Abstract
Accumulating evidence over the past 25 years depicts the healthy pulmonary system as a limiting factor of whole body endurance exercise performance. This brief overview emphasizes three respiratory system-related mechanisms which impair O2 transport to the locomotor musculature [arterial O2 content (CaO2) × leg blood flow (QL)], i.e. the key determinant of an individual’s aerobic capacity and ability to resist fatigue. First, the respiratory system often fails to prevent arterial desaturation substantially below resting values and thus compromises CaO2. Especially susceptible to this threat to convective O2 transport are well-trained endurance athletes characterized by high metabolic and ventilatory demands and, likely due to anatomical and morphologic gender differences, active females. Second, fatiguing respiratory muscle work (Wresp) associated with strenuous exercise elicits sympathetically-mediated vasoconstriction in limb-muscle vasculature which compromises QL. This impact on limb O2 transport is independent of fitness level and affects all individuals, however, only during sustained, high-intensity endurance exercise performed above ~85% VO2max. And third, excessive fluctuations in intrathoracic pressures accompanying Wresp can limit cardiac output and therefore QL. Exposure to altitude exacerbates the respiratory system limitations observed at sea level and further reduces CaO2 and substantially increases exercise-induced Wresp. Taken together, the intact pulmonary system of healthy endurance athletes impairs locomotor muscle O2 transport during strenuous exercise by failing to ensure optimal arterial oxygenation and compromising QL. This respiratory system-related impact exacerbates the exercise-induced development of fatigue and compromises endurance performance.
Keywords: pulmonary ventilation, blood flow distribution, gas exchange, exercise-induced arterial hypoxemia, arterial oxygen saturation, work of breathing
The purpose of this short report is to provide a general overview of endurance exercise limitations primarily pertaining to the pulmonary system. Although an exhaustive discussion of underlying mechanisms cannot be provided given space limitations, the article outlines key characteristics of the human respiratory system which have the potential to impact well-known determinants of endurance performance.
Numerous previous studies substantiate the role of muscle O2 transport [arterial O2 content (CaO2) × locomotor muscle blood flow (QL)] as a major determinant of high-intensity endurance exercise performance in humans. Reductions in muscle O2 transport attenuate VO2max, exaggerate the rate of fatigue development and deteriorate endurance exercise performance (Amann & Calbet, 2008). In contrast, increases in muscle O2 transport evoke the opposite effects. Consequently, any given factor impeding arterial oxygenation and/or QL impairs a human’s endurance exercise capacity and performance.
The Respiratory System: Well Designed for the Needs of Most Healthy Young Individuals
In contrast to the heart, the respiratory system of healthy young individuals is usually not considered a major limiting factor for high-intensity endurance exercise. This stems from the fact that the capacity of the healthy pulmonary system in most humans is sufficient to cope with the demands associated with ventilation and gas exchange – even during strenuous endurance exercise. The majority of untrained (VO2max ≤55 ml/kg/min), but even most well trained, individuals are characterized by only a small 2-3 fold increase in the alveolar to arterial O2 difference (A-aDO2; surrogate of gas exchange efficiency) from rest (~5-8 mmHg) to VO2max (<30 mmHg). This small change indicates a largely uncompromised and adequate rate of O2 diffusion across the alveolar-capillary membrane (Dempsey & Wagner, 1999).
Furthermore, in most humans, alveolar ventilation during exercise can rise unrestricted and out of proportion to CO2 production as arterial PCO2 (PaCO2) is reduced to ≥10 mmHg below resting levels. In other words, alveolar hyperventilation can increase sufficiently and raise alveolar PO2 (PAO2) high enough to enable a compensation for the widened A-aDO2. The net effect is a nearly unchanged PaO2 from rest to VO2max and only a fairly small reduction in arterial haemoglobin saturation (SaO2) which is, however, nearly exclusively caused by the exercise-induced increases in core temperature and metabolic acidosis (see below). Also, airway resistance and lung compliance during exercise are maintained near resting levels and, in untrained subjects, breathing requires only ≤10% of both VO2max and maximum cardiac output (Aaron et al., 1992; Harms et al., 1998b), and intrathoracic pressure changes developed by the respiratory muscles approximate only 40-50% of their maximal dynamic capacity (Johnson et al., 1992).
