Abstract
The 5-HT2A receptor mediates the effects of serotonergic hallucinogens and may play a role in the pathophysiology of certain psychiatric disorders, including schizophrenia. Given these findings, there is a need for animal models to assess the behavioral effects of 5-HT2A receptor activation. Our previous studies demonstrated that the phenylalkylamine hallucinogen and 5-HT2A/2C agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) produces dose-dependent effects on locomotor activity in C57BL/6J mice, increasing activity at low to moderate doses and reducing activity at high doses. DOI did not increase locomotor activity in 5-HT2A knockout mice, indicating the effect is a consequence of 5-HT2A receptor activation. Here, we tested a series of phenylalkylamine hallucinogens in C57BL/6J mice using the Behavioral Pattern Monitor (BPM) to determine whether these compounds increase locomotor activity by activating the 5-HT2A receptor. Low doses of mescaline, 2,5-dimethoxy-4-ethylamphetamine (DOET), 2,5-dimethoxy-4-propylamphetamine (DOPR), 2,4,5-trimethoxyamphetamine (TMA-2), and the conformationally restricted phenethylamine (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine (TCB-2) increased locomotor activity. By contrast, the non-hallucinogenic phenylalkylamine 2,5-dimethoxy-4-tert-butylamphetamine (DOTB) did not alter locomotor activity at any dose tested (0.1-10 mg/kg i.p.). The selective 5-HT2A antagonist M100907 blocked the locomotor hyperactivity induced by mescaline and TCB-2. Similarly, mescaline and TCB-2 did not increase locomotor activity in 5-HT2A knockout mice. These results confirm that phenylalkylamine hallucinogens increase locomotor activity in mice and demonstrate that this effect is mediated by 5-HT2A receptor activation. Thus, locomotor hyperactivity in mice can be used to assess phenylalkylamines for 5-HT2A agonist activity and hallucinogen-like behavioral effects. These studies provide additional support for the link between 5-HT2A activation and hallucinogenesis.
Keywords: hallucinogen, 5-HT2A receptor, locomotor activity, mescaline, rearing, hyperactivity, investigatory behavior
1. INTRODUCTION
There are 7 classes of serotonin (5-HT) receptors containing 14 identified receptor subtypes (Hannon and Hoyer., 2008). 5-HT receptors are involved in a wide variety of physiological process, both peripherally and centrally. The 5-HT2A receptor, which is coupled to Gq and activates phospholipase C, is widely distributed in the central nervous system, where it regulates neuronal excitability and transmitter release. There is evidence that the 5-HT2A receptor plays a role in neuropsychiatric disorders, including schizophrenia, bipolar disorder, depression, suicide, panic disorder, and drug dependence (Saiz et al., 2008a,b; Yoon et al., 2008; Quednow et al., 2010; Abdolmaleky et al., 2011; Vikki et al., 2011). Additionally, 5-HT2A receptors are believed to play a role in the therapeutic effects of atypical antipsychotics, as well as certain antidepressant drugs (Wilkie et al., 2009; Chen et al., 2009; Quednow et al., 2010; Kishi et al., 2010; Rasmussen et al., 2011).
In addition to its putative involvement in psychiatric illnesses, the 5-HT2A receptor mediates the effects of hallucinogens (reviewed by: Nichols 2004; Halberstadt and Geyer, 2011). Serotonergic hallucinogens act as 5-HT2A agonists, and there is a significant correlation between 5-HT2A receptor affinity and hallucinogen-like behavioral activity (Glennon et al., 1984; Titeler et al., 1988; Sadzot et al., 1989). Furthermore, 5-HT2A antagonists block most of the effects of hallucinogens in rodents and humans (Schreiber et al., 1995; Fiorella et al., 1995; Vollenweider et al., 1998; Carter et al., 2005, 2007). Serotonergic hallucinogens belong to two classes of compounds, indoleamines and phenylalkylamines. Examples of indoleamine hallucinogens include the ergoline lysergic acid diethylamide (LSD) and the tryptamines N,N-dimethyltryptamine (DMT) and psilocybin (4-phosphoryloxy-DMT). The phenylalkylamine class includes phenethylamines such as mescaline and phenylisopropylamines such as 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-methylamphetamine (DOM). Recently, potent analogs of the phenylalkylamines have been developed in which the ethylamine side-chain or one or more of the alkoxy ring substituents is conformationally constrained by incorporation into a ring structure (e.g., (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine (TCB-2); McLean et al., 2006b). Although the indoleamines are nonselective 5-HT receptor agonists, the phenylalkylamines are relatively selective for 5-HT2A and 5-HT2C receptors.
A variety of behavioral paradigms have been used to characterize 5-HT2A agonist/hallucinogen effects in rats, including drug discrimination, head and body shakes, locomotor and investigatory behavior, and prepulse inhibition of startle (Halberstadt and Nichols 2010; Halberstadt and Geyer, 2011). Relatively little is known about the behavioral effects of hallucinogens in mice, although relevant reports have appeared recently (Smith et al., 2003; Winter et al., 2005; Gonzalez-Maeso et al., 2007; Halberstadt et al., 2009, 2011a; Fantegrossi et al., 2010). The Behavioral Pattern Monitor (BPM), which provides quantitative and qualitative assessments of unconditioned locomotor and investigatory activity, has been used extensively to study the effects of hallucinogens in rats (Adams and Geyer, 1985; Wing et al., 1990; Krebs-Thompson et al., 1998) and more recently in mice. BPM studies in C57BL/6J mice demonstrated that low to moderate doses of DOI and DOM increase locomotor activity, whereas higher doses reduce activity (Halberstadt et al, 2009; Halberstadt and Geyer, 2011). Importantly, DOI does not increase locomotor activity in 5-HT2A knockout mice, indicating that the effect is a consequence of 5-HT2A receptor activation (Halberstadt et al, 2009). Although other groups have also found that DOI and DOM increase locomotor activity in mice (Yamamoto and Ueki et al., 1975; Darmani et al., 1996; Brookshire and Jones, 2009), Fox and colleagues recently reported that DOI and the conformationally restricted 5-HT2A agonist TCB-2 have no effect on locomotor activity in C57BL/6J mice (Fox et al., 2010). Given these discrepant findings, we tested a larger series of phenylalkylamine hallucinogens and related substances (Figure 1) in the BPM to determine whether those compounds increase locomotor activity by activating the 5-HT2A receptor. In addition, to determine whether the 5-HT2A-mediated increase in locomotor activity is specific to phenylalkylamines with hallucinogenic activity, we tested the nonhallucinogenic DOM homologue 2,5-dimethoxy-4-tert-butylamphetamine (DOTB; Shulgin and Dyer, 1975), which acts as a partial agonist at the 5-HT2A receptor (Glennon et al., 1992).
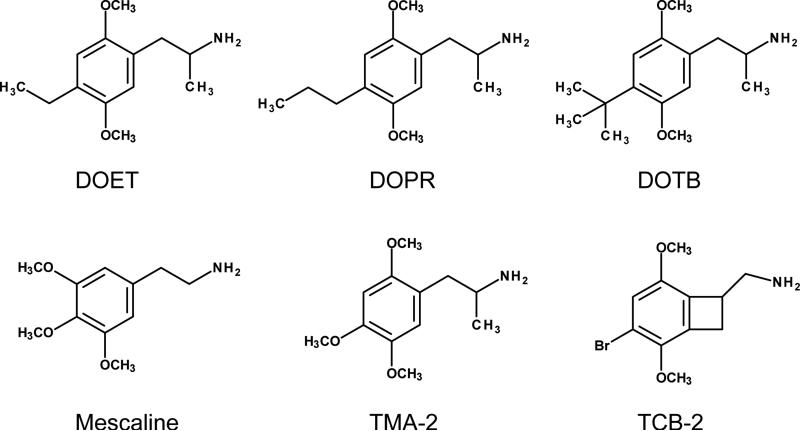
Figure 1.
Chemical structures of the phenylalkylamines.
