Abstract
Aims: Fibromyalgia (FM) is a prevalent chronic pain syndrome characterized by generalized hyperalgesia associated with a wide spectrum of symptoms such as fatigue and joint stiffness. Diagnosis of FM is difficult due to the lack of reliable diagnostic biomarkers, while treatment is largely inadequate. We have investigated the role of coenzyme Q10 (CoQ10) deficiency and mitochondrial dysfunction in inflammasome activation in blood cells from FM patients, and in vitro and in vivo CoQ10 deficiency models. Results: Mitochondrial dysfunction was accompanied by increased protein expression of interleukin (IL)-1β, NLRP3 (NOD-like receptor family, pyrin domain containing 3) and caspase-1 activation, and an increase of serum levels of proinflammatory cytokines (IL-1β and IL-18). CoQ10 deficiency induced by p-aminobenzoate treatment in blood mononuclear cells and mice showed NLRP3 inflammasome activation with marked algesia. A placebo-controlled trial of CoQ10 in FM patients has shown a reduced NLRP3 inflammasome activation and IL-1β and IL-18 serum levels. Innovation: These results show an important role for the NLRP3 inflammasome in the pathogenesis of FM, and the capacity of CoQ10 in the control of inflammasome. Conclusion: These findings provide new insights into the pathogenesis of FM and suggest that NLRP3 inflammasome inhibition represents a new therapeutic intervention for the disease. Antioxid. Redox Signal. 20, 1169–1180.
Introduction
Fibromyalgia (FM) is a common chronic pain syndrome accompanied by other symptoms such as fatigue and joint stiffness, which pathophysiological mechanisms are difficult to identify. Despite being a common disorder that affects at least 5 million individuals in the United States (11), routine laboratory investigations usually yield normal results (Table 2), and the diagnosis is not easy and may be frequently overlooked. Therefore, new diagnostic markers in FM are needed. Several pathological changes have been well documented in FM patients. For example, recent studies have implicated oxidative stress in the pathogenesis of FM (1, 2, 7, 9, 18), indicating that mitochondrial dysfunction may be associated with this syndrome. Furthermore, there are some hypotheses suggesting that cytokines may play a role in FM (4, 22, 24). Indeed, a decrease in mitochondrial mass and coenzyme Q10 (CoQ10) levels, as well as increased production of mitochondrial reactive oxygen species (ROS), have been detected in blood mononuclear cells (BMCs) from FM patients (7, 9). On the other hand, ROS have also been shown to be an important activator of inflammasome-mediated inflammation (31). The NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome is a molecular platform activated upon signs of cellular danger to trigger innate immune defenses through the maturation of proinflammatory cytokines such as interleukin (IL)-1β and IL-18 (30). To investigate a possible implication of mitochondria dysfunction in inflammasome activation in FM, we studied both pathological mechanisms in BMCs from FM patients and in two models, in vitro and in vivo, of CoQ10 deficiency (20, 21).
Table 2.
Serum Biochemical Parameters in Fibromyalgia Patients
| Biochemical parameter | Control | FM patients |
|---|---|---|
| Glucose (mg/dL) | 93.8±15.2 | 98.1±12.2 |
| Uric acid (mg/dL) | 3.9±1.5 | 3.1±1.6 |
| Aspartate aminotransferase (mU/ml) | 21.9±8.8 | 25.2±7.3 |
| Alanine aminotransferase (mU/ml) | 23.5±5.1 | 24.6±6.1 |
| Creatine kinase (IU/L) | 671.1±42.3 | 623.5±34.4 |
| Cholesterol (mg/dl) | 179.9±53.4 | 176.9±59.1 |
| Triglycerides (mg/dl) | 157.5±60.1 | 144.9±52.3 |
n=20 for controls and n=30 for FM patients, respectively.
Innovation.
In recent years, inflammasome has attracted increasing interest among basic and clinical researchers. Its implication in the pathophysiology of several diseases generates new therapeutic strategies. Our study reveals that the inflammasome complex is implicated in the pathophysiology of fibromyalgia (FM) mediated by coenzyme Q10 (CoQ10) deficiency. The oral CoQ10 treatment reduced inflammasome activation. These findings provide new insights into the pathogenesis of FM and indicate new potential molecular targets for the therapy of this disease.