Overall, the respiratory system in healthy young individuals might generally be considered as sufficiently “equipped” to handle the pulmonary gas exchange requirements associated with even high intensity endurance exercise.
Weaknesses and Limits of the Healthy Respiratory System
In some, not all, trained endurance athletes, the metabolic requirement associated with high intensity exercise demands extreme ventilation and pulmonary gas exchange which can actually reach and outstrip the functional capacity of their respiratory system and eventually compromise arterial oxygenation and limb O2 transport (Dempsey et al., 1984; Williams et al., 1986; Powers et al., 1988; Harms et al., 1997). I briefly cover three respiratory system related mechanisms which present significant limitations to locomotor muscle O2 transport during exercise.
1. Exercise-Induced Arterial Oxyhaemoglobin Desaturation
High-intensity endurance exercise in some fit athletes causes a time-dependant decrease in SaO2 of greater than 5% from resting levels (~98%) – extreme drops into the mid 80% range have been reported (Dempsey & Wagner, 1999). The oxyhaemoglobin desaturation during exercise is based on both respiratory and non-respiratory influences. Briefly, non-respiratory influences encompass the rightward shift of the oxyhaemoglobin dissociation curve mediated by metabolic acidosis and hyperthermia (Wasserman et al., 1967; Rasmussen et al., 1991).
In a minority of athletes, frequently those characterized by the greatest fitness (Williams et al., 1986), arterial oxyhaemoglobin desaturation also occurs due to a fall in PaO2 (Holmgren & Linderholm, 1958) secondary to an abnormally widened A-aDO2 (Hopkins & McKenzie, 1989; Dempsey & Wagner, 1999). At maximal exercise in healthy untrained individuals, A-aDO2 is usually up to 20-30 mmHg, however, in some elite athletes, this difference might be as wide as 35-50 mmHg (Dempsey et al., 1984).
Arterial desaturation during exercise can also occur due to an inadequate hyperventilatory response secondary to low chemoresponsiveness [i.e. attenuated response to circulating chemical stimuli like protons, catecholamines, adenosine, or potassium (Lumb & Nunn, 2000) – and maybe also O2 and CO2 (Harms & Stager, 1995; Guenette et al., 2004)] and/or mechanical constraints presented by the airways (Dempsey et al., 1984; Johnson et al., 1992; Dempsey & Wagner, 1999). Inadequate ventilatory responses during exercise have been shown to reduce PAO2 which negatively affects arterial blood gas status and SaO2 (Johnson et al., 1992).
Some recent studies indicate a greater prevalence of arterial oxyhaemoglobin desaturation in active females compared to their male counterpart (Harms et al., 1998a; Hopkins et al., 2000; Hopkins & Harms, 2004). Various pulmonary structural and functional differences have been found between females and age- and height-matched males (Hopkins & Harms, 2004). For example, women are characterized by smaller lung volumes and airways, a lower resting lung diffusion capacity, and lower maximal expiratory flow rates compared men (McClaran et al., 1998; Guenette et al., 2007). Although the exact effects of these anatomical and morphologic gender differences remain elusive, they are considered as key contributors to the greater gas exchange disturbances and ventilatory limitations during exercise in females vs males.
Remaining issues - from a personal communication with Prof. Jerry Dempsey: “In the minority of trained individuals characterized by a reduction in SaO2 secondary to an excessive A-aDO2 and the resulting fall in PaO2, it remains unresolved why the reductions in PaO2 already occur during submaximal exercise and why it only seems to occur in trained rather than untrained individuals – especially runners. The idea that we originally had (i.e. that the extraordinary demand for pulmonary O2 transport exceeds the ordinary structural capacity of the lung in these athletes) does not apply under submaximal conditions because the athletes are not anywhere near maximal demands for O2 transport and the ‘capacity’ of the lungs for gas exchange are not being challenged in the usual sense. The cause(s) of this arterial hypoxemia during submaximal exercise in the absence of hypoventilation in these types of endurance trained athletes remains a mystery to me. It is also a mystery to me that arterial hypoxemia occurs most often during running and only rarely during bicycle exercise. A further key unresolved issue is the huge variability in exercise-induced A-aDO2 difference and oxyhaemoglobin desaturation amongst athletes. Many athletes are hardly affected even at maximal exercise, whereas others are characterized by a fall in PaO2 even during submaximal exercise which worsens at higher workloads.”
