.1 mg/mL, 0.3 mg/mL, 1 mg/mL, 3 mg/mL, 5 mg/mL, and 10 mg/mL) is used as a positive control.
3.6. Scavenging of Superoxide Radical Anion
Figure 5 plots the scavenging activity of superoxide radical anion. While a marginal inhibition of 4.10% appeared at a WEP concentration of 0.01 mg/mL, this inhibition increased 83.65% in a dose response manner at WEP concentrations of 1 mg/mL (Figure 5). The WEP IC50 for superoxide anion-scavenging activity was 0.48 ± 0.04 mg/mL. The superoxide scavenging activity of chlorogenic acid and quercetin was significantly greater than that of WEP (P < 0.05) (Figure 5). However, with 1 mg/mL, we did not observe any significant difference between WEP, chlorogenic acid, and quercetin on superoxide anion-scavenging activity (Figure 5).
Figure 5.
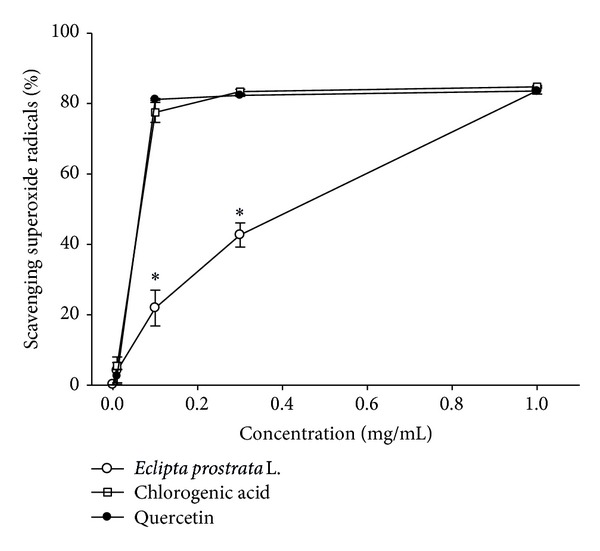
Scavenging activity of superoxide radical of WEP and chlorogenic acid. Values are means ± SD (n = 3). Samples with different lower case letters indicate a significant difference from quercetin (P < 0.05). Quercetin (0.1 mg/mL, 0.3 mg/mL, and 1 mg/mL) is used as a positive control.
3.7. UV Spectrum of WEP
WEP exhibits two UV absorbances (Figure 6). One band occurs in the UVC range at 200–275 nm, which is also absorbed by the air. The other band, at 275–400 nm, is classified as UVB/UVA. This absorption range comprises more than 95% of terrestrial UV radiation and is responsible for UV skin damage. WEP peak absorbance of UVB/UVA occurs at 273 nm (Figure 6). The absorbance of WEP at 273 nm is dose-dependent. WEP absorbance at concentrations of 0.01, 0.03, 0.1, 0.2, and 0.3 mg/mL at 273 nm were 0.086, 0.25, 0.83, 1.66, and 2.63, respectively (Figure 6), demonstrating that WEP may serve as an effective UVA and UVB filter.
Figure 6.
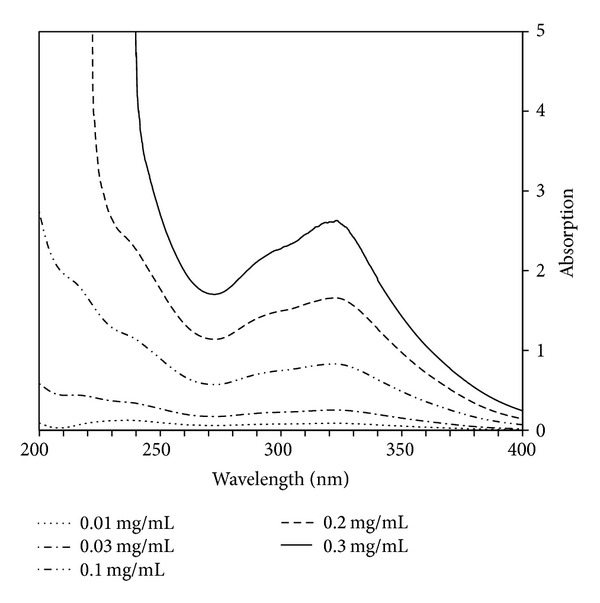
UV spectrum of WEP. The WEP peak absorbance of UVB/UVA occurs at 273 nm in a dose-dependent manner.