2. MATERIALS AND METHODS
2.1. Subjects
Mice were housed at the University of California San Diego (UCSD), in an AAALAC-approved animal facility that meets Federal and State requirements for care and treatment of laboratory animals. Male C57BL/6J mice were purchased from Jackson Labs (Bar Harbor, ME) and allowed to acclimate to the vivarium for at least 1 week after arrival. Male and female 5-HT2A wild-type (WT) and knockout (KO) mice, backcrossed (N10) onto the inbred C57BL/6 line (see: Halberstadt et al., 2009), were bred in-house using heterozygous breeding pairs to remove the possibility of genetic drift between WT and KO mice and to ensure that all mice received equivalent maternal care. The 5-HT2A WT and KO mice were weaned at 21-24 days of age, at which time part of the tail (~1.5 cm) was removed for genotyping by polymerase chain reaction (PCR). Animals were housed in a climate-controlled room with a reversed light-dark cycle (lights on at 2000 hours, off at 0800 hours), separated by sex (n=4/cage). Food and water were available ad libitum, except during behavioral testing. Behavioral experiments were conducted between 1000 and 1800 hours. All experiments were conducted in accord with the “Principles of laboratory Animal Care” NIH guidelines and were approved by the UCSD Institutional Animal Care and Use Committee (IACUC).
2.2. Apparatus
As described previously (Risbrough et al., 2006; Halberstadt et al., 2009), spontaneous exploratory and investigatory behavior were recorded in 9 mouse BPM chambers (San Diego Instruments, San Diego, CA). Briefly, each mouse BPM chamber is a clear Plexiglas box with an opaque 30 × 60 cm floor; each chamber is enclosed in a ventilated isolation box. The BPM chambers contain 11 1.4-cm holes (3 in the floor, 3 in each long wall, and 2 in each short wall); each hole is equipped with an infrared photobeam to detect holepoking behavior. The x,y position of the mouse in the chamber is recorded by a grid of 12 × 24 infrared photobeams located 1 cm above the floor. A second row of 16 infrared photobeams (parallel with the long axis of the chamber, 2.5 cm above the floor) detects rearing behavior. Photobeam status is sampled every 55 ms, and data recorded to a Windows PC for off-line analysis. The measures assessed in these experiments are distance traveled (a measure of horizontal locomotor activity), total holepokes, and total rearings (measures of investigatory behavior).
Mice were tested in the dark during the dark phase of their light/dark cycle. The animals were brought into the testing room at least 1 h before testing. Injections were made under red lights in the testing room. During BPM sessions, a white noise generator in the testing room was used to produce background noise at 65 dB(A). The chambers were cleaned with water between testing sessions.
2.3. Experimental Design
Animals were placed in the BPM chambers 10 min after treatment with mescaline or TMA-2, 15 min after treatment with DOI, DOET, DOPR, DOTB, or TCB-2, and/or 30 min after treatment with M100907. The mice were tested in the BPM for 60 min. Details of the individual BPM experiments are listed in Table 1.
Table 1.
Details of individual Behavioral Pattern Monitor (BPM) experiments.
| Experiment | Pretreatment | Treatment | Animals | Design |
|---|---|---|---|---|
| 1 | — | Vehicle or mescaline (6.25, 12.5, 25, 50, 100 mg/kg) | n = 9–10 (59 total) | Between-subjects |
| 2 | — | Vehicle or DOET (0.3, 1, 3, 10 mg/kg) | n = 11–12 (56 total) | Between-subjects |
| 3 | — | Vehicle of DOPR (0.3, 1, 3, 10 mg/kg) | n = 11–12 (59 total) | Between-subjects |
| 4 | — | Vehicle or TMA-2 (2.5, 5, 10, 15 mg/kg) | n = 11–12 (59 total) | Between-subjects |
| 5 | — | Vehicle or TCB-2 (0.1, 0.3, 1, 3, 10 mg/kg) | n = 9–10 (59 total) | Between-subjects |
| 6 | — | Vehicle or DOTB (0.1, 0.3, 1, 3, 10 mg/kg) | n = 10 (60 total) | Between-subjects |
| 7 | — | Vehicle or mescaline (25 mg/kg) | WT and 5-HT2A KO mice, n = 11–19 (9 WT and 12 KO male mice, and 2 WT and 7 KO female mice) | 2-way semi-randomized crossover with 1 week between tests |
| 8 | — | Vehicle or TCB-2 (3 mg/kg) | WT and 5-HT2A KO mice, n = 9–11 (9 WT and 11 KO male mice) | 2-way semi-randomized crossover with 1 week between tests |
| 9 | Vehicle or M100907 (0.03, 0.1 mg/kg) | Vehicle or mescaline (25 mg/kg) | n = 9–10 (59 total) | Between-subjects |
| 10 | Vehicle or M100907 (0.03, 0.1 mg/kg) | Vehicle or TCB-2 (3 mg/kg) | n = 8–10 (56 total) | Between-subjects |
2.4. Data Analysis
Distance traveled was examined in 10- and 30-min time blocks, and rearings and holepokes were analyzed in 30-min time blocks. In Experiments 1–6, 9, and 10, data were analyzed by using two- or three-way analyses of variance (ANOVAs) with treatment or pretreatment and treatment as between-subject factors and time as a repeated measure. Specific post hoc comparisons between selected groups were done using Dunnett's test or Tukey's studentized range method. Significance was demonstrated by surpassing an α-level of 0.05. In Experiments 7 and 8, genotype was the between-subject variable and drug treatment and time were within-subject variables. Sex was an additional between-subject variable in Experiment 7. One-way ANOVAs at each time-point were used for post-hoc analysis of Experiments 7 and 8.
2.5. Drugs
Drugs used were mescaline hydrochloride, 2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI; Sigma Chemical Co., St. Louis, MO); 2,5-dimethoxy-4-ethylamphetamine hydrochloride (DOET; donated by the National Institute on Drug Abuse (NIDA) Drug Supply Program, Bethesda, MD); 2,5-dimethoxy-4-propylamphetamine hydrochloride (DOPR), 2,5-dimethoxy-4-tert-butylamphetamine hydrochloride (DOTB, donated by Dr. A. T. Shulgin, Lafayette, CA); 2,4,5-trimethoxyamphetamine hydrochloride (TMA-2; donated by Dr. S. Knapp); (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB-2; Tocris Bioscience, Ellisville, MO); and (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol (M100907; donated by Hoechst Marion Roussel Inc., Kansas City, MO). Mescaline, DOI, DOET, DOPR, DOTB, TMA-2, and TCB-2 were dissolved in isotonic saline and administered intraperitoneally (i.p.) at a volume of 5 mL/kg body weight. M100907 was dissolved in water containing 5% Tween 80 and administered subcutaneously (s.c.) at a volume of 5 mL/kg body weight.
3. RESULTS
3.1. Effect of phenylalkylamine hallucinogens
3.1.1. Locomotor activity
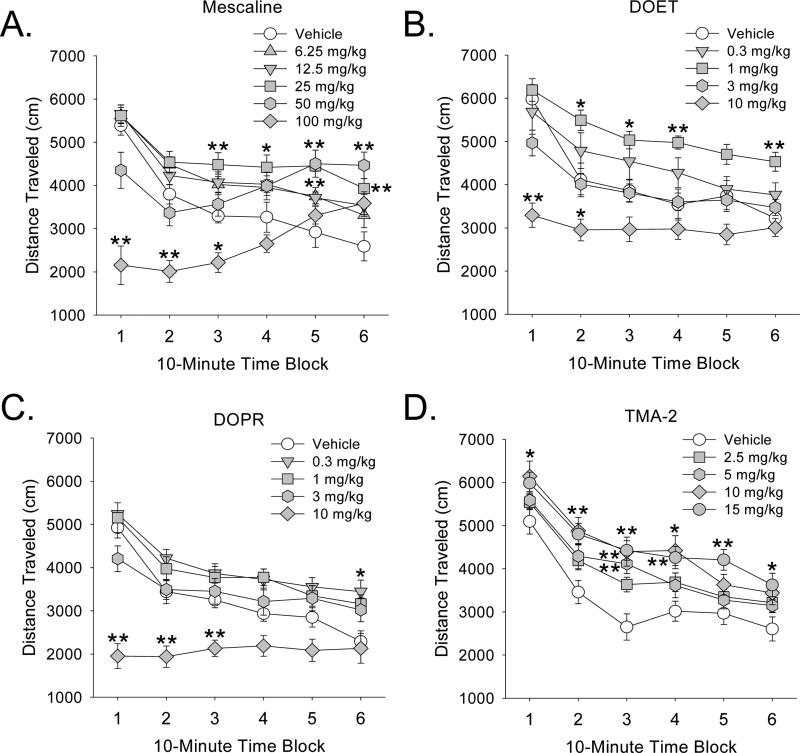
The effects of mescaline, DOET, DOPR, and TMA-2 on distance traveled, a measure of locomotor activity, are illustrated in Figure 2. We previously reported that the effects of DOI and DOM on locomotor activity follow an inverted U-shaped dose-response function, with low and moderate doses producing a delayed increase in locomotor activity, and high doses (≥10 mg/kg) reducing activity at the beginning of the test session (Halberstadt et al., 2009; Halberstadt and Geyer, 2011). The effects of mescaline (Drug × Block: F(25,265)=10.09, p<0.0001), DOET (Drug × Block: F(20,255)=4.60, p<0.0001), and DOPR (Drug × Block: F(20,470)=7.30, p<0.0001) on locomotor activity show a similar dose- and time- dependence. For example, 25 and 50 mg/kg of mescaline significantly increased locomotor activity during the last 40 minutes of the 1-h test session (p<0.01, 0.05, Dunnett’s test), and 100 mg/kg mescaline reduced locomotor activity during the first 30 minutes of testing (p<0.01, 0.05, Dunnett’s test; Fig. 2a). The dose-response of DOET was very similar to that of DOI, with 1 mg/kg DOET significantly increasing activity during the last 50 min of testing, and 10 mg/kg DOET significantly reducing activity during the first 20 min (p<0.01, 0.05, Dunnett’s test; Fig. 2b). DOPR was slightly more potent than DOET, and 0.3 mg/kg DOPR induced hyperactivity (Fig. 2c) whereas the same dose of DOET was inactive. At the dose range tested (2.5–15 mg/kg), TMA-2 increased locomotor activity (Drug effect: F(4,54)=5.21, p<0.002), with the 5, 10, and 15 mg/kg doses of TMA-2 inducing hyperactivity throughout the 1-h session (p<0.01, 0.05, Tukey’s test; Fig. 2d).