2. Exercise-Induced Wresp and Associated Metaboreflex-Mediated Impact on QL
A further threat to locomotor muscle O2 delivery is Wresp associated with heavy sustained exercise (>85% VO2max). The ventilatory response during heavy exercise, which is often accompanied and impaired by expiratory flow limitations and dynamic hyperinflation (Johnson et al., 1992), requires substantial increases in both inspiratory and expiratory muscle work, often leading to respiratory muscle fatigue. Even though diaphragm force, during tidal breathing, falls during the latter stages of sustained heavy exercise, alveolar ventilation is not compromised, presumably due to accessory muscle recruitment. However, fatiguing contractions and associated accumulation of metabolites in the inspiratory and expiratory muscles activate unmylenated group IV phrenic afferents (Hill, 2000) which reflexly increase sympathetic vasoconstrictor activity (St Croix et al., 2000) and vasoconstriction of the vasculature of the exercising limb (Harms et al., 1997) (Figure 1). The result is a reduction in QL, and (presumably) an increase in blood flow to the respiratory muscles, indicating a competitive relationship for a limited cardiac output (Manohar, 1986; Musch, 1993). These effects do not occur during exercise at intensities lower than ~80% VO2max (Wetter et al., 1999). During intense exercise (>85% VO2max) in the highly-trained subject, the respiratory muscles now require up to 15-16% of VO2max and cardiac output (Harms et al., 1998b) – versus ≤10% in the untrained. Thus, in contrast to arterial desaturation, Wresp induced by heavy, sustained exercise has no effect on CaO2, but the reduction in O2 transport is caused by reduced QL.
Figure 1.
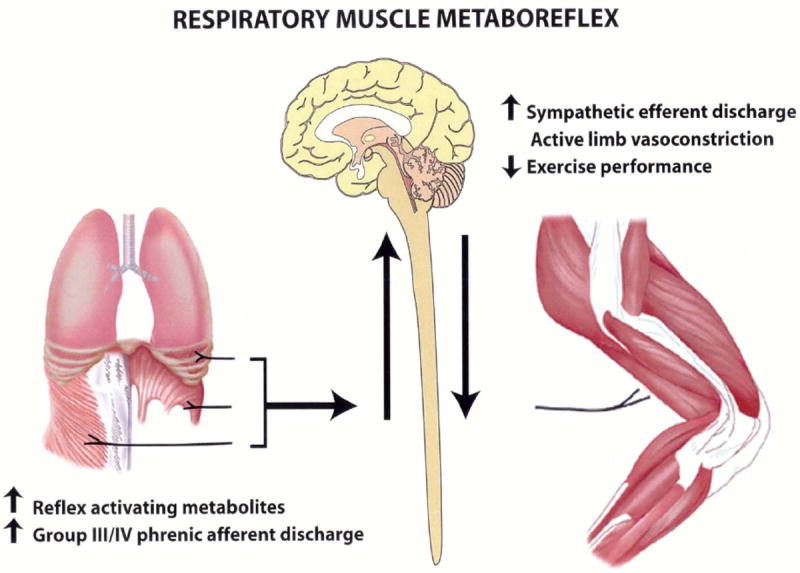
Relationship between respiratory muscle work and leg blood flow. Fatigue related metabolite accumulation in respiratory muscles activate group III/IV phrenic afferents which reflexly cause increased sympathetic efferent discharge and limb vasoconstriction. This sequence facilitates locomotor muscle fatigue and limits endurance exercise performance. Adapted from (Dempsey et al., 2002).