3.8. Protection against UV-Induced Cytotoxicity
Ultraviolet irradiation induces oxidative stress, resulting in cell damage or cell death [33]. UVB irradiation at 30 mJ/cm2 was significantly attenuated by 0.1 mg/mL WEP, and cell viability of HaCaT cells improved from 72.05 ± 6.58%, without WEP protection, to 96.88 ± 10.64%. Furthermore, WEP prevented UVB-induced cytotoxicity from occurring under 60 mJ/cm2 irradiation in a dose response manner. At concentrations of 0.3 mg/mL and above, WEP completely prevents UVB-induced cell death (Figure 7(a)). Fibroblast 3T3 cells were more sensitive to UVB-induced cell death compared with HaCaT cells. However, the results were consistent with those of the HaCaT cells; 0.1 mg/mL WEP can significantly attenuate 30 mJ/cm2 UVB irradiation and provide significant protection against UVB-induced cell death (Figure 7(b)). Thus, WEP prevents 60 mJ/cm2 UVB-induced 3T3 cell death in a dose response manner, and 0.3 mg/mL WEP and greater can almost completely prevent UVB-induced cell death (Figure 7(b)).
Figure 7.
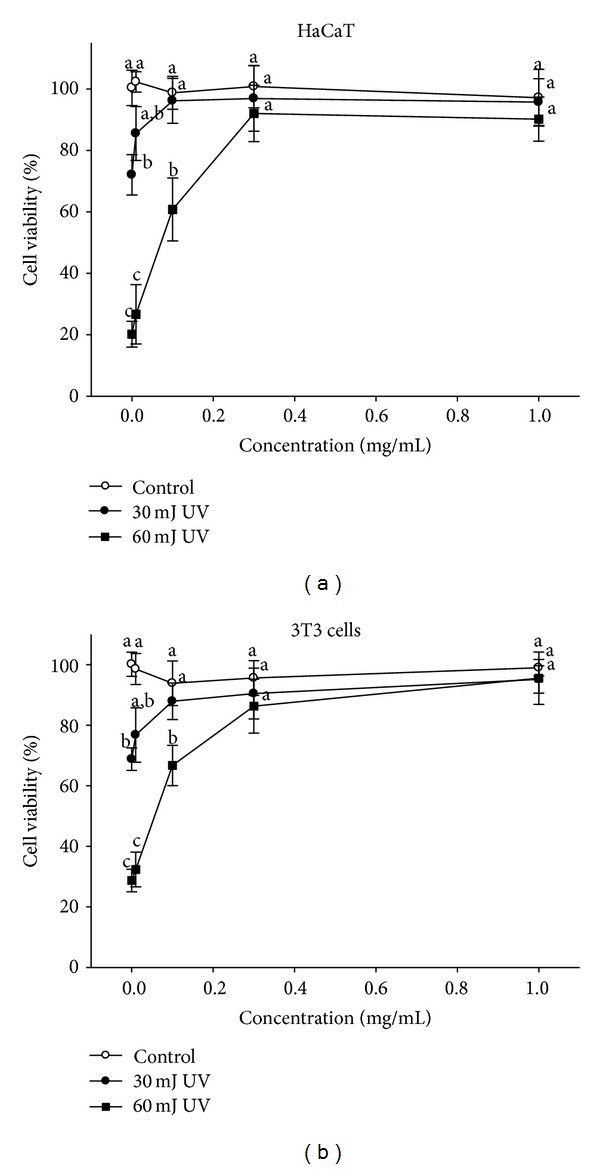
Protective effects of WEP on UVB-induced cytotoxicity. (a) WEP protects against 30 mJ/cm2 and 60 mJ/cm2 UVB-induced cytotoxicity with a dose-dependent response in HaCaT cells. (b) WEP protects against 30 mJ/cm2 and 60 mJ/cm2 UVB-induced cytotoxicity with a dose-dependent response in 3T3 cells. Mean values not sharing the same lower case letter are significantly different (P < 0.05).