Results
Mitochondrial characteristic in BMCs from FM patients
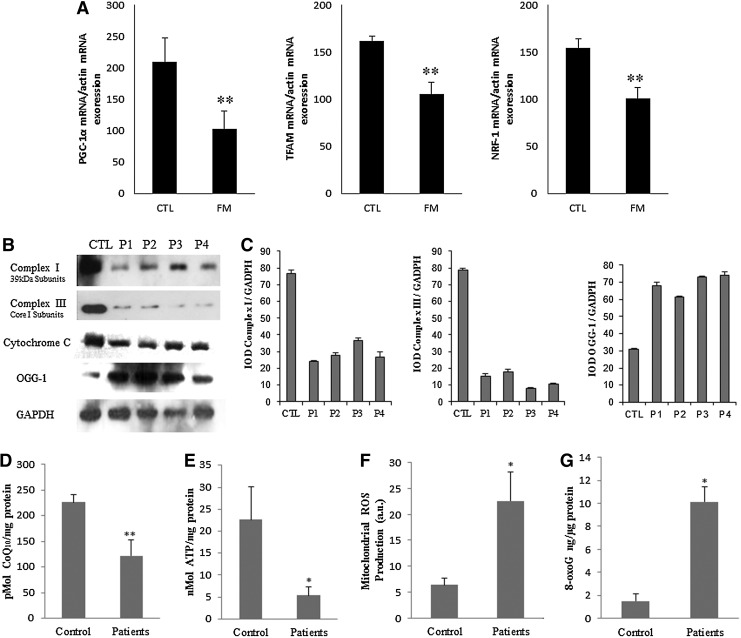
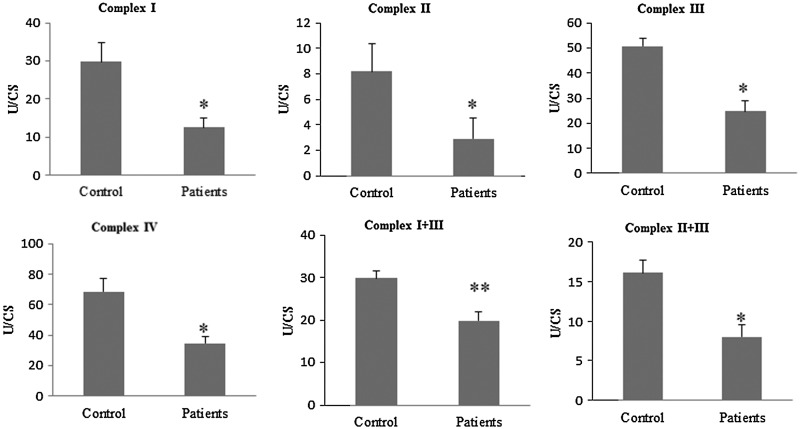
BMCs from FM patients showed a significant downregulation of several genes related to mitochondrial biogenesis (PGC-1α, TFAM, NRF1) (Fig. 1A). In parallel, BMCs showed a reduction of mitochondrial complex protein expression levels (complex I, complex III, and cytochrome c) (Fig. 1B, C) accompanied by reduced activities of the mitochondrial chain complex between 50%–60% patients compared with control (Fig. 2). CoQ10 is a key component of the mitochondrial respiratory chain transferring reducing equivalents from complexes I and II to complex III, and deficiencies of this electron and proton carrier are associated with mitochondrial dysfunction in a variety of human disorders (3). According to this, CoQ10 levels were reduced to 46% of the average control value in BMCs from FM patients (Fig. 1D). Moreover, to determine the effect of mitochondrial dysfunction on cellular bioenergetics, we measured intracellular ATP levels in BMCs from control and FM patients. ATP levels were reduced to 76% respect to controls (p<0.001) (Fig. 1E).
FIG. 1.
