Abstract
Anemia, usually due to iron deficiency, is highly prevalent among patients with colorectal cancer. Inflammatory cytokines lead to iron restricted erythropoiesis further decreasing iron availability and impairing iron utilization. Preoperative anemia predicts for decreased survival. Allogeneic blood transfusion is widely used to correct anemia and is associated with poorer surgical outcomes, increased post-operative nosocomial infections, longer hospital stays, increased rates of cancer recurrence and perioperative venous thromboembolism. Infections are more likely to occur in those with low preoperative serum ferritin level compared to those with normal levels. A multidisciplinary, multimodal, individualized strategy, collectively termed Patient Blood Management, minimizes or eliminates allogeneic blood transfusion. This includes restrictive transfusion policy, thromboprophylaxis and anemia management to improve outcomes. Normalization of preoperative hemoglobin levels is a World Health Organization recommendation. Iron repletion should be routinely ordered when indicated. Oral iron is poorly tolerated with low adherence based on published evidence. Intravenous iron is safe and effective but is frequently avoided due to misinformation and misinterpretation concerning the incidence and clinical nature of minor infusion reactions. Serious adverse events with intravenous iron are extremely rare. Newer formulations allow complete replacement dosing in 15-60 min markedly facilitating care. Erythropoiesis stimulating agents may improve response rates. A multidisciplinary, multimodal, individualized strategy, collectively termed Patient Blood Management used to minimize or eliminate allogeneic blood transfusion is indicated to improve outcomes.
Keywords: Colorectal cancer, Anemia, Allogeneic blood transfusion, Intravenous iron, Erythropoiesis stimulating agents, Patient Blood Management
Core tip: Anemia, usually due to iron deficiency, is highly prevalent among patients with colorectal cancer. Both anemia and allogeneic blood transfusion are associated with poorer outcomes. Anemia management, within a multidisciplinary, multimodal, individualized strategy to minimize or eliminate allogeneic blood transfusion, is indicated to improve outcomes. Intravenous iron is safe and effective but underused, despite the extremely low risk of causing serious adverse events. Newer intravenous iron formulations allow complete replacement dosing in 15-60 min markedly facilitating care. Erythropoiesis stimulating agents may improve response rates.
PREVALENCE OF ANEMIA AND IRON DEFICIENCY
Anemia is one of the most frequent extraintestinal manifestations of colorectal cancer (CRC), and may be present in 30%-75% of patients, predicated on the level of hemoglobin (Hb) used to define anemia and tumor localization and stage[1-7]. A study on 358 patients with CRC reported a 25% prevalence of moderate to severe anemia (Hb < 10 g/dL). The multivariate analysis revealed that age, tumor site (right colon), and tumor size (large size), but not clinical stage or histological type, were significant contributing factors[2]. These results were corroborated by a study of 1189 Norwegian patients[7]. Lastly, a recent study showed that iron deficiency (ID) in CRC was associated with poor performance and more advanced disease[8].
CONSEQUENCES OF ANEMIA
Preoperative anemia is the major predictive factor for allogeneic blood transfusion (ABT) in surgeries with moderate to high perioperative blood loss, which is causative in postoperative anemia and aggravates pre-existing anemia[9]. In CRC resection, a hematocrit of less than 30% has been shown to be an independent risk factor for perioperative ABT[6,7,10,11]. Even mild-to-moderate preoperative anemia has been linked to increased postoperative morbidity and length of hospital stay as well as decreased disease-free survival after resection[12-14]. This is supported by a recent publication in which preoperative anemia significantly worsened overall survival (P = 0.040) in the univariate analysis[15]. However, in the multivariate analysis the difference did not approach statistical significance.
Both preoperative anemia and ID without anemia increase the rate of postoperative nosocomial infection. Following abdominal surgery, infections were significantly more likely with low preoperative serum ferritins compared with normal levels. The data were especially poignant in that confounders including Hb level were taken into account in the analysis[16]. Zago et al[17] evaluated the relationship of vitamin and mineral levels to wound complications in 100 abdominal surgical procedures, and noted low plasma retinol (a marker of low vitamin A intake) and high erythrocyte protoporphyrin (an early marker of ID) to be surrogates of increased complications. Further evaluation of the benefits of these measurements as a standard of care appears warranted.
PATHOPHYSIOLOGY OF ANEMIA
In CRC, preoperative anemia can be attributed to chronic hemorrhage, neoadjuvant chemotherapy or radiotherapy, and nutritional deficiencies. These may be exacerbated by activation of the immune system with release of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and interleukins (IL)-1, -6, -8 and -10[18-20]. These inflammatory mediators cause anemia via a variety of pathophysiological mechanisms (Figure 1): (1) decreased red cell half-life due to dyserythropoiesis with red cell damage and increased erythrophagocytosis (TNF-α); (2) inadequate EPO response for the severity of anemia; (3) impaired responsiveness of erythroid cells to EPO (IFN-γ, IL-1, and TNF-α); (4) inhibited proliferation and differentiation of erythroid cells (IFN-γ, IL-1, TNF-α, and α-1-antitrypsin); and (5) pathologic iron homeostasis due to increased divalent metal transport 1 (IFN-γ) and transferrin receptor expression (IL-10) in macrophages, reduced ferroportin 1 expression (IFN-γ and IL-6-induced high hepcidin levels) in enterocytes and macrophages, and increased ferritin synthesis (TNF-α, IL-1, IL-6, IL-10).
Figure 1.
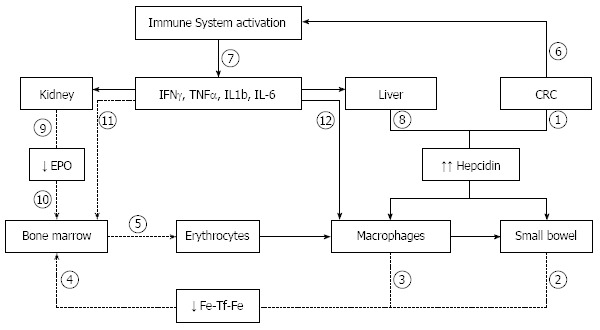
Pathophysiological mechanisms of anemia of inflammation in colorectal cancer. 1: Hepcidin release by colorectal cancer cells (CRC); 2,3: Decreased release of iron via ferroportin: leading to decreased transferrin-bound iron; 4: Decreased iron availability; 5: Reduced erythrocyte production; 6: Activation of immune system by CRC; 7: Release of immune and inflammatory cytokines; 8: Interleukin-6 (IL-6) induced hepcidin release; 9: Decreased erythropoietin (EPO) production; 10: Decreased erythropoietic stimulation; 11: Inhibition of erythroid cell proliferation; 12: Augmented erythrofagocytosis. IFN-γ: Interferon-γ; TNF-α: Tumor necrosis factor-α.
Transferrin-bound iron is the primary iron source for erythropoiesis, entering the erythroblast by a process involving transferrin receptor-mediated endocytosis. This iron may be obtained by absorption of dietary iron and/or mobilization of iron stores at macrophages and liver. Dietary non-hem iron primarily exists in an oxidised (Fe3+) form which is reduced to the Fe2+ form by a ferrireductase enzyme, before being transported across the intestinal epithelium by a carrier protein called divalent metal transporter 1. Dietary heme iron enters the enterocyte by heme carrier protein, is metabolised by heme oxygenase to release Fe2+, which enters a common pathway with dietary non-hem iron before being exported by ferroportin 1 across the basolateral membrane of the enterocyte (absorbed iron). Iron export from the stores at macrophages and hepatocytes is also accomplished primarily by ferroportin 1. Iron is then oxidized, released into the circulation, bound to transferrin and transported to sites of use (Figure 2)[19,20].
Figure 2.
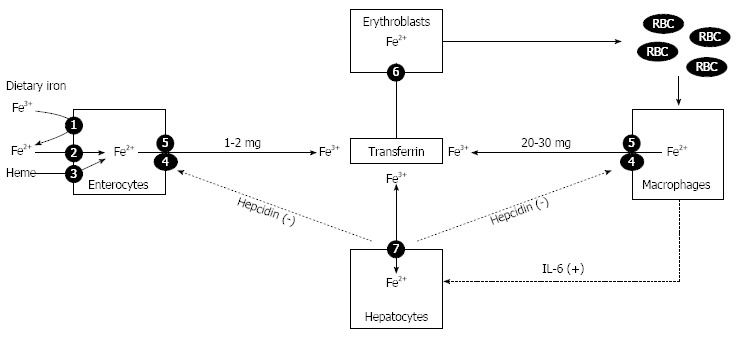
A simplified scheme of main pathways of iron metabolism. 1: Ferrireductase; 2: Divalent metal transporter (DMT1); 3: Heme protein carrier 1; 4: Ferroportin; 5: Hephastin/ceruloplasmin; 6: Transferrin receptor-1 (TfR1); 7: Several mechanisms; IL-6: Interleukin 6; RBC: Red blood cell.