4. Discussion
Chronic diseases such as neurodegeneration, cardiovascular disorder, diabetes, and cancers have become major health issues in numerous countries and demand considerable healthcare resources. These diseases mainly result from endogenous production of oxidative species. UV radiation is an exogenous ROS-inducing factor and also a critical factor in the initiation and development of a number of skin diseases [34]. According to a WHO report [35, 36], approximately 48,000 melanoma-related deaths occur worldwide per year. A number of antioxidants such as vitamin E and N-acetylcysteine (NAC) exist and are effective in the prevention of reactive oxidative stress- and UV-induced diseases [37]. In recent years, natural product extracts of potent antioxidative activity have arisen, in particular, extracts with high polyphenol contents, such as grape seed proanthocyanidins, resveratrol, silymarin, and genistein, which have demonstrable activity against UV-induced skin inflammation, oxidative stress, and DNA damage [38].
Numerous reports of studies investigate the antioxidative activities of methanol extracts such as those from rosemary or the oil fraction of plants. However, essential oils and methanol extracts of natural products exhibit different biological properties depending on the collection location and reveal seasonal variations [12, 21, 39]. It has been found recently that rosemary constitutes a biomass available for the development of oil-free extracts. The biological activity of oil-free rosemary extract is not sensitive to where the plant was grown [40]. In this study, we used water extract of Eclipta prostrata L. which can avoid differences arising from the plant's origin and avoid contamination with organic solvent residues in the final product.
We were unable to find any literature reports that E. prostrata L. (Asteraceae) is effective as a remedy for UV-induced skin disease. However, another Asteraceae plant, Gynura procumbens Merr., is used as a traditional remedy for various skin diseases in areas of southeast Asia. The ethanolic extract of Gynura procumbens inhibits metalloprotease-1 (MMP-1) and metalloprotease-9 (MMP-9) expression induced by UVB irradiation through the inhibition of proinflammatory cytokine mediator release and ROS production [41]. The antioxidative assay results from our study demonstrate that the WEP has potent activity against ROS (Figures 2 to 6) and has rich polyphenol content that includes chlorogenic acid as a major component (Figure 1 and Table 1). WEP protection against UVB-induced cytotoxicity produced similar results in HaCaT and 3T3 cells (Figures 7(a) and 7(b)), indicating that WEP can prevent epidermal (HaCaT) and dermal (3T3) cells against UV-induced cytotoxicity. Chlorogenic acid has been demonstrated as a potent antioxidative polyphenol compound. The scavenging of DPPH radical activity by chlorogenic acid is more effective than that of rutin and ferulic acid, although it is less efficient than caffeic acid and epicatechin gallate [42]. These study results are consistent with previous findings that chlorogenic acid is a potent DPPH radical scavenger, with an IC50 of 0.05 ± 0.002 mg/mL (Figure 2). Chlorogenic acid is photostable to UV light and does not degrade under UVA or UVB irradiation [43]. the use of hydrophilic chlorogenic acid with oil in a water-based microemulsion as a vehicle to protect guinea pig dorsal skin against UV-induced oxidative damage [44] has been demonstrated. Chlorogenic acid may play an important role in antioxidative activity and contribute to WEP protection against UVB-induced cytotoxicity [44]. Most of chlorogenic acid's antioxidative activities outperform those of WEP, with the exception of ion-chelating activity (Figure 4). Therefore, we propose that WEP's antioxidative properties and protection against UV-induced cytotoxicity may result from a synergistic effect between chlorogenic acid and other WEP active antioxidants.
5. Conclusion
In general, these results suggest that WEP can offer benefits to the pharmaceutical, food, and cosmetics industries to alleviate oxidative and UV-induced skin diseases. However, the detailed mechanism of WEP action against UVB-induced cytotoxicity requires further investigation.