3. Intrathoracic Pressure Effects on Cardiac Output
The ventilatory response during high intensity exercise is associated with a substantial augmentation of negative and positive intrathoracic pressures. In the presence of expiratory flow limitation and hyperinflation in the well-trained young athlete, these inspiratory pressures may approach 95% and 30% of the maximum dynamic pressure available to the inspiratory and expiratory muscles, respectively (Johnson et al., 1992). The heart and great vessels are exposed to these substantial oscillatory pressures.
Recent studies in exercising humans and animals have used mechanical ventilation and threshold loads to reduce negative inspiratory or increase positive expiratory intrathoracic pressures, respectively. The results of these investigations suggest a substantial effect of these pressures on venous return, stroke volume, and cardiac output during exercise. For example, the normally occurring negative inspiratory intrathoracic pressures associated with high-intensity exercise have a significant facilitating contribution (up to 10%) to end-diastolic volume and subsequently stroke volume and cardiac output. Importantly, no additional effects on cardiac output are observed when negative inspiratory intrathoracic pressures are increased beyond normal by imposing additional inspiratory negative pressure via resistive loading (Harms et al., 1998b; Miller et al., 2007). In contrast, even small increases in positive intrathoracic pressures on expiration (5-10 cm H2O) have been shown to decrease ventricular transmural pressure which reduces the rate of ventricular filling during diastole and thereby impairs stroke volume and cardiac output (Stark-Leyva et al., 2004; Miller et al., 2006). Increases in expiratory positive intrathoracic pressures of similar and even greater magnitudes occur during the transition from moderate to intense exercise in well-trained individuals and/or with the development of expiratory flow limitations (Johnson et al., 1992).
Taken together, negative inspiratory pressures during exercise appear to promote cardiac output via increasing ventricular preload and therefore stroke volume, whereas expiratory positive pressures during exercise limit cardiac output via increasing the ventricular afterload and thereby decreasing stroke volume. The net effect of intrathoracic pressure changes on cardiac output during high intensity exercise in well-trained endurance athlete will depend upon the degree to which the functional consequences of negative inspiratory pressures (i.e. facilitating cardiac output) balance the mechanical consequences of positive expiratory pressures (i.e. limiting cardiac output).
In summary, it should be emphasized that a threat to locomotor muscle O2 transport secondary to arterial desaturation >4-5% from rest is experienced only by a subgroup of well-trained endurance athletes and can develop even at submaximal exercise intensities. However, the threat to O2 delivery via the respiratory muscle metaboreflex occurs in all healthy subjects, but only at sustained, high-intensity endurance exercise (>85% VO2max). Furthermore, the reduction in QL imposed by the respiratory muscle metaboreflex is potentially even further exacerbated via potentially negative effects of intrathoracic pressure excursions on cardiac output.
Respiratory System Limitations: Consequences for Endurance Performance and Fatigue
Consequences of Exercise-Induced Arterial Desaturation on Endurance Performance
The impact of arterial desaturation on endurance performance has been revealed by adding just sufficient O2 to the inspired air to prevent the fall in SaO2 during exercise. The measurable threshold of SaO2-related limitations to peak aerobic power occurs at a desaturation of >4-5% from rest (Squires & Buskirk, 1982; Powers et al., 1989; Harms et al., 2000a). Beyond this threshold, a linear association between the changes in saturation and VO2max is observed, such that each further 1% reduction in SaO2 causes a 1-2% reduction in peak aerobic power.
Similarly, exercise-induced arterial desaturation also limits endurance performance achieved during a time trial-like test modality (Koskolou & McKenzie, 1994; Nielsen et al., 2002). For example, Amann et al. (Amann et al., 2006) have recently demonstrated a significant limiting effect of arterial desaturation on 5-km cycling time trial performance (Figure 2). CaO2, and thus O2 delivery, was increased by ~8% when the exercise-induced fall in SaO2 (to ~91%) was prevented by increasing the fraction of O2 in the inspired air. This resulted in a substantial 2-5% reduction in the time to completion, and up to a 5% increase in mean power output.
Figure 2.