The amount of iron required for daily renewal of red blood cells (20-30 mg) is provided mostly by senescent erythrocyte iron recycling at macrophages. Therefore, as daily absorption (1-2 mg) just balances daily loss, internal turnover of iron is essential to meet the bone marrow requirements for erythropoiesis.
Hepcidin, a 25-amino acid peptide produced mainly by hepatocytes in response IL-6 levels, plays a major role in dysregulation of iron homeostasis during inflammation. Once synthesised, hepcidin is secreted into the bloodstream and interacts with ferroportin 1 (the only known iron exporting protein) at enterocyte basolateral membrane, hepatocytes and macrophages (Figure 2). The binding of hepcidin to ferroportin 1 causes internalization and lysosomal degradation of the carrier protein. Thus, hepcidin regulates the rate of iron absorption by villous enterocytes and the rate of iron recirculation from macrophages and hepatocytes, resulting in hypoferremia. In addition, inflammatory mediators increased divalent metal transporter 1 (IFN-γ), transferrin receptor expression (IL-10) and ferritin synthesis (TNF-α, IL-1, IL-6, IL-10) in macrophages leading to increased iron storage[18-20].
Hypoferremia and increased reticuloendothelial iron result in decreased iron availability referred to as iron restricted erythropoiesis or functional iron deficiency (FID); formerly referred to as anemia of chronic disease. This is characterized by low serum iron and decreased transferrin saturation, in the face of adequate body iron stores defined by the presence of stainable iron in the bone marrow and/or a serum ferritin value within or above normal limits. Finally, when persisting decreased iron absorption and/or chronic blood loss are present, FID may evolve to absolute iron deficiency (FID + ID). While hepcidin affects iron trafficking in FID and FID + ID, individuals suffering from FID + ID have significantly lower hepcidin levels than those with FID without ID[21]. Individuals with both, in contrast to FID alone, absorb some dietary iron from the gut and mobilize some iron from macrophages. Thus, hepcidin levels may be useful in differentiating between FID and FID + ID and in selecting appropriate therapy for these patients[21]. This is supported by a recent presentation by Steensma et al[22] who noted a 92% response rate to intravenous (iv) iron in chemotherapy induced anemia patients with low pretreatment hepcidin levels. Hepcidin levels have been also shown useful in predicting non-responsiveness to oral iron therapy in patients with IDA[23]. Ward et al[24], in a cohort of 56 CRC patients, measured hepcidin in urine and determined hepcidin mRNA expression and hepcidin cellular localization in CRC tissue. Hepcidin immunoreactivity was found in 34% specimens from patients with CRC and was correlated with ferroportin inhibition. Urinary hepcidin was positively associated with increasing CRC tumor stage, but not with anemia. This suggests that CRC hepcidin, rather than hepatic hepcidin, is involved in a proportion of cases of CRC-associated anemia more likely to be IDA (or FID + ID) rather than FID and will respond to iv iron rather than oral iron[25].
DIAGNOSIS
The prevalence of gastrointestinal (GI) pathology among IDA patients varies from 43% to 86%, the most common being benign erosive lesions in the upper GI tract, accounting for 39%-57% of upper GI with CRC, accounting for 42%-69% of lower GI lesions[26]. IDA is a known harbinger of CRC mandating evaluation. Whenever clinically feasible, at four weeks prior to elective CRC surgery an anemia evaluation including standard iron parameters should be performed. If indicated, appropriate intervention should be implemented, as they decreases perioperative morbidity[27,28]. Laboratory screening for anemia in CRC should include, at least, a CBC, reticulocytes and assessment of iron parameters (Fe, TSAT, ferritin) (Table 1), and C-reactive protein (CRP), which is useful in determining the presence or absence of inflammation[29-33]. The presence of anemia should be considered when the Hb level is < 13 g/dL for men and < 12 g/dL for women. However, a normal Hb level does not exclude ID, as blood loss is nearly always the cause and a significant amount must occur before iron deficient erythropoiesis begins. In non-anemic ID patients, the symptom of chronic fatigue is non-specific and a laboratory finding of a low serum ferritin provides an indirect estimate of body iron stores (1 ng/mL of ferritin = 8 mg of stored iron)[34]. ID is defined as a ferritin level < 30 ng/mL regardless patient’s inflammatory status.
Table 1.
Main laboratory tests for the assessment of iron depletion
| Laboratory test |
Normal values |
||
| Conventional units | Conversion factor2 | SI units | |
| Iron status in the body | |||
| Serum iron | 50-180 g/dL | × 0.179 | 9-32 mol/L |
| Transferrin | 200-360 mg/dL | × 0.01 | 2-3.6 g/L |
| Transferrin saturation | 20%-50% | ||
| Ft | 30-300 ng/mL | × 2.247 | 65-670 pmol/L |
| sTfR1 | 0.76-1.76 mg/L | 6.4-25.7 nmol/L | |
| sTfR/log Ft | < 1 | ||
| Iron deficient red cell production | |||
| Hb | 12-16 g/dL ♀ | × 0.62063 | 7.5-10 mmol/L |
| 13-17 g/dL ♂ | 8-10.5 mmol/L | ||
| Mean corpuscular volume | 80-100 fL | ||
| Red cell distribution width | 11-15 | ||
| Mean corpuscular Hb | 28-35 pg | ||
| Hypochromic red cells | < 5% | ||
| Reticulocyte Hb content | 28-35 pg | ||
Normal values may differ depending on the assay used;
To convert the concentrations values in conventional unit into SI units multiply figures by the conversion factor;
In fact, although widely used, this factor allows for the calculation the molar concentration of hemoglobin subunits. Thus, the molar concentration of hemoglobin (Mw 64 kDa) is 4-fold times lower (2-3 mmol/L). Ft: Ferritin; sTfR: Soluble transferrin receptors; Hb: Hemoglobin; sTfR/log Ft: Ratio of sTfR to serum Ft.
In the presence of inflammation, TSAT < 20% and ferritin 30-100 ng/mL suggest absolute ID, whereas FID is generally defined by TSAT < 20% and ferritin ≥ 100 ng/mL[30,32,33,35] (Figure 3). However, though ferritin values > 100 ng/mL argue against concurrent absolute ID in the setting of inflammation, this test is imperfect due to its acute phase reactivity. Other tests can be utilized to evaluate for a component of ID, which if present suggests a benefit toward iron supplementation. These tests include measurement of the ratio of the sTfR to log ferritin and percent circulating hypochromic erythrocytes (increased in IDA and FID + ID), and the reticulocyte Hb content (low with IDA and FID + ID) (Table 1). Where available, hepcidin will help the diagnostic evaluation in this regard; if suppressed, a component of absolute ID is implied. IDA should be considered if anemia with a TSAT < 20% and/or ferritin 30 ng/mL, with or without inflammation (Figure 3). Low MCV (< 80 fL) is a reliable and routinely part of the automated CBC, but is a late indicator. In addition, IDA without microcytosis may occur early in iron deficiency anemia prior to the development of iron deficient erythropoiesis, when there is coexistinmg vitamin B12 or folate deficiency, post-bleeding reticulocytosis, initial response to iron treatment, alcohol intake or mild myelodysplasia.
Figure 3.
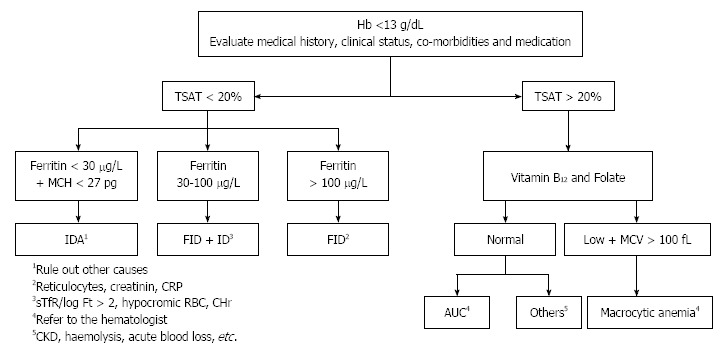
An algorithm for anemia diagnosis. Modified from Muñoz et al[20]. ACD: Anemia of chronic disease; AUC: Anemia of unknown cause; CHr: Reticulocyte hemoglobin; CKD: Chronic kidney disease; CRP: C-reactive protein; Ft: Ferritin; Hb: Hemoglobin; ID: iron deficiency; IDA: Iron deficiency anemia; MCH: Mean corpuscular hemoglobin; MCV: Mean corpuscular volume; sTfR: Serum transferrin receptor; TSAT: Transferrin saturation; FID: Functional iron deficiency.