Acknowledgment
The authors thank the National Science Council, Taiwan, for its financial support (NSC 102-2622-E-241-006-CC3).
Abbreviations
- UV:
Ultraviolet
- WEP:
Water extract of Eclipta prostrata L.
- DPPH:
2,2-Diphenyl-2-picrylhydrazyl
- GAE:
Gallic acid equivalents
- HPLC:
High-performance liquid chromatography
- HaCaT cells:
Human keratinocytes
- 3T3:
Mouse fibroblast cells
- ROS:
Reactive oxidative stress
- PMS:
Phenazine methosulfate
- NADH:
Nicotinamide adenine dinucleotide
- NBT:
Nitro blue tetrazolium
- FeCl3:
Ferrozine, ferric chloride
- K3Fe (CN)6:
Potassium ferricyanide
- MTT:
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- BHT:
Butylated hydroxytoluene
- EDTA:
Ethylenediaminetetraacetate
- DMEM:
Dulbecco's Modified Eagle's Medium
- FBS:
Fetal bovine serum
- ELISA:
The enzyme-linked immunosorbent assay
- NAC:
N-Acetylcysteine
- MMP-1:
Metalloprotease-1
- MMP-9:
Metalloprotease-9
- IC50:
Half maximal inhibitory concentration.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Róth E. Oxygen free radicals and their clinical implications. Acta chirurgica Hungarica. 1997;36(1–4):302–305. [PubMed] [Google Scholar]
- 2.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radical Biology and Medicine. 2009;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Birch-Machin MA, Swalwell H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis. 2010;25(2):101–107. doi: 10.1093/mutage/gep061. [DOI] [PubMed] [Google Scholar]
- 4.Haidari M, Ali M, Gangehei L, Chen M, Zhang W, Cybulsky MI. Increased oxidative stress in atherosclerosis-predisposed regions of the mouse aorta. Life Sciences. 2010;87(3-4):100–110. doi: 10.1016/j.lfs.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves TL, Erthal F, Corte CLD, et al. Involvement of oxidative stress in the pre-malignant and malignant states of cervical cancer in women. Clinical Biochemistry. 2005;38(12):1071–1075. doi: 10.1016/j.clinbiochem.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Tas F, Hansel H, Belce A, et al. Oxidative stress in breast cancer. Medical Oncology. 2005;22(1):11–15. doi: 10.1385/MO:22:1:011. [DOI] [PubMed] [Google Scholar]
- 7.Mehta R, Shangari N, O’Brien PJ. Preventing cell death induced by carbonyl stress, oxidative stress or mitochondrial toxins with vitamin B anti-AGE agents. Molecular Nutrition and Food Research. 2008;52(3):379–385. doi: 10.1002/mnfr.200600190. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama Y, Lindholm B, Stenvinkel P. Inflammation and oxidative stress in ESRD—the role of myeloperoxidase. Journal of Nephrology. 2004;17(8):S72–S76. [PubMed] [Google Scholar]
- 9.Carvour M, Song C, Kaul S, Anantharam V, Kanthasamy A, Kanthasamy A. Chronic low-dose oxidative stress induces caspase-3-dependent PKCδ proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Annals of the New York Academy of Sciences. 2008;1139:197–205. doi: 10.1196/annals.1432.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD. Increased oxidative stress and antioxidant expression in mouse keratinocytes following exposure to paraquat. Toxicology and Applied Pharmacology. 2008;231(3):384–392. doi: 10.1016/j.taap.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes AS, Serejo J, Gaspar J, et al. Oxidative injury in V79 Chinese hamster cells: Protective role of the superoxide dismutase mimetic MnTM-4-PyP. Cell Biology and Toxicology. 2010;26(2):91–101. doi: 10.1007/s10565-009-9120-3. [DOI] [PubMed] [Google Scholar]
- 12.Mors WB, Do Nascimento MC, Parente JP, Da Silva MH, Melo PA, Suarez-Kurtz G. Neutralization of lethal and myotoxic activities of south american rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae) Toxicon. 1989;27(9):1003–1009. doi: 10.1016/0041-0101(89)90151-7. [DOI] [PubMed] [Google Scholar]