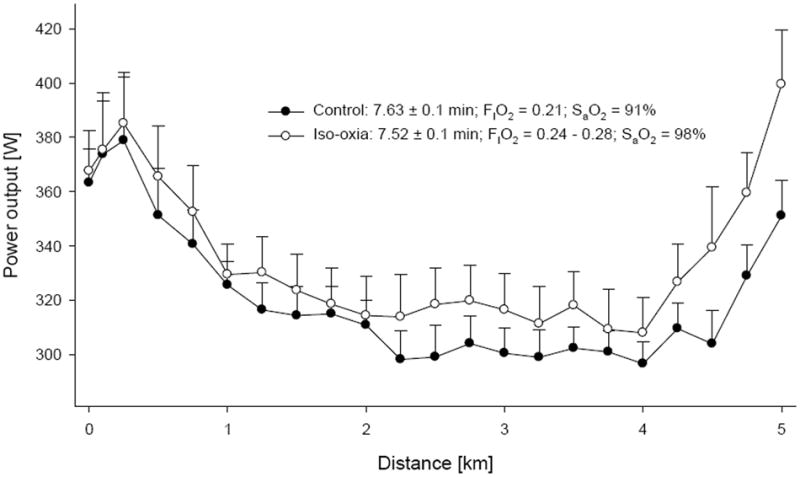
Effect of exercise-induced arterial desaturation on 5 km cycling time trial performance. During the iso-oxic trial, SaO2 was maintained at resting levels (~98%) via progressive increases in inspiratory O2 content (FIO2). Time to completion and mean power output (331 ± 13 W vs 314 ± 13 W) were significantly improved during the iso-oxic time trial. Adapted from (Amann et al., 2006).
Consequences of Wresp on Endurance Performance
The effects of the Wresp on endurance performance have been revealed by reducing the normally occurring Wresp during constant-load exercise via mechanical ventilatory assist or heliox breathing. At exercise intensities corresponding to ≤80% of VO2max, significant 20-40% reductions in Wresp have no effect on endurance performance (Gallagher & Younes, 1989; Marciniuk et al., 1994; Krishnan et al., 1996). These observations are not surprising given the fact that blood flow redistribution between the respiratory muscles and the locomotor muscles only occurs at exercise intensities >85-90% of VO2max (Harms et al., 1997) – and not at or below 80% of VO2max (Wetter et al., 1999). However, when constant-load exercise is performed at intensities greater than 85-90% VO2max, respiratory muscle unloading was found to significantly increase endurance time to exhaustion (Wilson & Welch, 1980; Johnson et al., 1996).
For example, during constant-load cycling at 90% VO2max, a 60% reduction in Wresp resulted in increased limb vascular conductance and 3-4% increases in leg O2 transport and uptake – even in the face of a reduced cardiac output (Harms et al., 1997). Time to exhaustion was increased by ~14% when Wresp was reduced by ~50%. This significant effect on exercise performance has indirectly been confirmed by increasing Wresp by ~28%, resulting in ~15% reduction in time to exhaustion (Harms et al., 2000b).
Consequences of Pulmonary System Limitations on the Development of Locomotor Muscle Fatigue
Even the relatively small reductions in O2 transport associated with exercise-induced haemoglobin desaturation >5% from rest, or the high Wresp, exacerbate the rate of development of peripheral locomotor muscle fatigue during exercise (Amann & Calbet, 2008). For example, during constant-load exercise (>90% VO2max), increases in locomotor muscle O2 transport secondary to a ~60% reduction in Wresp (via proportional assist ventilation) alleviated end-exercise quadriceps fatigue by 25-30% compared to control exercise (Romer et al., 2006b) (Figure 3). Furthermore, when exercise-induced arterial desaturation was prevented during constant-load leg cycling (>90% VO2max; via adding supplemental O2 to the inspired air), end-exercise quadriceps fatigue was nearly 50% less compared to control conditions (Romer et al., 2006a). In contrast, no effect of maintaining resting SaO2 on peripheral fatigue was observed in those individuals who sustained haemoglobin saturation above 95% during the exercise (Romer et al., 2006a).
Figure 3.
