Abstract
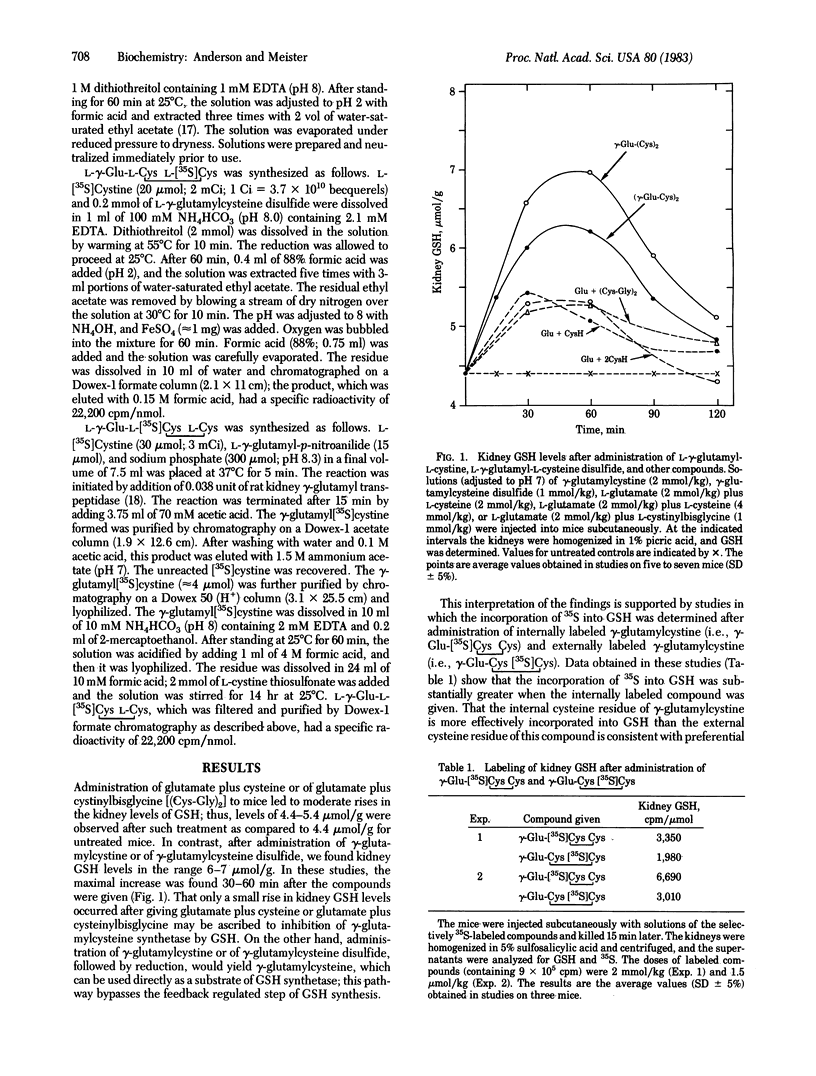
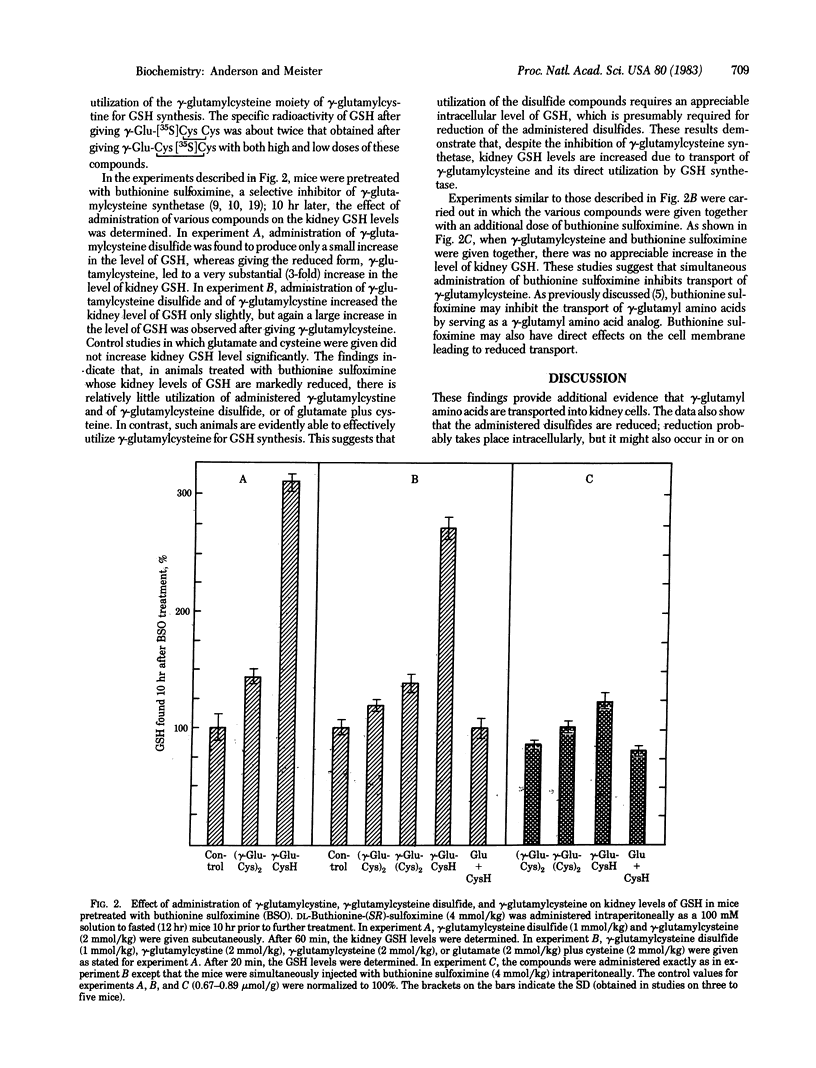
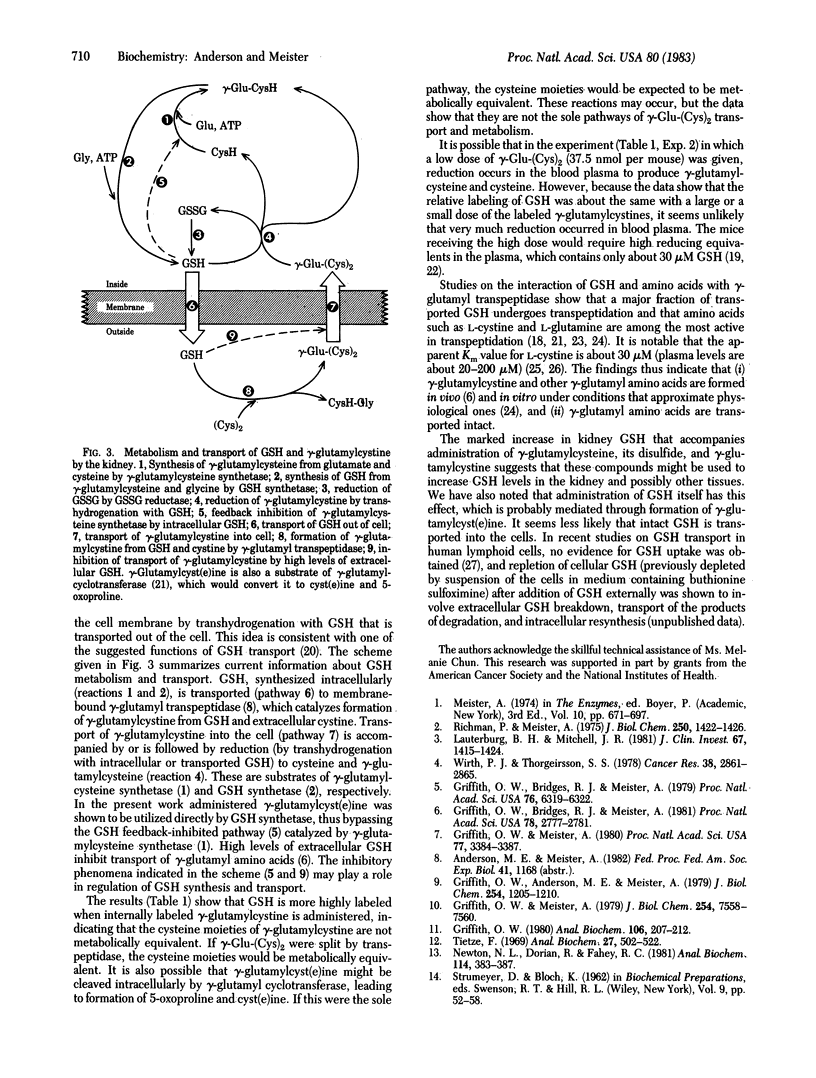
Administration of gamma-glutamylcystine or of gamma-glutamylcysteine disulfide to mice leads to significantly increased levels of glutathione in the kidney as compared to controls given glutamate plus cysteine (or cystinylbisglycine). Studies with gamma-glutamylcystine selectively labeled with 35S in either the internal or external S atom indicate preferential utilization of the gamma-glutamylcysteine moiety of this compound for glutathione synthesis. Mice depleted of glutathione by treatment with buthionine sulfoximine do not significantly use the disulfides gamma-glutamylcystine or gamma-glutamylcysteine disulfide but do use gamma-glutamylcysteine for glutathione synthesis. These findings suggest a pathway in which gamma-glutamylcystine, formed by transpeptidation between glutathione and cystine, is transported and reduced by transhydrogenation with glutathione to cysteine and gamma-glutamylcysteine; the latter is used directly for glutathione synthesis. The findings show transport of gamma-glutamyl amino acids, indicate an alternative pathway of glutathione synthesis, and demonstrate a means of increasing kidney glutathione