- 13.Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, Schauen M, Blaudschun R, Wenk J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biological Chemistry. 1997;378(11):1247–1257. [PubMed] [Google Scholar]
- 14.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochemistry and Photobiology. 1996;63(4):356–357. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 15.Scharffetter-Kochanek K, Brenneisen P, Wenk J, et al. Photoaging of the skin from phenotype to mechanisms. Experimental Gerontology. 2000;35(3):307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 16.Chan WH, Yu JS. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. Journal of Cellular Biochemistry. 2000;78:73–84. doi: 10.1002/(sici)1097-4644(20000701)78:1<73::aid-jcb7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Hariharakrishnan J, Satpute RM, Prasad GBKS, Bhattacharya R. Oxidative stress mediated cytotoxicity of cyanide in LLC-MK2 cells and its attenuation by alpha-ketoglutarate and N-acetyl cysteine. Toxicology Letters. 2009;185(2):132–141. doi: 10.1016/j.toxlet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Harvey CJ, Thimmulappa RK, Singh A, et al. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radical Biology and Medicine. 2009;46(4):443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna VG, Kannabiran K, Getti G. Leishmanicidal activity of saponins isolated from the leaves of Eclipta prostrata and Gymnema sylvestre . Indian Journal of Pharmacology. 2009;41(1):32–35. doi: 10.4103/0253-7613.48891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin X-H, Wu Y-B, Lin S, Zeng J-W, Zeng P-Y, Wu J-Z. Effects of volatile components and ethanolic extract from Eclipta prostrata on proliferation and differentiation of primary osteoblasts. Molecules. 2010;15(1):241–250. doi: 10.3390/molecules15010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MK, Ha NR, Yang H, Sung SH, Kim GH, Kim YC. Antiproliferative activity of triterpenoids from Eclipta prostrata on hepatic stellate cells. Phytomedicine. 2008;15(9):775–780. doi: 10.1016/j.phymed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Kumari CS, Govindasamy S, Sukumar E. Lipid lowering activity of Eclipta prostrata in experimental hyperlipidemia. Journal of Ethnopharmacology. 2006;105(3):332–335. doi: 10.1016/j.jep.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Rangineni V, Sharada D, Saxena S. Diuretic, hypotensive, and hypocholesterolemic effects of Eclipta alba in mild hypertensive subjects: a pilot study. Journal of Medicinal Food. 2007;10(1):143–148. doi: 10.1089/jmf.2006.0000. [DOI] [PubMed] [Google Scholar]
- 24.Banji D, Banji OJF, Annamalai AR, Shanthmurthy M. Impact of the aqueous extract of Eclipta alba on maternal aggression in rats. Pakistan Journal of Pharmaceutical Sciences. 2010;23(2):138–142. [PubMed] [Google Scholar]
- 25.Abdelwahab SI, Mohan S, Mohamed Elhassan M, et al. Antiapoptotic and antioxidant properties of orthosiphon stamineus benth (Cat’s Whiskers): intervention in the Bcl-2-mediated apoptotic pathway. Evidence-based Complementary and Alternative Medicine. 2011;2011:10 pages. doi: 10.1155/2011/156765.156765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao WC, Lai Y-C, Yuan M-C, Hsu Y-L, Chan C-F. Antioxidative activity of water extract of sweet potato leaves in Taiwan. Food Chemistry. 2011;127(3):1224–1228. doi: 10.1016/j.foodchem.2011.01.131. [DOI] [PubMed] [Google Scholar]
- 27.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics. 1994;315(1):161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 28.Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 29.Cantín CM, Moreno MA, Gogorcena Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) batsch] breeding progenies. Journal of Agricultural and Food Chemistry. 2009;57(11):4586–4592. doi: 10.1021/jf900385a. [DOI] [PubMed] [Google Scholar]