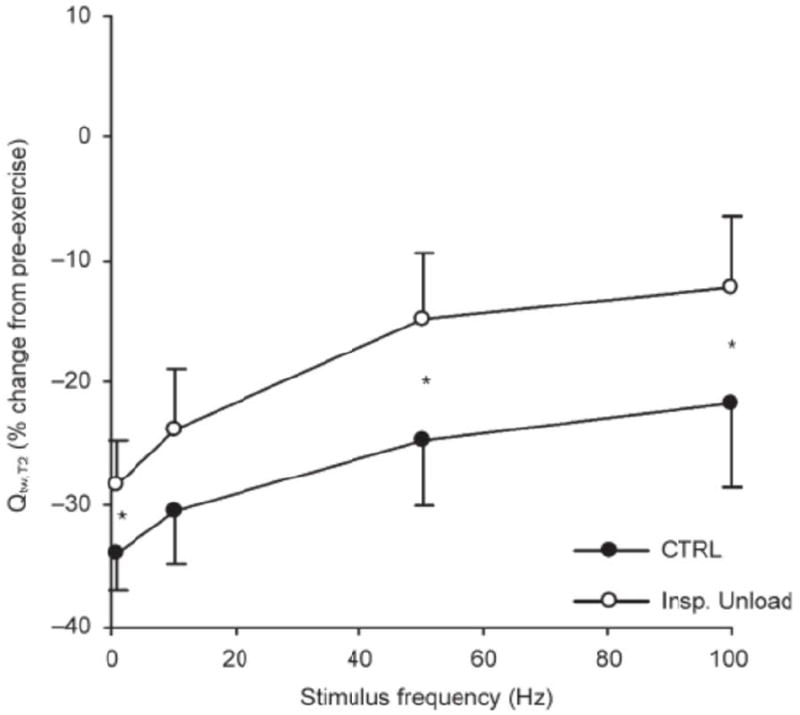
Effects of a 60% reduction in inspiratory muscle work (‘Insp. Unload’) on the pre- to post-exercise change in the force-frequency curve of the quadriceps muscle. The y-axis represents the change for the second of the paired quadricep twitch amplitude (Qtw,T2). The work rate and exercise time was identical during control exercise and inspiratory unloadin (≥90% VO2max; 292 W, 13 min). Adapted from (Romer et al., 2006b).
The effect of O2 delivery on peripheral fatigue development has been shown to be a key determinant of endurance exercise performance (Amann et al., 2006; Amann et al., 2011). We recently proposed that exercise-induced alterations of locomotor muscle fatigue affect, in a dose-dependent manner, the firing rate – and thus the central projection – of group III/IV muscle afferents which are known to provide inhibitory feedback to the determination of central motor drive during exercise (Amann et al., 2006; Amann et al., 2009; Amann, 2011). In other words, acting via inhibitory feedback to higher motor areas, the highly O2 delivery-sensitive peripheral locomotor muscle fatigue influences central motor drive and therefore exercise performance.
Exercise at Altitude
Additional respiratory limits to exercise performance at or near sea level occur during acute or chronic exposure to the hypoxia associated with altitudes beyond ~1500 m (Buskirk et al., 1967). Hypoxia aggravates the proposed threats to limb O2 delivery in two ways. First, the alveolar-capillary diffusion limitation becomes more pronounced, due to a decreased PAO2 at any given alveolar ventilation. Second, acute, but especially chronic, hypoxic exposures potentiate the hyperventilatory response to exercise and markedly increase Wresp (Thoden et al., 1969; Amann et al., 2007). Therefore, hypoxia exacerbates the rate of development of peripheral locomotor muscle fatigue elicited via high intensity exercise and reduces exercise performance in two ways, namely, via reductions in SaO2 and increases in Wresp (Amann et al., 2007). For example, we recently studied identical submaximal constant-load bike exercise (273 W, 8.6 min) performed at sea-level and simulated altitude (inspiratory O2 content = 0.15). Haemoglobin saturation was substantially lower during the exercise in acute hypoxia (~95% vs ~81%) whereas Wresp was about 40% higher compared to sea level (Amann et al., 2007). These drastic changes nearly doubled the rate of development of locomotor muscle fatigue during the cycling exercise and compromised the subjects’ endurance performance (Amann et al., 2006).