Figure 2.
Effects of phenylalkylamine hallucinogens on distance traveled (in cm), a measure of locomotor activity. (A) Mescaline, (B) DOET, (C) DOPR, (D) TMA-2. Mice used were male C57BL/6J. Data are presented as group means±SEM for successive 10-min intervals. *p<0.05, **p<0.01, significant difference from vehicle control group.
3.1.2. Investigatory behavior
As shown in Table 2, mescaline (F(5,53)=11.52, p<0.0001), DOET (F(4,51)=7.20, p=0.0001), and DOPR (F(4,54)=19.85, p<0.0001) produced dose-dependent reductions in holepoking behavior. Rearings were also reduced dose-dependently by mescaline (F(5,53)=43.93, p<0.0001), DOET (F(4,51)=22.71, p<0.0001), and DOPR (F(4,54)=36.76, p<0.0001). There was a trend toward an interaction of TMA-2 treatment and time block for holepokes (F(4,54)=2.36, p<0.07) and rearings (F(4,54)=2.33, p<0.07), but post-hoc analysis failed to confirm this effect for any specific 30-min time block (see Table 2).
Table 2.
Effect of phenylalkylamines on investigatory behavior
| Rearingsa | Holepokesa | ||||
|---|---|---|---|---|---|
| Drug | Dose | 0-30 Min | 30-60 Min | 0-30 Min | 30-60 Min |
| Mescaline | Vehicle | 184.8±11.0 | 165.9±18.1 | 82.9±5.9 | 94.0±10.7 |
| 6.25 | 181.1±14.6 | 185.2±19.9 | 104.7±10.1 | 129.1±20.7 | |
| 12.5 | 178.6±7.7 | 208.2±16.3 | 95.4±12.1 | 110.9±16.5 | |
| 25 | 142.2±10.5b | 182.6±8.7 | 90.9±6.8 | 115.6±14.6 | |
| 50 | 49.2±11.7c | 111.9±9.8b | 46.0±9.5b | 83.3±9.2 | |
| 100 | 3.5±2.0c | 31.8±7.1c | 12.7±5.8c | 27.6±6.9c | |
| DOET | Vehicle | 241.0±28.9 | 244.9±18.5 | 95.3±9.3 | 89.8±11.7 |
| 0.3 | 193.0±23.1 | 211.4±21.4 | 112.5±23.5 | 130.4±25.2 | |
| 1 | 165.4±14.3b | 194.3±12.1 | 101.9±9.8 | 140.2±12.9 | |
| 3 | 89.1±20.3c | 145.5±20.3c | 67.4±10.4 | 75.7±15.3 | |
| 10 | 15.5±3.4c | 37.1±8.4c | 23.3±5.0c | 44.9±12.7 | |
| DOPR | Vehicle | 187.9±12.7 | 171.7±19.2 | 119.2±13.4 | 127.3±14.8 |
| 0.3 | 165.9±14.2 | 171.4±18.3 | 135.0±11.1 | 156.1±10.8 | |
| 1 | 110.8±6.6c | 107.5±9.9c | 100.4±13.9 | 102.7±16.0 | |
| 3 | 54.8±9.9c | 88.4±14.0c | 63.8±8.0c | 60.9±9.1c | |
| 10 | 7.1±2.7c | 21.9±4.4c | 19.4±5.0c | 28.1±7.4c | |
| TMA-2 | Vehicle | 138.5±17.1 | 136.8±12.2 | 95.0±7.8 | 115.5±10.8 |
| 2.5 | 178.4±16.5 | 185.5±19.4 | 124.3±13.8 | 150.0±15.2 | |
| 5 | 160.9±12.4 | 157.9±13.9 | 109.4±21.2 | 140.9±18.1 | |
| 10 | 159.4±18.8 | 190.3±15.8 | 106.8±21.2 | 107.0±21.5 | |
| 15 | 123.8±12.6 | 159.9±22.47 | 111.2±12.7 | 152.0±18.7 | |
| TCB-2 | Vehicle | 116.3±17.0 | 134.0±29.2 | 97.1±6.8 | 118.0±9.5 |
| 0.1 | 128.1±20.0 | 125.3±26.8 | 96.3±11.3 | 119.3±18.1 | |
| 0.3 | 158.9±13.9 | 168.1±18.4 | 120.7±15.6 | 145.3±21.9 | |
| 1 | 148.3±16.0 | 185.5±18.5 | 84.8±7.8 | 106.5±11.8 | |
| 3 | 86.3±13.6 | 131.4±19.7 | 75.0±9.9 | 101.4±15.1 | |
| 10 | 15.3±5.5b | 55.8±10.5 | 22.0±3.0c | 44.0±6.2b | |
Data are reported as the mean number of events ± S.E.M.
p<0.05 vs. vehicle.
p<0.01 vs. vehicle.
3.2. Effect of TCB-2
3.2.1. Locomotor activity
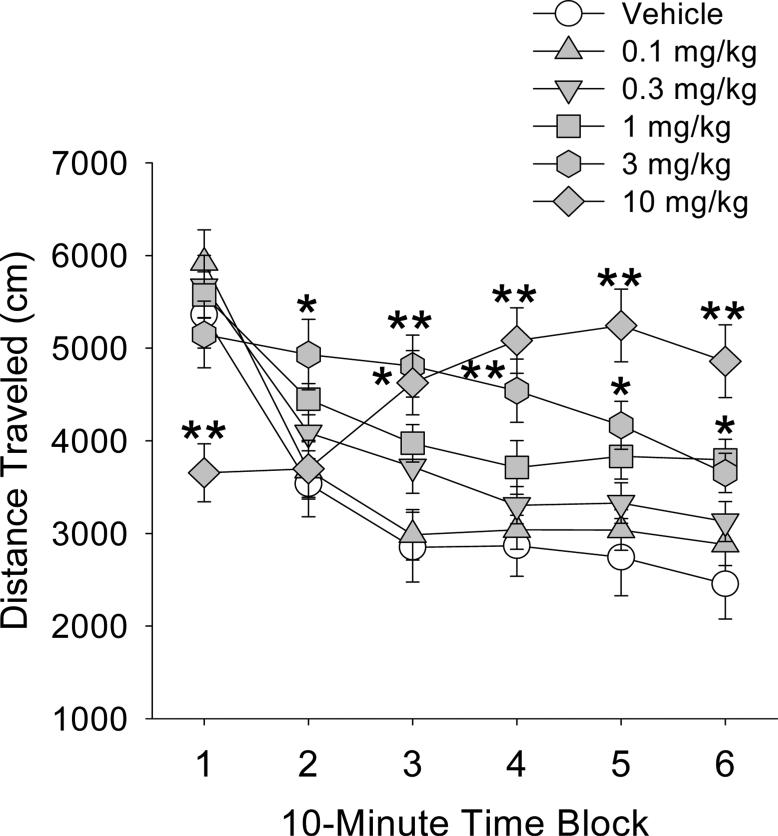
There was a main effect of the benzocyclobutene derivative TCB-2 on locomotor activity (F(5,53)=3.43, p<0.01), and an interaction between treatment and time (F(25,265)=21.67, p<0.0001). Like mescaline, DOET, and DOPR, low doses of TCB-2 produced a delayed increase in distance traveled. Post-hoc analysis showed that 1 mg/kg TCB-2 significantly increased distance traveled during block 6 (p<0.01, Tukey’s test; Fig. 3), and 3 mg/kg increased distance traveled during blocks 2–5 (p<0.01, 0.05, Tukey’s test). Conversely, the highest dose tested, 10 mg/kg, produced biphasic effects on locomotor activity, reducing activity during block 1 (p<0.01, Tukey’s test) and then increasing activity during blocks 3–6 (p<0.01, Tukey’s test).