levels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. D., Meister A. Evidence that transpeptidation is a significant function of gamma-glutamyl transpeptidase. J Biol Chem. 1981 Mar 25;256(6):2988–2992. [PubMed] [Google Scholar]
- Bulter J., Spielberg S. P., Schulman J. D. Reduction of disulfide-containing amines amino acids, and small peptides. Anal Biochem. 1976 Oct;75(2):674–675. doi: 10.1016/0003-2697(76)90129-9. [DOI] [PubMed] [Google Scholar]
- DICKINSON J. C., ROSENBLUM H., HAMILTON P. B. ION EXCHANGE CHROMATOGRAPHY OF THE FREE AMINO ACIDS IN THE PLASMA OF THE NEWBORN INFANT. Pediatrics. 1965 Jul;36:2–13. [PubMed] [Google Scholar]
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B., Eriksson S. A. Synthesis and characterization of the L-cysteine-glutathione mixed disulfide. Acta Chem Scand. 1967;21(5):1304–1312. doi: 10.3891/acta.chem.scand.21-1304. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W., Bridges R. J., Meister A. Formation of gamma-glutamycyst(e)ine in vivo is catalyzed by gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1981 May;78(5):2777–2781. doi: 10.1073/pnas.78.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Bridges R. J., Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6319–6322. doi: 10.1073/pnas.76.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterburg B. H., Mitchell J. R. Regulation of hepatic glutathione turnover in rats in vivo and evidence for kinetic homogeneity of the hepatic glutathione pool. J Clin Invest. 1981 May;67(5):1415–1424. doi: 10.1172/JCI110170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton G. L., Dorian R., Fahey R. C. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Identity of maleate-stimulated glutaminase with gamma-glutamyl transpeptidase in rat kidney. J Biol Chem. 1975 Jun 25;250(12):4619–4627. [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Hydrolysis and transfer reactions catalyzed by gamma-glutamyl transpeptidase; evidence for separate substrate sites and for high affinity of L-cystine. Biochem Biophys Res Commun. 1976 Jul 12;71(1):32–36. doi: 10.1016/0006-291x(76)90245-x. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Utilization of L-cystine by the gamma-glutamyl transpeptidase-gamma-glutamyl cyclotransferase pathway. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1985–1988. doi: 10.1073/pnas.72.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wirth P. J., Thorgeirsson S. S. Glutathione synthesis and degradation in fetal and adult rat liver and Novikoff hepatoma. Cancer Res. 1978 Sep;38(9):2861–2865. [PubMed] [Google Scholar]