When anemia in CRC cannot be explained by IDA or FID + ID, it is important to consider other causes that would demand specific treatment. In these cases, further testing should include B12, lactate dehydrogenase, and serum creatinine in order to exclude other nutritional deficiencies, hemolysis or renal disease[29-33]. If malabsorption or severe malnutrition, a red cell folate may also be useful.
TREATMENT OPTIONS
Allogeneic blood transfusion
After CRC surgery, perioperative blood loss and postoperative blunted erythropoiesis, due to surgery-induced inflammation, may lead to severe postoperative anemia, especially in those presenting with low preoperative Hb. In this context, ABT continues to be the most frequently used treatment for acute intra- and post-operative anemia, although its quick and effective increase in Hb levels is transitory, and is associated with poorer outcomes. Subsequently, ABT should be restricted to those with severe anemia, poor physiological reserve and/or acute symptoms requiring immediate correction.
Perioperative ABT is associated with increased rates of cancer recurrence[36,37]. In a meta-analysis, 23 out of 36 studies on 12127 patients showed a detrimental effect of ABT. After ABT a higher rate of tumor recurrence compared to those not transfused with a clustered OR of 1.42[38] was observed. In a more recent meta-analysis, ABT has been shown to increase all-cause mortality (OR = 1.72), cancer-related mortality (OR = 1.71) and morbidity, such as wound infection (OR = 3.27), after CRC resection[39]. Subsequently, many medical scientific societies recommend a restrictive approach for perioperative ABT, in which the level of Hb below which an ABT unit is transfused (i.e., the “transfusion trigger”) should be intimately related to the ability to tolerate normovolemic anemia relative to available cardiopulmonary reserve[40,41]. In non-bleeding, euvolemic anemic patients, ABT is recommended to maintain Hb levels between 7 and 9 g/dL (8-10 g/dL for those with cardiac and/or central nervous system dysfunction)[40,41].
Malignancy and surgery are known prothrombotic stimuli, and perioperative ABT may further increase the risk for venous thromboembolism (VTE). The analysis of two databases of almost 3000 CRC resections demonstrated that intraoperative ABT was a significant risk factor for the development of VTE, increasing with increased number of units transfused[42,43]. In this analysis the risk for VTE in women was statistically greater than in men. Preoperative hematocrit did not enter the multivariable model as an independent predictor of VTE, or did open versus laparoscopic resection or wound class[43]. The diagnosis of almost one third of postoperative VTE occurred after discharge[44]. Subsequently, the Enhanced Recovery after Surgery (ERAS) program recommends extended postoperative prophylaxis with low-molecular weight heparin for 28 d in CRC patients[45].
In summary, available data strongly recommend minimizing ABT in CRC surgery, using restrictive transfusion policies and implementing ABT alternatives, with emphasis on thromboprophylaxis after discharge.
Pharmacologic treatment
Objectives: The goal of preoperative anemia therapy should be normalization of the Hb levels, in accordance with World Health Organization criteria. However, as CRC resections are procedures with a moderate-to-high blood loss, it would be desirable to achieve a Hb of 13 g/dL for both genders to minimize the risk for transfusion. Similarly, postoperative anemia treatment should be aimed to attain Hb levels which avoid or reduce the exposure to ABT, followed by its correction in the shortest possible period, to favourably influence sensitivity to adjuvant treatments, facilitate the functional recovery and improve quality of life.
Iron replacement: In CRC, ID with or without anemia should be corrected pre-operatively by iv iron, with or without an erythropoiesis stimulating agent (ESA), preferably two to four weeks prior the scheduled procedure. While at least four studies explored the efficacy of preoperative oral iron[46-49] (Table 2), the results are routinely inferior to those with the iv iron route[50].
Table 2.
Characteristics of the clinical studies examining the role of preoperative iron replacement in colorectal cancer included in this review
| Study | Study design | Patients | Baseline Hb (g/dL) | Iron compound dose (mg) | Duration (wk) | Hb (g/dL) | ABT (% or U/pt) |
| Oral iron | |||||||
| Okuyama et al[46] | OBS | Iron: 32 | 8.1 ± 1.4 | Ferrous citrate | ≥ 2 | 2.0 | 9.4% |
| No iron: 84 | 8.0 ± 1.6 | (200 mg/d) | 0.9 | 27.4% | |||
| Lidder et al[47] | RCT | Iron: 23 | 13.4 ± 1.9 | FS | 2-8 | -0.3 | 26.0% |
| No iron: 22 | 12.4 ± 2.1 | (200 mg TDS) | -0.6 | 59.0% | |||
| Quinn et al[48] | OBS | Iron: 103 | 12 (10-14) | FS | 1-9 | 0.69 U/pt | |
| No iron: 167 | NS | (200 mg TDS) | 1.69 U/pt | ||||
| Ferrari et al[49] | RCT | FB: 12 | 11.6 ± 1.6 | FB | 8 | 1 (2) mo | NS |
| (28-14 mg/d) | 0.8 (1.4) | ||||||
| FS: 12 | 11.3 ± 1.2 | FS | 0.7 (1.4) | ||||
| (105 mg/d) | |||||||
| Intravenous iron | |||||||
| Edwards et al[57] | RCT | Iron: 34 | 13.7 ± 0.5 | IS | 2 | -0.2 | 14.7% |
| Placebo: 26 | 13.4 ± 0.4 | (2 × 300 mg) | -0.5 | 19.2% | |||
| Bisbe et al[64] | OBS | IS: 30 | 10.1 ± 1.2 | IS | 2-6 | 0.9 | 7.0% |
| (100-200 mg, 6 ± 3 doses) | |||||||
| FCM: 15 | 9.2 ± 1.0 | FCM | 2.5 | 40.0% | |||
| (500-1000 mg, 3 ± 1 doses) | |||||||
| Todman et al[68] | Case series | Iron: 22 | < 12 | Iron isomaltoside-1000 | 2-6 | 0.7, 1-2 w | NS |
| (20 mg/kg bw) | 1.4, 3-4 w | ||||||
| 3.1, 6-8 w |
RCT: Randomized controlled trial; OBS: Observational cohort study; Hb: Increment from baseline; ABT: Allogeneic blood transfusion; FB: Ferrous bisglicinate; FS: Ferrous sulphate; IS: Iron sucrose; FCM: Ferric carboxymaltose.
Okuyama et al[46] compared preoperative Hb levels and transfusion requirements of anemic patients (Hb ≤ 10 g/dL), 32 who received oral iron supplementation (sodium ferrous citrate, 200 mg/d) for at least 2 wk preoperatively with those of 84 who did not. While iron supplementation resulted in higher Hb levels immediately before surgery (+1.2 g/dL; P < 0.05), and fewer required intraoperative ABT (9.4% vs 27.4%, P < 0.05), there were no significant differences in postoperative Hb levels or ABT volumes between the two groups. Lidder et al[47] conducted a randomized controlled trial (RCT) of oral ferrous sulphate (200 mg TDS) for a mean of 14 d pre-operatively (12-56 d) vs no iron therapy in patients with IDA or ID scheduled for CRC surgery. Oral iron was found to prevent Hb decrease from recruitment to admission, and to reduce ABT (25% vs 59%, for iron and control, respectively; P = 0.031), although these differences were not statistically significant for patients with IDA.
In a series of 103 patients receiving oral ferrous sulphate (200 mg TDS) for a median of 39 d pre-operatively (interquartile range = 7-63 d) and no preoperative ABT, Quinn et al[48] observed that: (1) fifty-eight (56.3%) patients who were anemic at presentation had a mean increment in Hb of 1.7 g/dL (P < 0.001); (2) those with right-sided tumors (lower mean Hb at presentation) responded more often to oral iron than those with left-sided tumors (P < 0.017); (3) increase in Hb was unrelated to tumor stage, but was greater when iron was administered for more than 14 days; and (4) ABT rate for all curative resections was 0.69 units/patient (compared to 1.69 units/patient using an historical cohort).