Figure 3.
Effect of TCB-2 on locomotor activity. Mice used were male C57BL/6J. Data are presented as group means±SEM for successive 10-min intervals. *p<0.05, **p<0.01, significant difference from vehicle control group.
3.2.2. Investigatory behavior
There was a significant main effect of drug on holepoking behavior (F(5,53)=8.32, p<0.0001). Specific comparisons demonstrated that 10 mg/kg TCB-2 significantly reduced holepoking during the first and second 30-min time blocks (Table 2; p<0.01, 0.05, Tukey’s test). Rearings were also reduced by TCB-2 (F(5,53)=7.58, p<0.0001); this effect occurred primarily during the first 30-min block (see Table 2), resulting in an interaction between treatment and block (F(5,53)=3.44, p<0.01).
3.3. Effect of DOTB
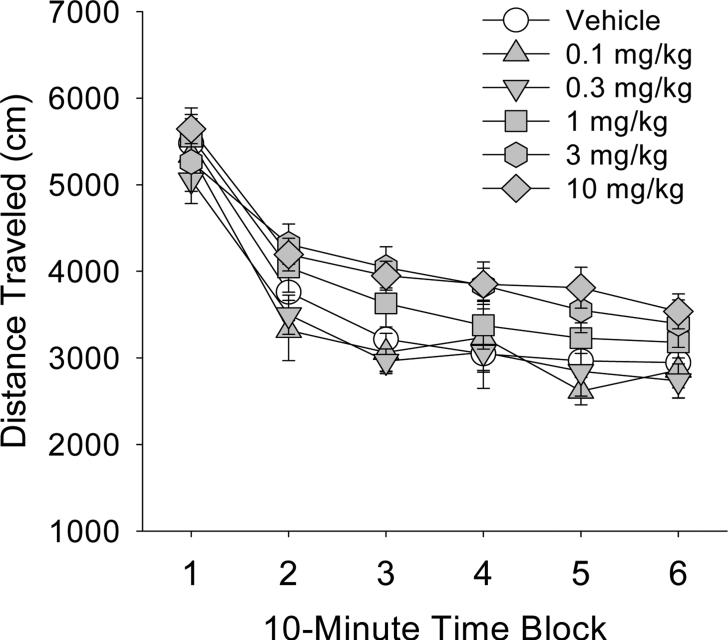
Administration of 0.1-10 mg/kg DOTB did not significantly alter locomotor activity (Figure 4). Although administration of the two highest doses of DOTB appeared to produce slight increases in locomotor activity, even the effect of the 10 mg/kg dose failed to attain significance (F(1,18)=3.04, p=0.098). Additionally, as shown in Table 3, DOTB had no effect on rearings or holepokes.
Figure 4.
Effect of DOTB on locomotor activity. Mice used were male C57BL/6J. Data are presented as group means±SEM for successive 10-min intervals.
Table 3.
Effect of DOTB on investigatory behavior
| Rearingsa | Holepokesa | |||
|---|---|---|---|---|
| 0-30 Min | 30-60 Min | 0-30 Min | 30-60 Min | |
| Vehicle | 195.8±31.9 | 189.4±34.6 | 115.0±10.6 | 136.3±12.0 |
| 0.1 | 143.1±20.9 | 156.8±34.8 | 130.2±12.7 | 142.4±20.0 |
| 0.3 | 175.6±20.9 | 184.6±24.9 | 121.5±7.1 | 129.4±16.5 |
| 1 | 175.4±17.5 | 205.4±25.4 | 128.7±16.9 | 151.3±30.4 |
| 3 | 179.1±9.9 | 217.5±20.7 | 143.2±10.1 | 141.0±10.6 |
| 10 | 139.1±9.9 | 143.3±12.3 | 127.5±18.5 | 139.4±27.2 |
Data are reported as the mean number of events ± S.E.M.
3.4. Effect of 5-HT2A receptor gene deletion on the response to mescaline
3.4.1. Locomotor activity
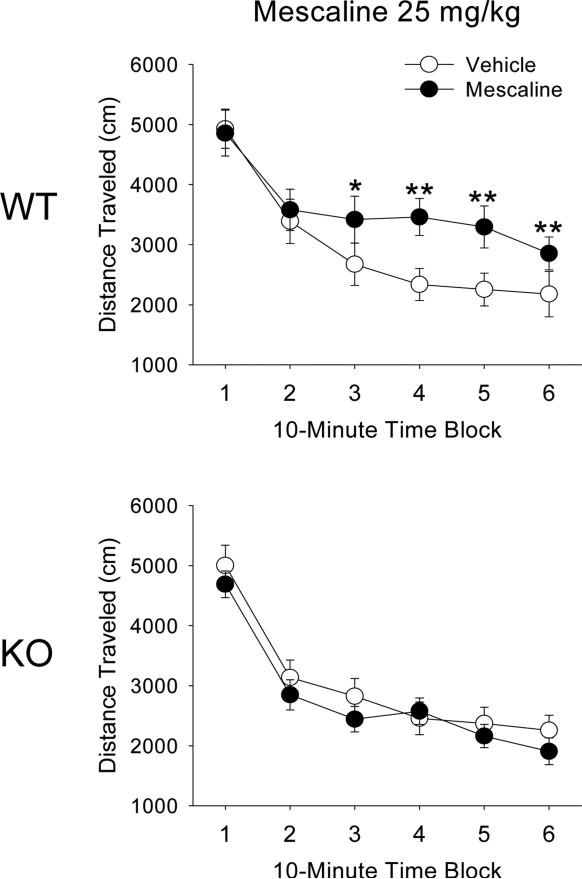
We previously reported that 1.0 mg/kg DOI induces locomotor hyperactivity in WT mice but not in 5-HT2A receptor KO mice, suggesting that the response is mediated by 5-HT2A receptors (Halberstadt et al., 2009). To determine whether the hyperactivity induced by mescaline is mediated by the 5-HT2A receptor, we compared the effect of 25 mg/kg mescaline in WT and 5-HT2A KO mice. As we previously observed (Halberstadt et al. 2009), there was a main effect of sex on distance traveled in the WT and 5-HT2A KO mice (F(1,26)=9.28, p<0.006). There were, however, no interactions between sex and gene, sex and drug, or sex, gene, and drug, so data were collapsed across sex. The effects of mescaline on locomotor activity in 5-HT2A WT and KO mice are illustrated in Figure 5. Treatment with 25 mg/kg mescaline had no effect on locomotor activity in 5-HT2A KO mice (Gene × Drug: F(1,28)=7.58, p<0.02). By contrast, 25 mg/kg mescaline increased distance traveled in WT mice (Drug × Block: F(5,140)=4.95, p=0.0003). Although there was a main effect of gene on distance traveled (F(1,28)=7.55, p<0.02) and an interaction of gene and time block (F(5,140)=11.56, p<0.0001), post hoc analysis revealed that there was no difference in the baseline level of locomotor activity in WT and 5-HT2A KO mice.
Figure 5.
Effect of 5-HT2A gene deletion on the locomotor response to mescaline. Effect of vehicle or 25 mg/kg mescaline on distance traveled in male and female WT mice (top panel) and male and female 5-HT2A KO mice (bottom panel). Data are presented as group means±SEM for successive 10-min intervals. *p<0.05, **p<0.01, significant difference from the respective vehicle control group.