- 30.Brunetti L, Menghini L, Orlando G, et al. Antioxidant effects of garlic in young and aged rat brain in vitro. Journal of Medicinal Food. 2009;12(5):1166–1169. doi: 10.1089/jmf.2008.0176. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y-C, Chang Y-H, Shao Y-Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chemistry. 2006;98(3):529–538. [Google Scholar]
- 32.Elmastaş M, Turkekul I, Öztürk L, Gülçin I, Isildak O, Aboul-Enein HY. Antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta) from North Turkey. Combinatorial Chemistry and High Throughput Screening. 2006;9(6):443–448. doi: 10.2174/138620706777698544. [DOI] [PubMed] [Google Scholar]
- 33.Jeon G-I, Yoon M-Y, Park H-R, Lee S-C, Park E. Neuroprotective activity of Viola mandshurica extracts on hydrogen peroxide-induced DNA damage and cell death in PC12 cells. Annals of the New York Academy of Sciences. 2009;1171:576–582. doi: 10.1111/j.1749-6632.2009.04889.x. [DOI] [PubMed] [Google Scholar]
- 34.Svobodova AR, Galandakova A, Sianska J, Dolezal D, Ulrichova J, Vostalova J. Acute exposure to solar simulated ultraviolet radiation affects oxidative stress-related biomarkers in skin, liver and blood of hairless mice. Biological & Pharmaceutical Bulletin. 2011;34:471–479. doi: 10.1248/bpb.34.471. [DOI] [PubMed] [Google Scholar]
- 35.Robyn L, Tony M, Wayne S, Bruce A. Solar Ultraviolet Radiation: Global Burden of Disease From Solar Ultraviolet Radiation. W. H. Organization; 2006. (Env Burd Dis Ser V 13). [Google Scholar]
- 36.Roy RK, Thakur M, Dixit VK. Hair growth promoting activity of Eclipta alba in male albino rats. Archives of Dermatological Research. 2008;300(7):357–364. doi: 10.1007/s00403-008-0860-3. [DOI] [PubMed] [Google Scholar]
- 37.Goodson AG, Cotter MA, Cassidy P, et al. Use of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress: towards a novel paradigm for melanoma chemoprevention. Clinical Cancer Research. 2009;15(23):7434–7440. doi: 10.1158/1078-0432.CCR-09-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Archives of Dermatological Research. 2010;302(2):71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogunbinu AO, Flamini G, Cioni PL, Ogunwande IA, Okeniyi SO. Essential oil constituents of Eclipta prostrata (L.) L. and Vernonia amygdalina Delile. Natural Product Communications. 2009;4(3):421–424. [PubMed] [Google Scholar]
- 40.Martin R, Pierrard C, Lejeune F, Hilaire P, Breton L, Bernerd F. Photoprotective effect of a water-soluble extract of Rosmarinus officinalis L. against UV-induced matrix metalloproteinase-1 in human dermal fibroblasts and reconstructed skin. European Journal of Dermatology. 2008;18(2):128–135. doi: 10.1684/ejd.2008.0349. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Lee C-W, Kim EK, et al. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. Journal of Ethnopharmacology. 2011;137(1):427–433. doi: 10.1016/j.jep.2011.04.072. [DOI] [PubMed] [Google Scholar]
- 42.Bandoniene D, Murkovic M. On-line HPLC-DPPH screening method for evaluation of radical scavenging phenols extracted from apples (Malus domestica L.) Journal of Agricultural and Food Chemistry. 2002;50(9):2482–2487. doi: 10.1021/jf011475s. [DOI] [PubMed] [Google Scholar]
- 43.Rivelli DP, Filho CAH, Almeida RL, Ropke CD, Sawada TCH, Barros SBM. Chlorogenic acid UVA-UVB photostability. Photochemistry and Photobiology. 2010;86(5):1005–1007. doi: 10.1111/j.1751-1097.2010.00776.x. [DOI] [PubMed] [Google Scholar]
- 44.Kitagawa S, Yoshii K, Morita S-Y, Teraoka R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chemical and Pharmaceutical Bulletin. 2011;59(6):793–796. doi: 10.1248/cpb.59.793. [DOI] [PubMed] [Google Scholar]