Several iv iron formulations are currently available (Table 3). iv iron therapy, with or without ESAs, as a safe and efficacious tool for treating anemia and reducing transfusion requirements in surgical and medical patients, has been extensively reviewed[51-54]. Randomized clinical trials have shown superior efficacy of iv iron over oral or no iron in reducing ABT, increasing Hb, and improving quality of life in ESA-treated anemic cancer patients[53,55]. In contrast, studies examining iv iron as sole anemia treatment in cancer patients are only just starting to emerge, and the role of iv iron for correcting perioperative anemia is frequently overlooked in the surgical care of cancer patients[56-60].
Table 3.
Some characteristics of the different intravenous iron formulations
| Iron gluconate | Iron sucrose | High molecular weight iron dextran | Low molecular weight iron dextran | Ferric carboxymaltose | Iron isomaltoside 1000 | Ferumoxytol | |
| Brand name | Ferrlecit® | Venofer® | Dexferrum® | Cosmofer® | Ferinject® | Monofer® | Rienso® |
| INFeD® | Injectafer® | FeraHeme® | |||||
| Carbohydrate shell | Gluconate (monosaccharide) | Sucrose (disaccharide) | Dextran (branched polysaccharide) | Dextran (branched polysaccharide) | Carboxymaltose (branched polysaccharide) | Isomaltoside (linear oligosaccharide) | Polyglucose sorbitol carboxymethylether |
| Molecular weight (kDa) | 289-440 | 30-60 | 265 | 165 | 150 | 150 | 750 |
| Plasma half-life (h) | 1 | 6 | 60 | 20 | 16 | 20 | 15 |
| Direct iron donation to transferrin (% injected dose) | 5-6 | 4-5 | 1-2 | 1-2 | 1-2 | < 1 | < 1 |
| Test dose required1 | No | Yes/No | Yes | Yes | No | No | No |
| Iron content (mg/mL) | 12.5 | 20 | 50 | 50 | 50 | 100 | 30 |
| Maximal single dose (mg) | 125 | 200-300 | 20 mg/kg | 20 mg/kg2 | 20 mg/kg (max 1000 mg in one infusion) | 20 mg/kg | 5103 |
| Premedication | No | No | TDI only | No | No | No | No |
| Life-threatening ADE (× 106 doses) | 0.9 | 0.6 | 11.3 | 3.3 | ?? | ?? | ?? |
Two case series illustrates the potential benefits of pre- or peri-operative iron supplementation in CRC resections. Campos et al[61] studied 43 CRC patients who received preoperative oral iron (100 mg/d) if Hb > 14 g/dL and iron deficiency was present; iron sucrose (200 mg/wk) if Hb 10-14 g/dL; or iron sucrose (200 mg twice a week) if Hb < 10 g/dL, during weeks 3-4. Seventeen received postoperative iron sucrose (200 mg on days 0, 2, and 4). A retrospective series not receiving iron was used as a control (n = 66). Despite a lower baseline Hb (12.3 g/dL vs 11.5 g/dL; P < 0.05), iron therapy reduced the transfusion index (4.0 unit/patient vs 1.3 unit/patient; P < 0.05) and the percentage of patients who received preoperative ABT (33% vs 9%; P < 0.05), but not the percentage of patients administered perioperative ABT (48% vs 35%; P = 0.161). However, the treatment was ineffective in patients with a high transfusion index (> 5 units/patient).
Díaz Espallardo et al[62] analyzed data from 437 CRC surgeries from 2005-2009. Patients presenting with Hb <13 g/dL and/or abnormal iron parameters, (group A, n = 242) received preoperative iron supplementation (178 received a mean of 867 mg iv iron sucrose, and 64 oral iron), whereas those presenting with Hb ≥ 13 g/dL and normal iron status, received no treatment (group B, n = 195). From diagnosis to surgery, Hb increased by 0.6 g/dL in group A, while it decreased by 0.8 g/dL in Group B (P < 0.05). From diagnosis to discharge, Hb decreased by 0.4 g/dL in group A, and by 2.5 g/dL in group B (P < 0.05). This tendency to progressive anemia observed in both groups may be secondary to the effects of CRC on erythropoiesis, chemo-radiotherapy treatment, and blood loss due to the tumor and later surgery. However, the differences between groups strongly suggest that iron therapy prevented patients from group A from reaching low Hb levels. The overall ABT rate was 8.6% (32/244, 13.1% vs 6/195, 3.1%; P = NS) and no differences in complications were observed.
In contrast, in a retrospective paired case-control study, Titos-Arcos et al[63] observed that postoperative administration of iv iron sucrose (592 ± 445 mg) did not decrease ABT rates (28.8% vs 30.8%, for case and control, respectively). In addition, for patients not receiving ABT, there were no differences in Hb concentration decrease between the first postoperative day and discharge (0.88 g/dL vs 0.82 g/dL, for case and control).
Higher vs lower dose intravenous iron administration: Bisbe et al[64] compared clinical and laboratory data of 15 anemic CRC receiving preoperative ferric carboxymaltose (FCM, 500-1000 mg/session; 3 ± 1 sessions) to those from a previous series of 30 CRC receiving preoperative iron sucrose (100-200 mg/session; 6 ± 1 sessions). Even though those in the FCM group had lower baseline Hb levels (9.2 g/dL vs 10.1 g/dL; P < 0.05), those from the FCM group showed a higher post-treatment Hb increment (+2.5 g/dL vs +0.9 g/dL; P < 0.05), and received fewer perioperative ABT (7% vs 40%; P > 0.05). While the total amount of iv iron infused in the FCM group was higher (1550 mg vs 1140 mg; P < 0.05) most clinical trials suggest 1000 mg is an adequate replacement dose[65-67]. Todman et al[68] administered a single dose iron infusion (Iron isomaltoside-1000, 20 mg/kg body weight) to 22 major cancer surgery patients with IDA either 2-6 wk before surgery, or post-operatively. Hemoglobin levels were monitored for up to 8 wk after infusion, or up to next blood transfusion, whichever was earlier. Mean Hb rise at 1-2 wk was 0.7 g/dL, 1.4 g/dL at 3-4 wk and 3.1g/dL at 6-8 wk, and no serious adverse effects were noted (Table 2).
This apparent superiority of “higher dose” over “lower dose” iv iron supplementation in improving erythropoiesis has been also reported for inflammatory bowel disease[69], and several factors might have account for the observed differences. Firstly, the “extra” amount of iron administered to the FCM group could have compensated for the ongoing blood loss from recruitment to surgery. Secondly, macrophage iron loading may also inhibit pro-inflammatory immune effector pathways[18,70]. Lastly, in iron balance, high hepcidin also reduces IL-6 production by macrophages, thus limiting the potential damage of an excessive inflammatory response[71]. As ID is highly prevalent among CRC, it is possible that rapid repletion with higher dose of iv iron can contribute in restoring an adequate immune response, improving the erythropoietic response.
As summarized in Table 2, initial results with preoperative oral or iv iron replacement therapy in CRC have been mixed, highlighting the need for large randomized controlled trials in preoperatively anemic patients.
Safety of intravenous iron: Although no serious adverse drug events (SAEs) have been reported in the studies reviewed, both the numbers and follow-up time were not large enough to draw definitive conclusions regarding the safety of iv iron agents in this clinical setting. In all published evidence extant, including millions of dialysis patients, no long term toxicity has been reported over the last two decades[72].
As of June 28th 2013, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) concluded that the benefits of iv exceed their risks, provided that adequate measures are taken to minimize the risk of allergic reactions. Data on the risk of hypersensitivity comes mainly from post-marketing spontaneous reports and the total number of life-threatening and fatal events reported is low, and cannot be used to detect any differences in the safety profile of the different iron formulations available in Europe (high molecular weight iron dextran and ferumoxytol were not included). The CHMP has issued recommendations for health care professionals which include: (1) intravenous iron medicines should only be administered when staff trained to evaluate and manage anaphylactic and anaphylactoid reactions are immediately available as well as resuscitation facilities; (2) a test dose is no longer recommended, as there are data indicating that allergic reactions may still occur in patients who have not reacted to a test dose; (3) patients should be closely observed for signs and symptoms of hypersensitivity reactions during and for at least 30 min following each injection of an iv iron medicine; and (4) intravenous iron-containing products are contraindicated in patients with hypersensitivity to a specific active substance or excipients, or other parenteral iron products.