3.4.2. Investigatory behavior
As expected, 25 mg/kg mescaline had no effect on rearings or holepokes in either WT or 5-HT2A KO mice (data not shown). There was no baseline difference between WT and 5-HT2A KO for either holepoking or rearing.
3.5. Effect of 5-HT 2A receptor gene deletion on the response to TCB-2
3.5.1. Locomotor activity
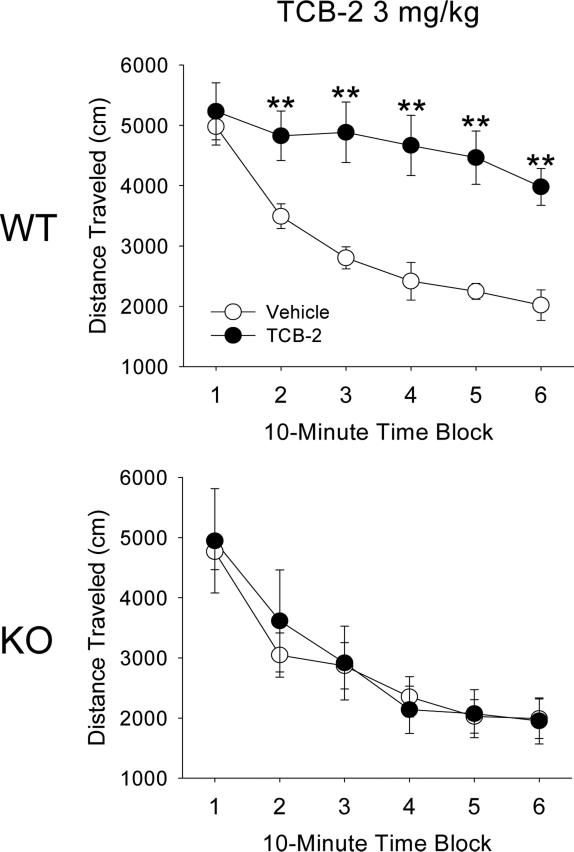
Treatment with 3 mg/kg TCB-2 had no effect on locomotor activity in 5-HT2A KO mice, resulting in an interaction of gene and drug (F(1,18)=6.25, p<0.03) and of gene, drug, and block (F(5,90)=3.73, p<0.005; Fig. 6). Conversely, in WT mice, 3 mg/kg TCB-2 increased distance traveled (F(1,18)=7.89, p<0.02). There were no baseline differences in the locomotor activity of WT and 5-HT2A KO mice.
Figure 6.
Effect of 5-HT2A gene deletion on TCB-2-induced increases in locomotor activity. Effect of vehicle or 3 mg/kg TCB-2 on distance traveled in male WT mice (top panel) and male 5-HT2A KO mice (bottom panel). Data are presented as group means±SEM for successive 10-min intervals. *p<0.05, **p<0.01, significant difference from the respective vehicle control group.
3.5.2. Investigatory behavior
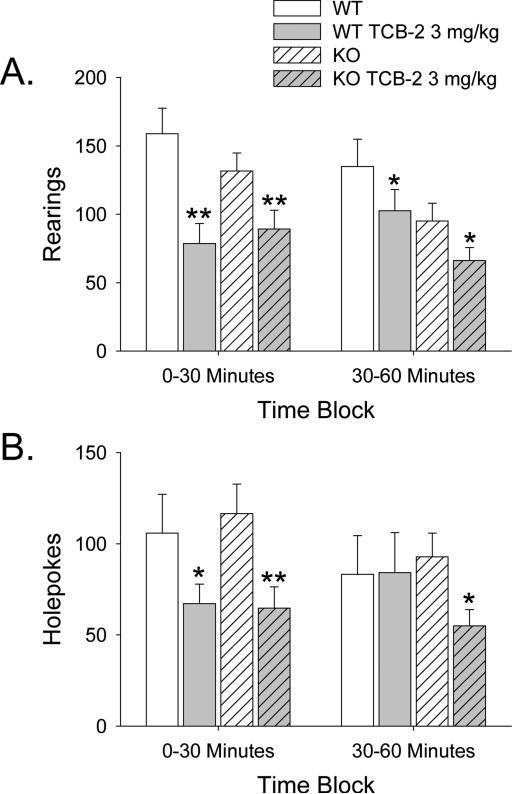
Administration of 3 mg/kg TCB-2 reduced rearing (F(1,18)=32.74, p<0.0001) and holepoking (F(1,18)=11.61, p<0.004), but there was no difference in the response to 3 mg/kg TCB-2 in WT and 5-HT2A KO mice with regard to investigatory behavior (Fig. 7a,b).
Figure 7.
Effect of 5-HT2A gene deletion on the changes in investigatory activity induced by TCB-2. TCB-2 was tested at 3 mg/kg in male 5-HT2A WT and KO mice. Data are presented as group means±SEM over 30-min blocks. *p<0.05, **p<0.01, significant difference from the respective vehicle control group.
3.6. Blockade of the effect of mescaline on locomotor activity with a selective 5-HT2A antagonist
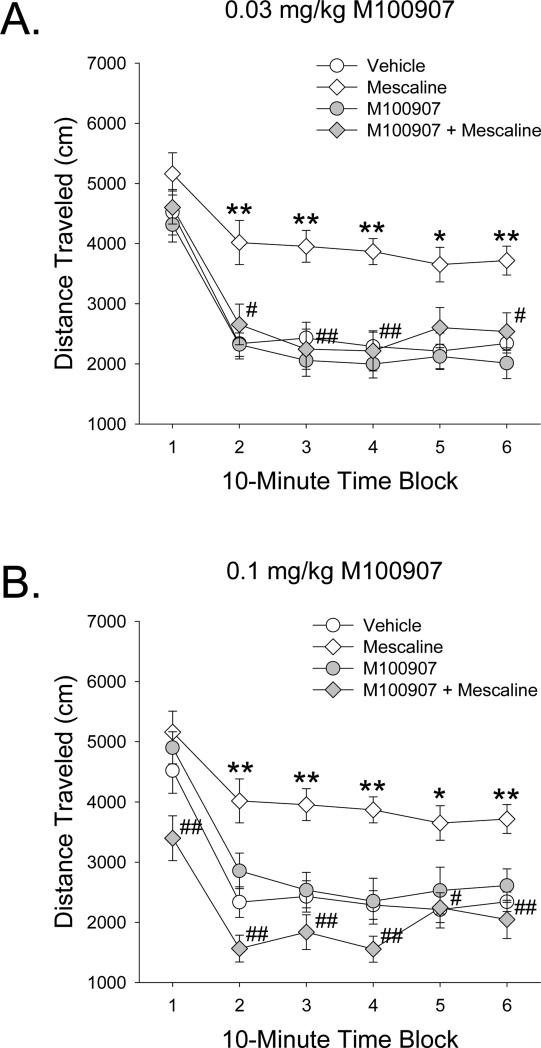
To confirm that the hyperactivity induced by mescaline is mediated by the 5-HT2A receptor, we examined whether the effect of 25 mg/kg mescaline is attenuated by pretreatment with the selective 5-HT2A antagonist M100907. As shown in Figure 8, 25 mg/kg mescaline increased locomotor activity during the last 50 min of the test session (Drug × Block: F(5,265)=3.70, p<0.003) (p<0.05, 0.01, Tukey's test), and this effect was completely blocked by 0.03 and 0.1 mg/kg M100907 (M100907 × mescaline: F(2,53)=10.03, p=0.0002) (p<0.01, Tukey's test). Although there was a main effect of pretreatment with M100907 (F(2,53)=6.71, p<0.003), this was not confirmed by post-hoc analysis.
Figure 8.
Effect of pretreatment with the 5-HT2A antagonist M100907 on the locomotor response to 25 mg/kg mescaline. Mice were pretreated with 0.03 mg/kg M100907 (A) or 0.1 mg/kg M100907 (B). Mice used were male C57BL/6J. Data are presented as group means±SEM for successive 10-min intervals. *p<0.05, **p<0.01, significant difference from vehicle control group. #p<0.05, ##p<0.001, significant difference from mescaline alone.