Conclusion
Accumulating evidence over the past 25 years indicates a substantial role of the healthy respiratory system in limiting high-intensity endurance exercise in humans. This influence is mediated via the effects of the respiratory system on locomotor muscle O2 delivery and associated consequences on the development of fatigue during exercise and an individual’s aerobic capacity. Reductions in O2 delivery are caused by the failure of the pulmonary system to maintain resting arterial oxygenation during exercise and/or a respiratory muscle metaboreflex which causes a sympathetically-mediated reduction in QL. Furthermore, intrathoracic pressure excursions associated with the high ventilatory work during intense exercise have been suggested to limit cardiac output. Taken together, the pulmonary system is a key – although highly variable – determinant of endurance performance in healthy individuals.
Acknowledgments
I thank Professor Jerry Dempsey for his comments on this manuscript. This work was supported by the US National Heart, Lung, and Blood Institute.
References
- Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol. 1992;72:1818–1825. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- Amann M. Central and Peripheral Fatigue: Interaction during Cycling Exercise in Humans. Med Sci Sports Exerc. 2011;43:2039–2045. doi: 10.1249/MSS.0b013e31821f59ab. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of Group III and IV Muscle Afferents for High Intensity Endurance Exercise Performance in Humans. J Physiol. 2011;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104:861–870. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006;575:937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2036–2045. doi: 10.1152/ajpregu.00442.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskirk ER, Kollias J, Akers RF, Prokop EK, Reategui EP. Maximal performance at altitude and on return from altitude in conditioned runners. J Appl Physiol. 1967;23:259–266. doi: 10.1152/jappl.1967.23.2.259. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol. 1984;355:161–175. doi: 10.1113/jphysiol.1984.sp015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87:1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- Gallagher CG, Younes M. Effect of pressure assist on ventilation and respiratory mechanics in heavy exercise. J Appl Physiol. 1989;66:1824–1837. doi: 10.1152/jappl.1989.66.4.1824. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Diep TT, Koehle MS, Foster GE, Richards JC, Sheel AW. Acute hypoxic ventilatory response and exercise-induced arterial hypoxemia in men and women. Respir Physiol Neurobiol. 2004;143:37–48. doi: 10.1016/j.resp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581:1309–1322. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998a;507(Pt 2):619–628. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Stager JM. Low chemoresponsiveness and inadequate hyperventilation contribute to exercise-induced hypoxemia. J Appl Physiol. 1995;79:575–580. doi: 10.1152/jappl.1995.79.2.575. [DOI] [PubMed] [Google Scholar]
- Harms CS, McClaran S, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Effect of exercise-induced arterial O2 desaturation on VO2max in women. Exerc Sci Med Sport. 2000a;32:1101–1108. doi: 10.1097/00005768-200006000-00010. [DOI] [PubMed] [Google Scholar]
- Harms CS, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998b;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CS, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000b;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res. 2000;856:240–244. doi: 10.1016/s0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Linderholm H. Oxygen and carbon dioxide tensions of arterial blood during heavy and exhaustive exercise. Acta Physiologica Scandinavica. 1958;44:203–215. doi: 10.1111/j.1748-1716.1958.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S, Wagner PD. Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol. 2000;89:721–730. doi: 10.1152/jappl.2000.89.2.721. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Harms CA. Gender and pulmonary gas exchange during exercise. Exerc Sport Sci Rev. 2004;32:50–56. doi: 10.1097/00003677-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, McKenzie DC. Hypoxic ventilatory response and arterial desaturation during heavy work. J Appl Physiol. 1989;67:1119–1124. doi: 10.1152/jappl.1989.67.3.1119. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Aaron EA, Babcock MA, Dempsey JA. Respiratory muscle fatigue during exercise: implications for performance. Med Sci Sports Exerc. 1996;28:1129–1137. doi: 10.1097/00005768-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol. 1992;73:874–886. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, McKenzie DC. Arterial hypoxemia and performance during intense exercise. Eur J Appl Physiol Occup Physiol. 1994;68:80–86. doi: 10.1007/BF00599246. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Zintel T, McParland C, Gallagher CG. Lack of importance of respiratory muscle load in ventilatory regulation during heavy exercise in humans. J Physiol. 1996;490(Pt 2):537–550. doi: 10.1113/jphysiol.1996.sp021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb A, Nunn JF. Nunn’s applied respiratory physiology. Butterworth-Heinemann; Oxford: 2000. [Google Scholar]
- Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol. 1986;377:25–35. doi: 10.1113/jphysiol.1986.sp016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniuk D, McKim D, Sanii R, Younes M. Role of central respiratory muscle fatigue in endurance exercise in normal subjects. J Appl Physiol. 1994;76:236–241. doi: 10.1152/jappl.1994.76.1.236. [DOI] [PubMed] [Google Scholar]
- McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol. 1998;84:1872–1881. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- Miller JD, Hemauer SJ, Smith CA, Stickland MK, Dempsey JA. Expiratory threshold loading impairs cardiovascular function in health and chronic heart failure during submaximal exercise. J Appl Physiol. 2006;101:213–227. doi: 10.1152/japplphysiol.00862.2005. [DOI] [PubMed] [Google Scholar]
- Miller JD, Smith CA, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H580–592. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- Musch TI. Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. Am J Physiol. 1993;265:H1721–1726. doi: 10.1152/ajpheart.1993.265.5.H1721. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Bredmose PP, Stromstad M, Volianitis S, Quistorff B, Secher NH. Bicarbonate attenuates arterial desaturation during maximal exercise in humans. J Appl Physiol. 2002;93:724–731. doi: 10.1152/japplphysiol.00398.2000. [DOI] [PubMed] [Google Scholar]
- Powers SK, Dodd S, Lawler J, Landry G, Kirtley M, McKnight T, Grinton S. Incidence of exercise induced hypoxemia in elite endurance athletes at sea level. Eur J Appl Physiol Occup Physiol. 1988;58:298–302. doi: 10.1007/BF00417266. [DOI] [PubMed] [Google Scholar]
- Powers SK, Lawler J, Dempsey JA, Dodd S, Landry G. Effects of incomplete pulmonary gas exchange on VO2 max. J Appl Physiol. 1989;66:2491–2495. doi: 10.1152/jappl.1989.66.6.2491. [DOI] [PubMed] [Google Scholar]
- Rasmussen J, Hanel B, Diamant B, Secher NH. Muscle mass effect on arterial desaturation after maximal exercise. Med Sci Sports Exerc. 1991;23:1349–1352. [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2006a;290:R365–375. doi: 10.1152/ajpregu.00332.2005. [DOI] [PubMed] [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006b;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires RW, Buskirk ER. Aerobic capacity during acute exposure to simulated altitude, 914 to 2286 meters. Med Sci Sports Exerc. 1982;14:36–40. doi: 10.1249/00005768-198201000-00007. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529(Pt 2):493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 2004;96:1920–1927. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- Thoden JS, Dempsey JA, Reddan WG, Birnbaum ML, Forster HV, Grover RF, Rankin J. Ventilatory work during steady-state response to exercise. Fed Proc. 1969;28:1316–1321. [PubMed] [Google Scholar]
- Wasserman K, Van Kessel AL, Burton GG. Interaction of physiological mechanisms during exercise. J Appl Physiol. 1967;22:71–85. doi: 10.1152/jappl.1967.22.1.71. [DOI] [PubMed] [Google Scholar]
- Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on VO(2) and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
- Williams JH, Powers SK, Stuart MK. Hemoglobin desaturation in highly trained athletes during heavy exercise. Med Sci Sports Exerc. 1986;18:168–173. [PubMed] [Google Scholar]
- Wilson GD, Welch HG. Effects of varying concentrations of N2/O2 and He/O2 on exercise tolerance in man. Med Sci Sports Exerc. 1980;12:380–384. [PubMed] [Google Scholar]