Early formulations of high molecular weight iron dextran were associated with rare occurrences of anaphylaxis and even death. The newer formulations, LMW ID, ferric gluconate, iron sucrose, ferumoxytol, iron isomaltoside and ferric carboxymaltose are much safer with SAEs vanishingly rare. None the less minor infusion reactions still occur and are often misinterpreted as SAEs[53]. Premedication with antihistamines has been reported to cause the majority of perceived reactions to iv iron in one large cohort[73]. Antihistamines can cause somnolence, diaphoresis, hypotension and tachycardia ostensibly attributed to the administered iron. Tryptase a marker of mast cell degranulation, levels are virtually always normal and subsequently the use of premedication with antihistamines should be proscribed. In contradistinction, all of the formulations can be associated with acute chest and back tightness, without accompanying hypotension, tachypnea, tachycardia, wheezing, stridor or periorbital edema[51,74]. These infrequent reactions abate without therapy and rarely recur with rechallenge. The reactions are more frequent in those with allergic diatheses[65]. It is important not to overreact in the event of these minor AEs. A few patients will experience self-limited arthralgias and myalgias the day after iron infusions. These reactions abate without therapy and never leave residua. Non-steroidal anti-inflammatory drugs may shorten their duration. When these tenets are adhered to the administration of iv iron is safe and much safer than most physicians realize.
Erythropoiesis stimulating agents: The role of iv iron for CRC-related anemia remains unclear. It is possible that the effectiveness of perioperative iron treatment could be enhanced by concomitant ESA administration, although in Europe this is an off-label use of these growth factors. Pooled data from six trials (621 patients) showed that perioperative treatment with recombinant human erythropoietin did not reduced ABT (33% vs 37%; OR = 0.89; P = 0.206) in GI cancer surgery[75-80] (Table 4). Norager et al[80] explored the effect of perioperative ESA administration in CRC surgery (Table 4). No significant benefits were found for postoperative fatigue, quality of life, muscle strength or ABT use, but improved work capacity and early restoration of postoperative Hb concentrations to preoperative levels were observed. The treatment was uniformly well tolerated. Unfortunately, the impact of ESA therapy on anemic CRC patients was not analyzed separately. However, a reduction of both the percentage of transfused patients and the number of transfused units was only observed for those receiving ESAs plus iv iron. Additionally, the use of iv iron allowed for a significant reduction in the total dose of ESAs (Table 4).
Table 4.
Effects of perioperative administration of erythropoietin and iron on transfusion requirements in patients undergoing elective colorectal cancer resection
| Study |
+ESA |
Placebo |
Iron | ESA | ||
| n | ABT, n (%) | n | ABT, n (%) | (route, type, dose, d) | (type, total dose, route, day) | |
| Braga et al[75] | 10 | 1 (10)1 | 10 | 5 (50) | iv iron gluconate, 125 mg/d, 4 d | Epoetin alfa, |
| 500 IU/kg, SC | ||||||
| (from day -12 to day +8) | ||||||
| Kettelhack et al[76] | 48 | 16 (33) | 54 | 15 (28) | Oral, NS, 5-10 d preOP | Epoetin beta, |
| iv, NS, 40 mg, 1 d postOP | 3000-4500 IU/kg, SC | |||||
| (from day -10 to day +4) | ||||||
| Qvist et al[77] | 38 | 13 (34)2 | 43 | 23 (53) | Oral, NS, | Epoetin alfa, |
| 200 mg/d, 4 d | 1350 IU/kg, SC | |||||
| (from day -4 to day +7) | ||||||
| Kosmadakis et al[78] | 31 | 9 (29)1 | 32 | 19 (59) | iv iron sucrose, | Epoetin alfa, |
| 100 mg/d, 14 d | 4200 IU/kg, SC | |||||
| (from day -7 to day +7) | ||||||
| Christodoulakis et al[79] | 69 (a) | 34 (49) | 68 | 35 (51) | Oral, NS, | Epoetin alfa, |
| 67 (b) | 27 (40)2 | 200 mg/d, 10 d | 1800 IU/kg, SC (a) | |||
| 3600 IU/kg, SC (b) | ||||||
| (from day -10 to day +1) | ||||||
| Norager et al[80] | 75 | 10 (13) | 76 | 9 (12) | Oral, NS, | Darbepoetin alfa, |
| 200 mg/d, 7d | 750-1500 g, SC | |||||
| (from day -10 to day +25) | ||||||
| Overall | 338 | 110 (33) | 283 | 106 (37) | OR = 0.89 (95%CI: 0.58-1.12; P = 0.206) | |
Reduction in both percentage of transfused patients and number of transfused units;
Reduction in the number of transfused units only. ABT: Allogeneic blood transfusion; ESA: Erythropoiesis stimulating agent; NS: Not stated; preOP: Preoperative; postOP: Postoperative; SC: Subcutaneous.
Safety of erythropoiesis stimulating agents: ESA prevent transfusions among chemotherapy-associated anemia patients. An ESA-associated increase in mortality and/or disease progression has also been reported in eight controlled studies conducted in CIA however in each of these the ESA use was off-label. Another meta-analysis of 60 studies (15323 patients) showed no significant effect of ESAs on survival or disease progression, but increased the risk for venous-thromboembolic events (44 studies: OR = 1.48; 95%CI: 1.28-1.72)[81]. However, venous-thromboembolic events in cancer patients receiving ESAs for chemotherapy induced anemia may be linked to thrombocytosis due to ESA induced iron restricted erythropoiesis, which can be reversed by administration of iv iron[82,83]. Nevertheless, product labels advise against administering ESAs with potentially curative chemotherapy (United States) or to conduct risk-benefit assessments (Europe/Canada) and, since 2007, fewer chemotherapy-associated anemia patients in the United States and Europe receive ESAs[84-87].
In CRC surgery, a recent systematic review and meta-analysis of 4 RCTs also found insufficient evidence to support the use of ESAs in the preoperative and post-operative period for improving anemia and decreasing ABT. There were no significant differences in post-operative mortality or thrombotic events between groups, but no included study evaluated recurrences, survival, or quality of life[88].
Vitamin replacement: Deficiencies of vitamin B12, with or without anemia, should be appropriately managed. The intramuscular route is preferred (hydroxyl-cobalamin, 1 mg/wk, 4-6 wk), except for vegans (oral route) or anticoagulated patients (iv route).
Adjuvant measures
Nutritional support: Poor pre-operative nutritional status has been linked consistently to an increase in post-operative complications and poorer surgical outcome. Patients should be screened for nutritional status and, if deemed to be at risk of under-nutrition, given active nutritional support[45].
Meta-analyses were undertaken on trials evaluating different preoperative nutritional interventions. Benefits on post-operative complications and length of hospital stay of preoperative immune enhancing nutrition or parenteral nutrition may not be generalized or are not applicable to current clinical practice, whereas trials evaluating enteral or standard oral supplements were inconclusive[89]. Therefore, except for the severely malnourished, whether or not nutritional intervention should be initiated earlier in the preoperative period remains unclear.
In contrast, post-operative management in gastrointestinal surgery is becoming well established with ERAS protocols starting 24 h prior to surgery with carbohydrate loading, minimization of preoperative fasting and early oral or enteral feeding given to patients the first day following surgery (with oral nutritional supplements if necessary). ERAS is aimed to reduce surgical stress, insulin resistance, unnecessary protein losses and postoperative complications. In comparison with traditional care, ERAS programs were associated with significantly decreased length of hospital stay and total and general complications, without affecting readmission rates, surgical complications, and mortality[90].
Preventing perioperative hypothermia: Perioperative maintenance of normothermia with a suitable warming device and warmed iv fluids to keep body temperature > 36 °C decreased intraoperative blood loss and postoperative shivering, and it has been associated with lower rates of postoperative infection and better pain scores[45,91-94].
Restrictive fluid replacement (fluid balance): Hypovolemia can lead to hypoperfusion of vital organs and the bowel, which can lead to complications, and appropriated fluid reposition with balanced crystalloid solutions should be performed. However, administering too much may result in bowel edema, increased interstitial lung water, and dilution anemia which can also lead to complications[95]. The evidence suggests that patients being in a state of ‘‘fluid balance’’ (goal-directed fluid replacement) fared better than those with ‘‘fluid imbalance’’[96-99]. Postoperative iv fluids should be aimed to maintain normovolemia and avoid fluid excess. The enteral route should be used in preference and the drip taken down at the earliest opportunity (preferably no later than the morning after surgery)[45].
Perioperative supplemental oxygen: Although the role of perioperative supplemental oxygen in anemia tolerance has not been properly investigated, it has been proposed to decrease the incidence of surgical site infection in CRC surgery. This positive effect was not confirmed by a recent meta-analysis of 5 RCTs[100]. However, supplemental oxygen appears to confer a mortality benefit, a previously unreported finding that needs to be confirmed.