3.7. Blockade of the effect of TCB-2 on locomotor activity with a selective 5-HT2A antagonist
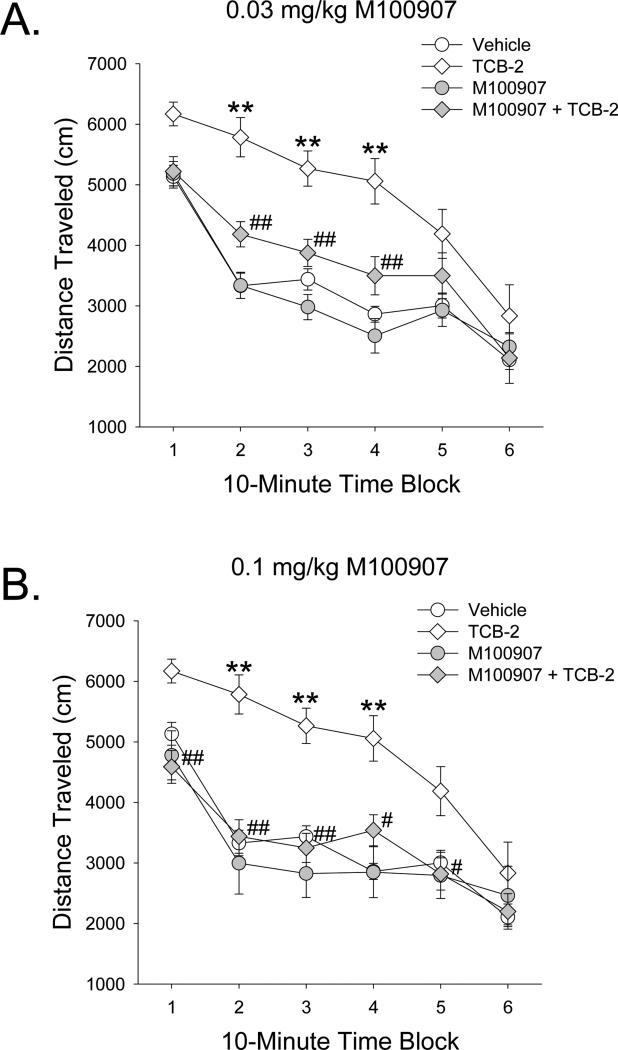
As we found with mescaline, pretreatment with M100907 attenuated the locomotor hyperactivity induced by 3 mg/kg TCB-2 (M100907 × TCB-2: F(2,50)=4.71, p<0.02). The increase in locomotor activity induced by TCB-2 (Drug effect: F(1,50)=16.25, p=0.0002; Drug × Block: F(5,250)=8.54, p<0.0001) was partially attenuated by 0.03 mg/kg M100907 and completely blocked by 0.1 mg/kg M100907 (p<0.05, 0.01, Tukey's test; Fig. 9). Although there was a main effect of pretreatment with M100907 (F(1,34)=10.11, p<0.004), post-hoc analysis did not confirm this effect.
Figure 9.
Effect of pretreatment with the 5-HT2A antagonist M100907 on the locomotor response to 3 mg/kg TCB-2. Mice were pretreated with 0.03 mg/kg M100907 (A) or 0.1 mg/kg M100907 (B). Mice used were male C57BL/6J. Data are presented as group means±SEM for successive 10-min intervals. *p<0.05, **p<0.01, significant difference from vehicle control group. #p<0.05, ##p<0.001, significant difference from TCB-2 alone.
4. DISCUSSION
There is considerable evidence that serotonergic hallucinogens produce their characteristic effects by activating the 5-HT2A receptor (Nichols 2004; Halberstadt and Geyer 2011). We have previously reported that the phenylalkylamine hallucinogen DOI produces an increase in locomotor activity in C57BL/6J mice that is ameliorated by 5-HT2A receptor gene deletion, indicating 5-HT2A receptor mediation (Halberstadt et al., 2009). In the present investigation, we examined whether a larger series of phenylalkylamine hallucinogens increase locomotor activity in C57BL/6J mice, and used a combination of genetic and pharmacological methods to determine whether the hyperactivity is a consequence of 5-HT2A receptor activation. These studies demonstrate that, like DOI, the phenethylamine hallucinogen mescaline and the phenylisopropylamine hallucinogens DOET, DOPR, and TMA-2 produce locomotor hyperactivity in mice. Likewise, administration of the conformationally restricted benzocyclobutene derivative TCB-2, previously shown to be a potent 5-HT2A agonist (McLean et al., 2006b), also provoked hyperactivity. By contrast, DOTB, a homologue of DOET and DOPR that exhibits very low efficacy at the 5-HT2A receptor (Glennon et al., 1992) and is inactive as a hallucinogen (Shulgin and Dyer, 1975), failed to alter any of the behavioral measures tested. To test for involvement of the 5-HT2A receptor in the behavioral response to mescaline and TCB-2, we compared the effects of these substances in WT and 5-HT2A receptor KO mice on a C57 background. The increase in locomotor activity normally induced by mescaline and TCB-2 was absent in 5-HT2A receptor KO mice, supporting the hypothesis that 5-HT2A receptors are responsible for mediating the locomotor-stimulating effects of mescaline and TCB-2. We used genetically modified mice because these animals are free from the problems of efficacy and selectivity that are found with many 5-HT2A receptor antagonists. The use of genetically engineered mice, however, is confounded by the possibility that developmental, compensatory, or epigenetic changes can occur in the animals. To verify that the findings in 5-HT2A KO mice are due specifically to the absence of the receptor, we also tested whether a 5-HT2A antagonist can block the hyperactivity induced by phenylalkylamines. This experiment demonstrated that the selective 5-HT2A antagonist M100907 completely blocks the increase in locomotor activity induced by mescaline and TCB-2. Taken together, these findings confirm that the phenylalkylamine class of hallucinogens produce hyperactivity in mice, and support the hypothesis that this effect is mediated by 5-HT2A receptor activation.
In addition to altering locomotor activity, DOI also reduces investigatory behavior (Halberstadt et al., 2009). The current experiments show that mescaline, DOET, DOPR, and TCB-2 produce DOI-like effects on rearing and holepoking. Although TMA-2 had no effect on investigatory behavior, it is possible that its inactivity may be a consequence of the limited dose range tested. Our earlier experiments demonstrated that the ability of DOI to reduce rearing behavior is attenuated in 5-HT2A KO mice (Halberstadt et al., 2009). Interestingly, there was no difference in TCB-2 effects on rearing in WT and 5-HT2A KO mice, indicating that TCB-2 influences this behavior through a non-5-HT2A receptor-dependent mechanism.
Similar to our findings, other groups have reported that DOI increases locomotor activity in mice (Darmani et al., 1996; Brookshire and Jones, 2009). There is also evidence that low doses of DOM can increase locomotor activity and reduce rearing behavior in ddN mice (Yamamoto and Ueki, 1975). Recently, it was shown that TCB-2 reduces rearings in C57BL/6J mice (Fox et al., 2010). In contrast to the present findings, those workers reported that DOI and TCB-2 have no effect on locomotor activity (both drugs were tested at 1.0 and 2.5 mg/kg). It is important to note, however, that Fox and colleagues assessed locomotor activity in an open field for 30 min immediately after administration of DOI and TCB-2. In our BPM studies, the mice were placed in the chambers 15 min after treatment with DOI or TCB-2, and there was a 10–30 min delay between the beginning of the test session and the onset of hyperactivity (i.e., the animals became hyperactive 25–45 min after dug administration). Therefore, these discrepant findings are most likely a consequence of differences in study design, and the duration of the open field studies with DOI and TCB-2 may not have been long enough to detect hyperactivity induced by those agents. In contrast to our BPM studies, which were conducted during the dark phase of the light-dark cycle, Fox et al. (2010) tested DOI and TCB-2 during the light phase; it is unlikely, however, that this contributed to the discrepant findings because DOI has also been shown to induce hyperactivity during the light-phase (Brookshire and Jones, 2009).