FROM LITERATURE TO BED-SIDE: A PRAGMATIC APPROACH TO CRC ASSOCIATED ANEMIA
“Time is gold for anemic patients waiting for CRC resection”
Early and aggressive treatment of anemia in CRC enables optimization of preoperative Hb, thus transforming a high transfusion risk to a low transfusion risk, which improves outcomes. Therefore, we developed a pragmatic, easy-to-follow protocol for diagnosis and treatment of preoperative CRC associated anemia, which is based upon the following considerations.
Diagnosis
Basic laboratory screening for anemia in CRC should comprise Hb, full blood counts (including reticulocytes), and assessments of body iron store (serum ferritin), iron availability (TSAT) and level of inflammation (CRP). Should anemia not be explained by initial work-up, further testing could comprise vitamin B12 and folic acid, haptoglobin, lactate dehydrogenase, and serum creatinine if other laboratory tests indicate their usefulness (Figure 3). These are low-cost, widely available tests which allow for correctly classifying most cases of CRC-associated anemia.
Treatment
Iron therapy: As IDA and FID + ID are the most frequent types of anemia in CRC, iron supplementation is of paramount importance and can be accomplished by following the algorithm depicted in Figure 4. The estimated total iron deficiency take into account the amount of iron needed to restore a Hb level of 13 g/dL and to replenish iron stores, as well as estimated iron loss due to ongoing chronic bleeding and perioperative blood loss.
Figure 4.
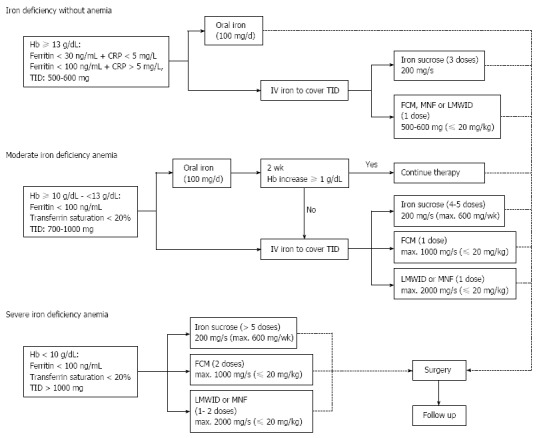
An algorithm for iron replacement. Modified from Muñoz et al[31]. FCM: Ferric caboxymaltose; Hb: Hemoglobin; LMWID: Low molecular weight iron dextran; MNF: Iron isomaltoside-1000; s: Session; TID: Total iron deficiency; CRP: C-reactive protein.
Erythropoiesis stimulating agents: Until more safety data in CRC are available, ESAs should be only used in the approved indications and following the recommendations of international guidelines.
Restrictive transfusion protocol: In most surgical CRC patients, ABT could be considered for maintaining Hb concentrations between 7 and 9 g/dL; for those with cardiac and/or central nervous system dysfunction, ABT could be considered for patients with symptoms or a Hb level of 8 g/dL or less, and ABT given for maintaining Hb concentrations between 8 and 10 g/dL[40,41] Carson 2011. However, whenever possible, avoidance of ABT is preferable.
Adjuvant therapies: All of above mentioned measures aimed to decrease blood loss, hemodilution and postoperative hyper-catabolism should also be implanted, as they may contribute to reduce the severity of and to hasten the recovery from postoperative anemia.
Follow-up
Patients should be followed-up for documenting the recovery from postoperative anemia, especially if adjuvant chemotherapy and/or radiotherapy were administered.
Footnotes
P- Reviewers: Petronella P, Steele SR S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
References
- 1.Cappell MS, Goldberg ES. The relationship between the clinical presentation and spread of colon cancer in 315 consecutive patients. A significant trend of earlier cancer detection from 1982 through 1988 at a university hospital. J Clin Gastroenterol. 1992;14:227–235. doi: 10.1097/00004836-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Sadahiro S, Suzuki T, Tokunaga N, Mukai M, Tajima T, Makuuchi H, Saito T. Anemia in patients with colorectal cancer. J Gastroenterol. 1998;33:488–494. doi: 10.1007/s005350050120. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, Kosmidis P, Krzakowski M, Nortier J, Olmi P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Beale AL, Penney MD, Allison MC. The prevalence of iron deficiency among patients presenting with colorectal cancer. Colorectal Dis. 2005;7:398–402. doi: 10.1111/j.1463-1318.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 5.Prutki M, Poljak-Blazi M, Jakopovic M, Tomas D, Stipancic I, Zarkovic N. Altered iron metabolism, transferrin receptor 1 and ferritin in patients with colon cancer. Cancer Lett. 2006;238:188–196. doi: 10.1016/j.canlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Konyalian V, Huynh R, Mittal R, Stamos M, Kumar R. Identification of predictive factors for perioperative blood transfusion in colorectal resection patients. Int J Colorectal Dis. 2007;22:1493–1497. doi: 10.1007/s00384-007-0347-2. [DOI] [PubMed] [Google Scholar]
- 7.Edna TH, Karlsen V, Jullumstrø E, Lydersen S. Prevalence of anaemia at diagnosis of colorectal cancer: assessment of associated risk factors. Hepatogastroenterology. 2012;59:713–716. doi: 10.5754/hge11479. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig H, Müldür E, Endler G, Hübl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24:1886–1892. doi: 10.1093/annonc/mdt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:58S–69S. doi: 10.1016/j.amjmed.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson KR, Berenholtz SM, Dorman T, Garrett E, Lipsett P, Kaufman HS, Pronovost PJ. Preoperative predictors of blood transfusion in colorectal cancer surgery. J Gastrointest Surg. 2002;6:753–762. doi: 10.1016/s1091-255x(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 11.Benoist S. [Perioperative transfusion in colorectal surgery] Ann Chir. 2005;130:365–373. doi: 10.1016/j.anchir.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 13.Kang CY, Chaudhry OO, Halabi WJ, Nguyen V, Carmichael JC, Stamos MJ, Mills S. Outcomes of laparoscopic colorectal surgery: data from the Nationwide Inpatient Sample 2009. Am J Surg. 2012;204:952–957. doi: 10.1016/j.amjsurg.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Zhen L, Zhe S, Zhenning W, Zhifeng M, Zhidong L, Xiaoxia L, Jianguang Y, Huimian X. Iron-deficiency anemia: a predictor of diminished disease-free survival of T3N0M0 stage colon cancer. J Surg Oncol. 2012;105:371–375. doi: 10.1002/jso.22032. [DOI] [PubMed] [Google Scholar]
- 15.Fjørtoft I, Furnes B, Hausken T, Storli KE, Eide GE, Søndenaa K. Pre-operative anaemia in colon cancer patients became normal after more than a year post-operatively but did not influence oncological outcome in the final analysis. Scand J Gastroenterol. 2013;48:663–671. doi: 10.3109/00365521.2013.781216. [DOI] [PubMed] [Google Scholar]
- 16.Harju E. Empty iron stores as a significant risk factor in abdominal surgery. JPEN J Parenter Enteral Nutr. 1988;12:282–285. doi: 10.1177/0148607188012003282. [DOI] [PubMed] [Google Scholar]
- 17.Zago L, Dupraz H, Torino F, Río ME. [Preoperative nutritional status and surgical risk. Identification of promissory biochemical markers] Nutr Hosp. 2010;25:91–98. [PubMed] [Google Scholar]
- 18.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J Gastroenterol. 2009;15:4617–4626. doi: 10.3748/wjg.15.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. J Clin Pathol. 2011;64:281–286. doi: 10.1136/jcp.2010.079046. [DOI] [PubMed] [Google Scholar]
- 21.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 22.Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, Stella PJ, Rowland KM, Novotny PJ, Loprinzi CL. Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J Clin Oncol. 2011;29:97–105. doi: 10.1200/JCO.2010.30.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88:97–101. doi: 10.1002/ajh.23354. [DOI] [PubMed] [Google Scholar]
- 24.Ward DG, Roberts K, Brookes MJ, Joy H, Martin A, Ismail T, Spychal R, Iqbal T, Tselepis C. Increased hepcidin expression in colorectal carcinogenesis. World J Gastroenterol. 2008;14:1339–1345. doi: 10.3748/wjg.14.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muñoz M, García-Erce JA, Díez-Lobo AI, Campos A, Sebastianes C, Bisbe E. [Usefulness of the administration of intravenous iron sucrose for the correction of preoperative anemia in major surgery patients] Med Clin (Barc) 2009;132:303–306. doi: 10.1016/j.medcli.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24:109–116. doi: 10.1097/MEG.0b013e32834f3140. [DOI] [PubMed] [Google Scholar]
- 27.Beris P, Muñoz M, García-Erce JA, Thomas D, Maniatis A, Van der Linden P. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 28.Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, Fergusson DA, Gombotz H, Habler O, Monk TG, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bermejo F, García-López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15:4638–4643. doi: 10.3748/wjg.15.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muñoz M, Botella-Romero F, Gómez-Ramírez S, Campos A, García-Erce JA. Iron deficiency and anaemia in bariatric surgical patients: causes, diagnosis and proper management. Nutr Hosp. 2009;24:640–654. [PubMed] [Google Scholar]
- 31.Muñoz M, Gómez-Ramírez S, García-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol. 2009;15:4666–4674. doi: 10.3748/wjg.15.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muñoz M, García-Erce JA, Remacha ÁF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol. 2011;64:287–296. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 33.Reinisch W, Staun M, Bhandari S, Muñoz M. State of the iron: how to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J Crohns Colitis. 2013;7:429–440. doi: 10.1016/j.crohns.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319–332. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, Cavill I. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161:639–648. doi: 10.1111/bjh.12311. [DOI] [PubMed] [Google Scholar]
- 36.Koch M, Antolovic D, Reissfelder C, Rahbari NN, Holoch J, Michalski I, Sweiti H, Ulrich A, Büchler MW, Weitz J. Leucocyte-depleted blood transfusion is an independent predictor of surgical morbidity in patients undergoing elective colon cancer surgery-a single-center analysis of 531 patients. Ann Surg Oncol. 2011;18:1404–1411. doi: 10.1245/s10434-010-1453-x. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen FV, Jensen LS, Sørensen HT, Pedersen L. Cause-specific mortality associated with leukoreduced, buffy coat-depleted, or no blood transfusion after elective surgery for colorectal cancer: a posttrial 15-year follow-up study. Transfusion. 2011;51:259–263. doi: 10.1111/j.1537-2995.2010.02825.x. [DOI] [PubMed] [Google Scholar]
- 38.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;(1):CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 40.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 41.Leal-Noval SR, Muñoz M, Asuero M, Contreras E, García-Erce JA, Llau JV, Moral V, Páramo JA, Quintana M. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilsson KR, Berenholtz SM, Garrett-Mayer E, Dorman T, Klag MJ, Pronovost PJ. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg. 2007;142:126–132; discussion 133. doi: 10.1001/archsurg.142.2.126. [DOI] [PubMed] [Google Scholar]
- 43.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129:568–572. doi: 10.1016/j.thromres.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 44.Davenport DL, Vargas HD, Kasten MW, Xenos ES. Timing and perioperative risk factors for in-hospital and post-discharge venous thromboembolism after colorectal cancer resection. Clin Appl Thromb Hemost. 2012;18:569–575. doi: 10.1177/1076029611433642. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, Macfie J, Liberman AS, Soop M, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37:259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 46.Okuyama M, Ikeda K, Shibata T, Tsukahara Y, Kitada M, Shimano T. Preoperative iron supplementation and intraoperative transfusion during colorectal cancer surgery. Surg Today. 2005;35:36–40. doi: 10.1007/s00595-004-2888-0. [DOI] [PubMed] [Google Scholar]
- 47.Lidder PG, Sanders G, Whitehead E, Douie WJ, Mellor N, Lewis SJ, Hosie KB. Pre-operative oral iron supplementation reduces blood transfusion in colorectal surgery - a prospective, randomised, controlled trial. Ann R Coll Surg Engl. 2007;89:418–421. doi: 10.1308/003588407X183364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinn M, Drummond RJ, Ross F, Murray J, Murphy J, Macdonald A. Short course pre-operative ferrous sulphate supplementation--is it worthwhile in patients with colorectal cancer. Ann R Coll Surg Engl. 2010;92:569–572. doi: 10.1308/003588410X12699663904277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrari P, Nicolini A, Manca ML, Rossi G, Anselmi L, Conte M, Carpi A, Bonino F. Treatment of mild non-chemotherapy-induced iron deficiency anemia in cancer patients: comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomed Pharmacother. 2012;66:414–418. doi: 10.1016/j.biopha.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Muñoz M, Campos A, Garcia Erce JA. Intravenous iron in colorrectal cancer surgery. Semin Hematol. 2006;43 Suppl 6:S36–S38. [Google Scholar]
- 51.Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet. 2007;369:1502–1504. doi: 10.1016/S0140-6736(07)60689-8. [DOI] [PubMed] [Google Scholar]
- 52.Muñoz M, Breymann C, García-Erce JA, Gómez-Ramírez S, Comin J, Bisbe E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008;94:172–183. doi: 10.1111/j.1423-0410.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 53.Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program. 2010;2010:338–347. doi: 10.1182/asheducation-2010.1.338. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz M, Gómez-Ramírez S, Martín-Montañez E, Pavía J, Cuenca J, García-Erce JA. Perioperative intravenous iron: an upfront therapy for treating anaemia and reducing transfusion requirements. Nutr Hosp. 2012;27:1817–1836. doi: 10.3305/nh.2012.27.6.6087. [DOI] [PubMed] [Google Scholar]
- 55.Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 2012;23:1954–1962. doi: 10.1093/annonc/mds112. [DOI] [PubMed] [Google Scholar]
- 56.Kim YT, Kim SW, Yoon BS, Cho HJ, Nahm EJ, Kim SH, Kim JH, Kim JW. Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecol Oncol. 2007;105:199–204. doi: 10.1016/j.ygyno.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Edwards TJ, Noble EJ, Durran A, Mellor N, Hosie KB. Randomized clinical trial of preoperative intravenous iron sucrose to reduce blood transfusion in anaemic patients after colorectal cancer surgery. Br J Surg. 2009;96:1122–1128. doi: 10.1002/bjs.6688. [DOI] [PubMed] [Google Scholar]
- 58.Dangsuwan P, Manchana T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol Oncol. 2010;116:522–525. doi: 10.1016/j.ygyno.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Steinmetz T, Tschechne B, Harlin O, Klement B, Franzem M, Wamhoff J, Tesch H, Rohrberg R, Marschner N. Clinical experience with ferric carboxymaltose in the treatment of cancer- and chemotherapy-associated anaemia. Ann Oncol. 2013;24:475–482. doi: 10.1093/annonc/mds338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Athibovonsuk P, Manchana T, Sirisabya N. Prevention of blood transfusion with intravenous iron in gynecologic cancer patients receiving platinum-based chemotherapy. Gynecol Oncol. 2013;131:679–682. doi: 10.1016/j.ygyno.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 61.Campos A, Sevilla I, Baca JJ, Romero A, Ramírez G, Muñoz M. Perioperative iron therapy and transfusion requirements in patients undergoing surgery for colon cancer. Preliminary results [abstract] Transfus Altern Transfus Med. 2005;8 Suppl 1:96. [Google Scholar]
- 62.Díaz Espallardo C, Laso Morales MJ, Colilles Calvet C, Mora López L, Roig Martínez I, Martínez Marín MT. [The multidisciplinary approach is useful for optimising preoperative haemoglobin in colorectal cancer surgery] Cir Esp. 2011;89:392–399. doi: 10.1016/j.ciresp.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Titos-Arcos JC, Soria-Aledo V, Carrillo-Alcaraz A, Ventura-López M, Palacios-Muñoz S, Pellicer-Franco E. Is intravenous iron useful for reducing transfusions in surgically treated colorectal cancer patients. World J Surg. 2012;36:1893–1897. doi: 10.1007/s00268-012-1589-x. [DOI] [PubMed] [Google Scholar]
- 64.Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–478. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 65.Fishbane S, Kowalski EA, Imbriano LJ, Maesaka JK. The evaluation of iron status in hemodialysis patients. J Am Soc Nephrol. 1996;7:2654–2657. doi: 10.1681/ASN.V7122654. [DOI] [PubMed] [Google Scholar]
- 66.Auerbach M, Winchester J, Wahab A, Richards K, McGinley M, Hall F, Anderson J, Briefel G. A randomized trial of three iron dextran infusion methods for anemia in EPO-treated dialysis patients. Am J Kidney Dis. 1998;31:81–86. doi: 10.1053/ajkd.1998.v31.pm9428456. [DOI] [PubMed] [Google Scholar]
- 67.Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, Balan S, Barker L, Rana J. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol. 2004;22:1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 68.Todman E, Mudan S, Rao Baikady R. Total dose iron infusion for iron deficiency anaemia in major cancer surgery - service evaluation [abstract] Tranfus Med. 2013;23 Suppl 1:38. [Google Scholar]