Given our previous findings with DOI (Halberstadt et al., 2009) and DOM (Halberstadt and Geyer, 2011; Halberstadt et al., 2011b) in the BPM, the fact that DOET, DOPR, TMA-2 and mescaline also induce locomotor hyperactivity in mice indicates that this effect may be a property common to phenylalkylamines that activate the 5-HT2A receptor and produce hallucinogenic effects. The relative potencies of the phenylalkylamines in the BPM (DOPR ≈ DOI ≈ DOET > TMA-2 > mescaline) are consistent with their potencies for eliciting DOM-like discriminative stimulus effects in rats (Glennon et al., 1983) and hallucinogenic effects in humans (Shulgin and Dyer, 1975; Shulgin and Shulgin, 1991). In rats and humans, DOET and DOI are ~10-fold more potent than TMA-2 and ~50-fold more potent than mescaline (see Table 4), which parallel our findings in mice. The fact that DOTB did not alter exploratory or investigatory behavior in mice is consistent with evidence that DOTB is not hallucinogenic (Shulgin and Dyer, 1975), and does not induce hallucinogen-like behavioral effects in rodents (Kulkarni, 1973; Morin et al., 1975; Glennon et al., 1982). DOTB has high affinity for the 5-HT2A receptor (Table 4), but exhibits weak efficacy, inducing phosphoinositide hydrolysis with ~50% of the intrinsic efficacy of R-(-)-DOB (Glennon et al., 1992). Given the weak partial agonist activity of DOTB at the 5-HT2A receptor, we hypothesized that it would not increase locomotor activity in mice. Indeed, DOTB did not significantly increase locomotor activity when tested at doses up to 10 mg/kg. Although it is possible that DOTB may be active in mice at doses >10 mg/kg, the present findings demonstrate that it is at least an order of magnitude less potent than DOI and DOET despite having similar affinity for 5-HT2A receptors (Table 5).
Table 4.
5-HT2A affinity and behavioral potency of phenylalkylamines
| Drug | 5-HT2A receptor affinity Ki (nM) | Drug discriminationj | Human dose range (mg)k | Doses that increase locomotor activity in mice (mg/kg) | ||
|---|---|---|---|---|---|---|
| Agonist radioliganda | Antagonist radioligandf | ED50 (mg/kg) | ED50 (μmol/kg) | |||
| Mescaline | 360b | 5,500g | 14.64 | 59.1 | 178–256 | 25–100 |
| TMA-2 | 81c | 1,650h | 3.59 | 13.7 | 20–40 | 5–15 |
| DOM | 8c | 100i | 0.44 | 1.79 | 3–10 | 1.0l |
| DOET | 1.50c | 100i | 0.23 | 0.89 | 2–6 | 1.0 |
| DOPR | 0.90c | 69i | 0.17 | 0.62 | 2.5–5.0 | 0.3 |
| DOTB | 1.7d | 19i | PS | PS | Inactive at 25 | Inactive at 10 |
| DOI | 0.70c | 18.9h | 0.42 | 1.17 | 1.5–3.0 | 0.625–5.0m |
| TCB-2 | 0.73e | ND | ND | ND | ND | 1–10 |
Doses listed refer to the hydrochloride salts, except for TCB-2 which was tested as the hydrobromide. ND, not determined. PS, produced only partial substitution for the training drug.
Affinity for [125I]DOI- or [3H]DOB-labeled 5-HT2A receptors.
Affinity for [3H]ketanserin-labeled 5-HT2A receptors.
Stimulus generalization in rats trained with 1.0 mg/kg DOM HCl (data taken from: Glennon et al., 1983).
Doses that produce hallucinogenic effects in humans (data taken from: Shulgin and Shulgin, 1991).
Although the BPM can be used to assess the behavioral effects of 5-HT2A receptor activation in mice, it is important to note that locomotor hyperactivity does not represent a model of human hallucinogenic effects. Multiple classes of drugs, including dopamine agonists and NMDA antagonists, can increase locomotor activity in mice, and it is not clear whether hallucinogens alter locomotor activity in humans. Furthermore, we have previously found that indoleamine hallucinogens such as psilocin and 5-methoxy-N,N-dimethyltryptamine reduce locomotor activity in C57BL/6J mice, an effect mediated by 5-HT1A receptor activation (Halberstadt et al., 2011a). There is evidence that the 5-HT1A receptor can suppress the behavioral response to 5-HT2A activation (Darmani et al., 1990). The fact that indoleamine hallucinogens do not produce hyperactivity in the BPM, despite acting as 5-HT2A agonists, indicates that 5-HT1A receptor activation can block 5-HT2A-induced hyperlocomotion.
In vitro and in vivo evidence demonstrates that TCB-2 is a potent and highly efficacious 5-HT2A agonist (McLean et al., 2006b; Fox et al., 2010). TCB-2 induces the head twitch response in C57BL/6J mice, an effect that is blocked by the highly selective 5-HT2A antagonist MDL 11,939 (Fox et al., 2010). Furthermore, the R isomer of TCB-2 substitutes in rats trained to discriminate LSD or DOI (McLean et al., 2006b). These findings indicate that TCB-2 may have hallucinogenic effects, although we are not aware of any studies that have tested this compound in humans. The current experiments extend those earlier behavioral findings by demonstrating that TCB-2 increases locomotor activity in mice by activating the 5-HT2A receptor. The 5-HT2A receptor is known to be coupled to multiple downstream signaling pathways (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003; Moya et al., 2007), including activation of phospholipase C (PLC) and phospholipase A2 (PLA2), but the specific effector mechanisms responsible for mediating the behavioral effects of hallucinogens have not been conclusively identified. Interestingly, TCB-2 preferentially activates PLC compared with PLA2 (McLean et al., 2006b), whereas phenylalkylamines such as mescaline, DOI, and DOB appear to be either relatively non-selective or selective for PLA2 (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003; Moya et al., 2007). The fact that TCB-2 potently increases locomotor activity despite having weak effects on PLA2 indicates that the PLA2 signal transduction pathway may not be responsible for mediating the locomotor-activating effects of 5-HT2A agonists in mice.
In summary, the present experiments confirm that 5-HT2A receptor activation increases locomotor activity in mice, as well as reducing rearing behavior and altering locomotor patterns. Given that a variety of phenylalkylamine 5-HT2A agonists produce virtually identical effects on locomotor activity, these results indicate that effects in the BPM can be used as a behavioral measure of 5-HT2A activation in mice. Given the putative role for the 5-HT2A receptor in the pathology and treatment of several psychiatric disorders, it is important to develop behavioral paradigms that can be used to assess 5-HT2A receptor-induced behavioral effects in mice. Although drug discrimination and head twitch response can be used to assess the response to 5-HT2A receptor activation in mice, there are advantages to using the BPM paradigm. For example, the drug discrimination paradigm involves extended operant training and head twitch studies require time-consuming behavioral scoring, whereas the BPM provides an automated assessment of unconditioned behavior. Furthermore, alterations in 5-HT2A receptor-induced locomotor hyperactivity can be used to probe interactions between 5-HT and other transmitter systems (Halberstadt et al., 2011b). Previous BPM studies have shown that the locomotor effects of hallucinogens in rats—including mescaline and DOI—are mediated by the 5-HT2A receptor (Wing et al., 1990; Krebs-Thomson et al., 1998). Importantly, our studies show that mescaline and DOI alter locomotor activity in mice through the same receptor mechanism. Therefore, these studies provide additional support for the link between 5-HT2A activation and hallucinogenic effects.
HIGHLIGHTS.