- 69.Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, Chopey IV, Gutzwiller FS, Riopel L, Gasche C. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846–853.e1-2. doi: 10.1053/j.gastro.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Weiss G, Meusburger E, Radacher G, Garimorth K, Neyer U, Mayer G. Effect of iron treatment on circulating cytokine levels in ESRD patients receiving recombinant human erythropoietin. Kidney Int. 2003;64:572–578. doi: 10.1046/j.1523-1755.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 71.Pagani A, Nai A, Corna G, Bosurgi L, Rovere-Querini P, Camaschella C, Silvestri L. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood. 2011;118:736–746. doi: 10.1182/blood-2011-02-337212. [DOI] [PubMed] [Google Scholar]
- 72.Kalantar-Zadeh K, Streja E, Miller JE, Nissenson AR. Intravenous iron versus erythropoiesis-stimulating agents: friends or foes in treating chronic kidney disease anemia. Adv Chronic Kidney Dis. 2009;16:143–151. doi: 10.1053/j.ackd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 73.Barton JC, Barton EH, Bertoli LF, Gothard CH, Sherrer JS. Intravenous iron dextran therapy in patients with iron deficiency and normal renal function who failed to respond to or did not tolerate oral iron supplementation. Am J Med. 2000;109:27–32. doi: 10.1016/s0002-9343(00)00396-x. [DOI] [PubMed] [Google Scholar]
- 74.Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis. 1996;28:529–534. doi: 10.1016/s0272-6386(96)90463-1. [DOI] [PubMed] [Google Scholar]
- 75.Braga M, Gianotti L, Gentilini O, Vignali A, Corizia L, Di Carlo V. Erythropoiesis after therapy with recombinant human erythropoietin: a dose-response study in anemic cancer surgery patients. Vox Sang. 1999;76:38–42. [PubMed] [Google Scholar]
- 76.Kettelhack C, Hönes C, Messinger D, Schlag PM. Randomized multicentre trial of the influence of recombinant human erythropoietin on intraoperative and postoperative transfusion need in anaemic patients undergoing right hemicolectomy for carcinoma. Br J Surg. 1998;85:63–67. doi: 10.1046/j.1365-2168.1998.00564.x. [DOI] [PubMed] [Google Scholar]
- 77.Qvist N, Boesby S, Wolff B, Hansen CP. Recombinant human erythropoietin and hemoglobin concentration at operation and during the postoperative period: reduced need for blood transfusions in patients undergoing colorectal surgery--prospective double-blind placebo-controlled study. World J Surg. 1999;23:30–35. doi: 10.1007/s002689900561. [DOI] [PubMed] [Google Scholar]
- 78.Kosmadakis N, Messaris E, Maris A, Katsaragakis S, Leandros E, Konstadoulakis MM, Androulakis G. Perioperative erythropoietin administration in patients with gastrointestinal tract cancer: prospective randomized double-blind study. Ann Surg. 2003;237:417–421. doi: 10.1097/01.SLA.0000055275.38740.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christodoulakis M, Tsiftsis DD, Hellenic Surgical Oncology Perioperative EPO Study Group. Preoperative epoetin alfa in colorectal surgery: a randomized, controlled study. Ann Surg Oncol. 2005;12:718–725. doi: 10.1245/ASO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 80.Norager CB, Jensen MB, Madsen MR, Qvist N, Laurberg S. Effect of darbepoetin alfa on physical function in patients undergoing surgery for colorectal cancer. A randomized, double-blind, placebo-controlled study. Oncology. 2006;71:212–220. doi: 10.1159/000106071. [DOI] [PubMed] [Google Scholar]
- 81.Glaspy J, Crawford J, Vansteenkiste J, Henry D, Rao S, Bowers P, Berlin JA, Tomita D, Bridges K, Ludwig H. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102:301–315. doi: 10.1038/sj.bjc.6605498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beguin Y. Erythropoietin and platelet production. Haematologica. 1999;84:541–547. [PubMed] [Google Scholar]
- 83.Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. Am J Hematol. 2012;87:308–310. doi: 10.1002/ajh.22262. [DOI] [PubMed] [Google Scholar]
- 84.Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist. 2008;13 Suppl 3:33–36. doi: 10.1634/theoncologist.13-S3-33. [DOI] [PubMed] [Google Scholar]
- 85.National Comprehensive Cancer Network®. NCCN clinical practice guidelines in oncology. Cancer- and chemotherapy-induced anemia. Version 1.2013, August 2012. Available from: http://www.nccn.org Accessed 17 July 2013.
- 86.Mikhael J, Melosky B, Cripps C, Rayson D, Kouroukis CT. Canadian supportive care recommendations for the management of anemia in patients with cancer. Curr Oncol. 2007;14:209–217. doi: 10.3747/co.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bennett CL, Spiegel DM, Macdougall IC, Norris L, Qureshi ZP, Sartor O, Lai SY, Tallman MS, Raisch DW, Smith SW, et al. A review of safety, efficacy, and utilization of erythropoietin, darbepoetin, and peginesatide for patients with cancer or chronic kidney disease: a report from the Southern Network on Adverse Reactions (SONAR) Semin Thromb Hemost. 2012;38:783–796. doi: 10.1055/s-0032-1328884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devon KM, McLeod RS. Pre and peri-operative erythropoietin for reducing allogeneic blood transfusions in colorectal cancer surgery. Cochrane Database Syst Rev. 2009;(1):CD007148. doi: 10.1002/14651858.CD007148.pub2. [DOI] [PubMed] [Google Scholar]
- 89.Burden S, Todd C, Hill J, Lal S. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. 2012;11:CD008879. doi: 10.1002/14651858.CD008879.pub2. [DOI] [PubMed] [Google Scholar]
- 90.Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56:667–678. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]
- 91.Camus Y, Delva E, Cohen S, Lienhart A. The effects of warming intravenous fluids on intraoperative hypothermia and postoperative shivering during prolonged abdominal surgery. Acta Anaesthesiol Scand. 1996;40:779–782. doi: 10.1111/j.1399-6576.1996.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 92.Scott EM, Buckland R. A systematic review of intraoperative warming to prevent postoperative complications. AORN J. 2006;83:1090–104, 1107-13. doi: 10.1016/s0001-2092(06)60120-8. [DOI] [PubMed] [Google Scholar]
- 93.Wong PF, Kumar S, Bohra A, Whetter D, Leaper DJ. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg. 2007;94:421–426. doi: 10.1002/bjs.5631. [DOI] [PubMed] [Google Scholar]
- 94.Pu Y, Cen G, Sun J, Gong J, Zhang Y, Zhang M, Wu X, Zhang J, Qiu Z, Fang F. Warming with an underbody warming system reduces intraoperative hypothermia in patients undergoing laparoscopic gastrointestinal surgery: a randomized controlled study. Int J Nurs Stud. 2014;51:181–189. doi: 10.1016/j.ijnurstu.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 95.Bundgaard-Nielsen M, Secher NH, Kehlet H. ‘Liberal’ vs. ‘restrictive’ perioperative fluid therapy--a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009;53:843–851. doi: 10.1111/j.1399-6576.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 96.Cohn SM, Pearl RG, Acosta SM, Nowlin MU, Hernandez A, Guta C, Michalek JE. A prospective randomized pilot study of near-infrared spectroscopy-directed restricted fluid therapy versus standard fluid therapy in patients undergoing elective colorectal surgery. Am Surg. 2010;76:1384–1392. [PubMed] [Google Scholar]
- 97.Futier E, Constantin JM, Petit A, Chanques G, Kwiatkowski F, Flamein R, Slim K, Sapin V, Jaber S, Bazin JE. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Arch Surg. 2010;145:1193–1200. doi: 10.1001/archsurg.2010.275. [DOI] [PubMed] [Google Scholar]
- 98.Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010;69:488–498. doi: 10.1017/S0029665110001734. [DOI] [PubMed] [Google Scholar]
- 99.Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112:281–289. doi: 10.1093/bja/aet307. [DOI] [PubMed] [Google Scholar]
- 100.Brar MS, Brar SS, Dixon E. Perioperative supplemental oxygen in colorectal patients: a meta-analysis. J Surg Res. 2011;166:227–235. doi: 10.1016/j.jss.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. 2011;86:860–862. doi: 10.1002/ajh.22153. [DOI] [PubMed] [Google Scholar]
- 102.Auerbach M, Strauss W, Auerbach S, Rineer S, Bahrain H. Safety and efficacy of total dose infusion of 1,020 mg of ferumoxytol administered over 15 min. Am J Hematol. 2013;88:944–947. doi: 10.1002/ajh.23534. [DOI] [PubMed] [Google Scholar]