 Mescaline and other phenylalkylamine hallucinogens increase locomotor activity in mice
Mescaline and other phenylalkylamine hallucinogens increase locomotor activity in mice The 5-HT2A agonist TCB-2 increases locomotor activity in mice
The 5-HT2A agonist TCB-2 increases locomotor activity in mice The non-hallucinogenic mescaline analog DOTB, a weak 5-HT2A partial agonist, does not significantly increase locomotor activity
The non-hallucinogenic mescaline analog DOTB, a weak 5-HT2A partial agonist, does not significantly increase locomotor activity The locomotor hyperactivity induced mescaline and TCB-2 is blocked by selective 5-HT2A antagonists
The locomotor hyperactivity induced mescaline and TCB-2 is blocked by selective 5-HT2A antagonists Mescaline and TCB-2 do not induce locomotor hyperactivity in 5-HT2A receptor knockout mice
Mescaline and TCB-2 do not induce locomotor hyperactivity in 5-HT2A receptor knockout mice
ACKNOWLEDGMENTS
Supported by National Institute on Drug Abuse Awards R01 DA002925 and F32 DA025412, and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdolmaleky HM, Yaqubi S, Papageorgis P, Lambert AW, Ozturk S, Sivaraman V, Thiagalingam S. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr. Res. 2011;129:183–190. doi: 10.1016/j.schres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Adams L, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1985;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Arranz MJ, Munro J, Owen MJ, Spurlock G, Sham PC, Zhao J, Kirov G, Collier DA, Kerwin RW. Evidence for association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and response to clozapine. Mol. Psychiatry. 1998;3:61–66. doi: 10.1038/sj.mp.4000348. [DOI] [PubMed] [Google Scholar]
- Arranz B, Rosel P, San L, Ramírez N, Dueñas RM, Salavert J, Centeno M, del Moral E. Low baseline serotonin-2A receptors predict clinical response to olanzapine in first-episode schizophrenia patients. Psychiatry Res. 2007;153:103–109. doi: 10.1016/j.psychres.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Brookshire BR, Jones SR. Direct and indirect 5-HT receptor agonists produce gender- specific effects on locomotor and vertical activities in C57 BL/6J mice. Pharmacol. Biochem. Behav. 2009;94:194–203. doi: 10.1016/j.pbb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J. Cogn. Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology. 2007;195:415–424. doi: 10.1007/s00213-007-0930-9. [DOI] [PubMed] [Google Scholar]
- Chen SF, Shen YC, Chen CH. HTR2A A-1438G/T102C polymorphisms predict negative symptoms performance upon aripiprazole treatment in schizophrenic patients. Psychopharmacology. 2009;205:285–292. doi: 10.1007/s00213-009-1538-z. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol. Biochem. Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Shaddy J, Gerdes CF. Differential ontogenesis of three DOI-induced behaviors in mice. Physiol. Behav. 1996;60:1495–1500. doi: 10.1016/s0031-9384(96)00323-x. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4- iodoamphetamine-elicited head twitch behavior in mice. J. Pharmacol. Exp. Ther. 2010;335:728–734. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs I: Antagonist correlation analysis. Psychopharmacology. 1995;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fox MA, French HT, Laporte JL, Blackler AR, Murphy DL. The serotonin 5-HT2A receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology. 2009;212:13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA. Indolealkylamine and phenalkylamine hallucinogens: a brief overview. Neurosci. Biobehav. Rev. 1982;6:489–497. doi: 10.1016/0149-7634(82)90030-6. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R. Drug-induced discrimination: a description of the paradigm and a review of its application in the study of hallucinogenic agents. Med Res Rev. 1983;3:289–340. doi: 10.1002/med.2610030305. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanisms of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Sanders-Bush E. Hallucinogens and serotonergic mechanisms. NIDA Res Monogr. 1992;119:131–135. [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. A comparison of the behavioral effects of DOM homologs. Pharmacol Biochem Behav. 1982;16:557–559. doi: 10.1016/0091-3057(82)90414-2. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–81. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2011a;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Lehmann-Masten VD, Geyer MA, Powell SB. Interactive effects of mGlu5 and 5-HT2A receptors on locomotor activity in mice. Psychopharmacology. 2011b;215:81–92. doi: 10.1007/s00213-010-2115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Nichols DE. Serotonin and serotonin receptors in hallucinogen action. In: Muller C, Jacobs B, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic; London: 2010. pp. 621–636. [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Kitajima T, Okochi T, Okumura T, Tsunoka T, Yamanouchi Y, Kinoshita Y, Kawashima K, Naitoh H, Nakamura J, Ozaki N, Iwata N. HTR2A is associated with SSRI response in major depressive disorder in a Japanese cohort. Neuromolecular. Med. 2010;12:237–242. doi: 10.1007/s12017-009-8105-y. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Kulkarni AS. Scratching response induced in mice by mescaline and related amphetamine derivatives. Biol. Psychiatry. 1973;6:177–180. [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J. Pharmacol. Exp. Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- McLean TH, Chambers JJ, Parrish JC, Braden MR, Marona-Lewicka D, Kurrasch-Orbaugh D, Nichols DE. C-(4,5,6-trimethoxyindan-1-yl)methanamine: a mescaline analogue designed using a homology model of the 5-HT2A receptor. J. Med. Chem. 2006a;49:4269–4274. doi: 10.1021/jm060272y. [DOI] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J. Med. Chem. 2006b;49:5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, Sorant AJ, Papanicolaou GJ, Laje G, Fava M, Trivedi MH, Wisniewski SR, Manji H. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am. J. Hum. Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte AP, Waldman SR, Marona-Lewicka D, Wainscott DB, Nelson DL, Sanders-Bush E, Nichols DE. Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J. Med. Chem. 1997;40:2997–3008. doi: 10.1021/jm970219x. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierraz-Hernandez, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamines and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J. Pharmacol. Exp. Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Morin RD, Benington F, Mitchell SR, Beaton JM, Bradley RJ, Smythies JR. The behavioral effects of 2,5-dimethoxy-4-alkyl amphetamines. Experentia. 1975;31:93–95. doi: 10.1007/BF01924697. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behav. Brain Res. 1993;53:11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT2A serotonin and D2 dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Geyer MA, Halberstadt AL. Serotonin and schizophrenia. In: Muller CP, Jacobs B, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic Press; London: 2010. pp. 585–620. [Google Scholar]
- Rasmussen H, Ebdrup BH, Erritzoe D, Aggernaes B, Oranje B, Kalbitzer J, Pinborg LH, Baaré WF, Svarer C, Lublin H, Knudsen GM, Glenthoj B. Serotonin2A receptor blockade and clinical effect in first-episode schizophrenia patients treated with quetiapine. Psychopharmacology. 2011;213:583–592. doi: 10.1007/s00213-010-1941-5. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M. Hallucinogenic drug interactions at human brains 5-HT2 receptor: implications for treating LSD-induced hallucinogenesis. Psychopharmacology. 1989;98:495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- Saiz PA, García-Portilla MP, Arango C, Morales B, Martínez-Barrondo S, Alvarez C, San Narciso G, Carreño E, Alvarez V, Coto E, Bobes J. Association between heroin dependence and 5-HT2A receptor gene polymorphisms. Eur. Addict. Res. 2008a;14:47–52. doi: 10.1159/000110410. [DOI] [PubMed] [Google Scholar]
- Saiz PA, García-Portilla MP, Paredes B, Arango C, Morales B, Alvarez V, Coto E, Bascaran MT, Bousoño M, Bobes J. Association between the A-1438G polymorphism of the serotonin 2A receptor gene and nonimpulsive suicide attempts. Psychiatr. Genet. 2008b;18:213–218. doi: 10.1097/YPG.0b013e3283050ada. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5- Dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Seggel MR, Yousif MY, Lyon RA, Titeler M, Roth BL, Suba EA, Glennon RA. A structure-affinity study of the binding of 4-substituted analogues of 1-(2,5-dimethoxyphenyl)-2-aminopropane at 5-HT2 serotonin receptors. J. Med. Chem. 1990;33:1032–1036. doi: 10.1021/jm00165a023. [DOI] [PubMed] [Google Scholar]
- Shannon M, Battaglia G, Glennon RA, Titeler M. 5-HT1 and 5-HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA). Eur. J. Pharmacol. 1984;102:23–29. doi: 10.1016/0014-2999(84)90333-9. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Dyer DC. Psychotomimetic phenylisopropylamines. 5. 4-Alkyl-2,5-dimethoxyphenylisopropylamines. J. Med. Chem. 1975;18:1201–1204. doi: 10.1021/jm00246a006. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PIHKAL: A Chemical Love Story. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/−)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 2003;166:61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon LA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropyl amine hallucinogens. Psychopharmacology. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Viikki M, Huuhka K, Leinonen E, Illi A, Setälä-Soikkeli E, Huuhka M, Mononen N, Lehtimäki T, Kampman O. Interaction between two HTR2A polymorphisms and gender is associated with treatment response in MDD. Neurosci. Lett. 2011;501:20–24. doi: 10.1016/j.neulet.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Wilkie MJ, Smith G, Day RK, Matthews K, Smith D, Blackwood D, Reid IC, Wolf CR. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics J. 2009;9:61–70. doi: 10.1038/sj.tpj.6500491. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology. 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Winter JC, Kieres AK, Zimmerman MD, Reissig CJ, Eckler JR, Ullrich T, Rice KC, Rabin RA, Richards JB. The stimulus properties of LSD in C57BL/6 mice. Pharmacol Biochem Behav. 2005;81:830–837. doi: 10.1016/j.pbb.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur. J. Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Miyara T, Matsushima T, Imanishi T. Desensitization of 5-HT2A receptor function by chronic administration of selective serotonin reuptake inhibitors. Brain Res. 2006;1067:164–169. doi: 10.1016/j.brainres.2005.10.075. [DOI] [PubMed] [Google Scholar]
- Yoon HK, Yang JC, Lee HJ, Kim YK. The association between serotonin-related gene polymorphisms and panic disorder. J. Anxiety Disord. 2008;22:1529–1534. doi: 10.1016/j.janxdis.2008.03.006. [DOI] [PubMed] [Google Scholar]