Mitochondrial dysfunction and oxidative stress in fibromyalgia blood mononuclear cells (BMCs). n=20 and 30 for control and fibromyalgia groups, respectively. (A) Relative expression of mitocondrial biogenesis genes (mean±SE) determined by quantitative PCR in BMCs from fibromyalgia (FM) patients. (B) Protein expression levels of mitochondrial complex I (39 kDa Subunit), complex III (Core I Subunit), cytochrome c, and 8-oxoguanine glycosylase (OGG-1, a DNA glycosylase enzyme responsible for the excision of 7,8-dihydro-8-oxoguanine (8-oxoG). (C) Protein levels were determined by densitometric analysis (IOD, integrated optical intensity) of three different western blots and normalized to the GADPH signal, using BMCs from four representative FM patients, compared with a pool of five healthy age- and sex-matched control subjects. (D) Coenzyme Q10 (CoQ10) levels in control and FM cells were determined by hexane extraction and high-performance liquid chromatography (HPLC) separation as described in Material and Methods section. (E) ATP levels in control and FM BMCs were analyzed by bioluminescence as described in Material and Methods section. (F, G) Mitochondrial reactive oxygen species (ROS) production and 8-oxoG were analyzed in BMCs from control and FM patients by flow cytometry and EIA kit as described in Material and Methods section. Data represent the mean±SD of three separate experiments. *p<0.001, **p<0.01 between control and FM patients.
FIG. 2.
Mitochondrial complexes enzymatic activities in fibromyalgia BMCs. n=20 and 30 for control and fibromyalgia groups, respectively. Mitochondrial enzymatic activities were determined as described in Material and Methods section. Results (mean±SD) are expressed in U/CS (units per citrate synthase). Data represent the mean±SD of three separate experiments. *p<0.001, **p<0.01 between control and FM patients.
Oxidative stress in BMCs from FM patients
It is well established that mitochondrial dysfunction is associated with induction of ROS production in mitochondria. Furthermore, oxidative stress has been proposed as a relevant event in the pathogenesis of FM showing a significant positive correlation with clinical symptoms (5). To assess oxidative stress in FM, we determined mitochondrial ROS production in BMCs from control and FM patients by using MitoSOX, a mitochondrial superoxide indicator. Mitochondrial superoxide production was significantly increased in BMCs from FM patients respect to controls (p<0.001) (Fig. 1F). Additionally, as an oxidative stress marker, we determined the expression levels of 8-oxoguanine glycosylase (OGG1), a DNA glycosylase enzyme responsible for the excision of 7,8-dihydro-8-oxoguanine (8-oxoG), a mutagenic base byproduct that occurs as a result of exposure of DNA to ROS, and 8-oxoG levels in BMCs from patients. On average, FM patients significantly showed higher levels of OGG1 and 8-oxoG in BMCs from FM patients (Fig. 1B, G).
Inflammasome activation by oxidative stress involved in pain of FM
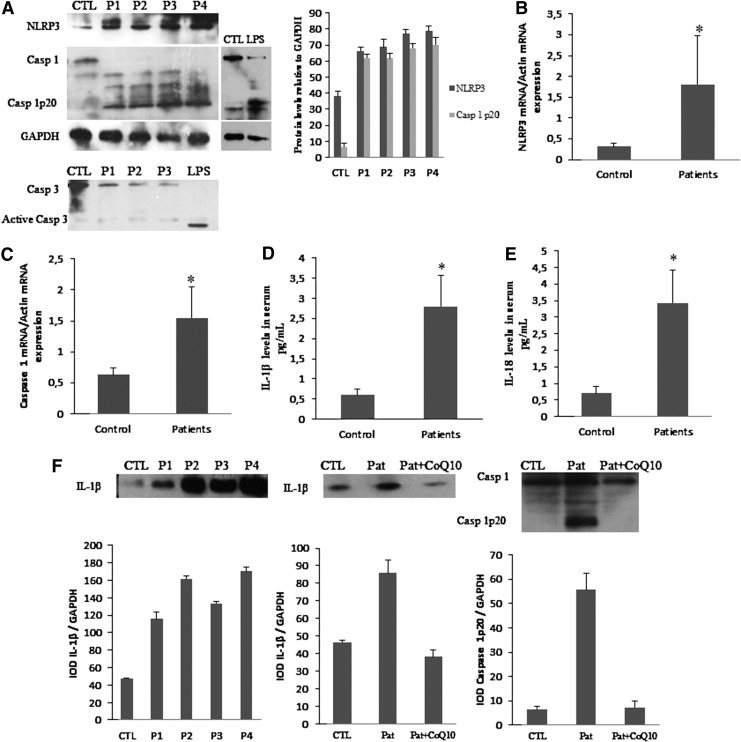
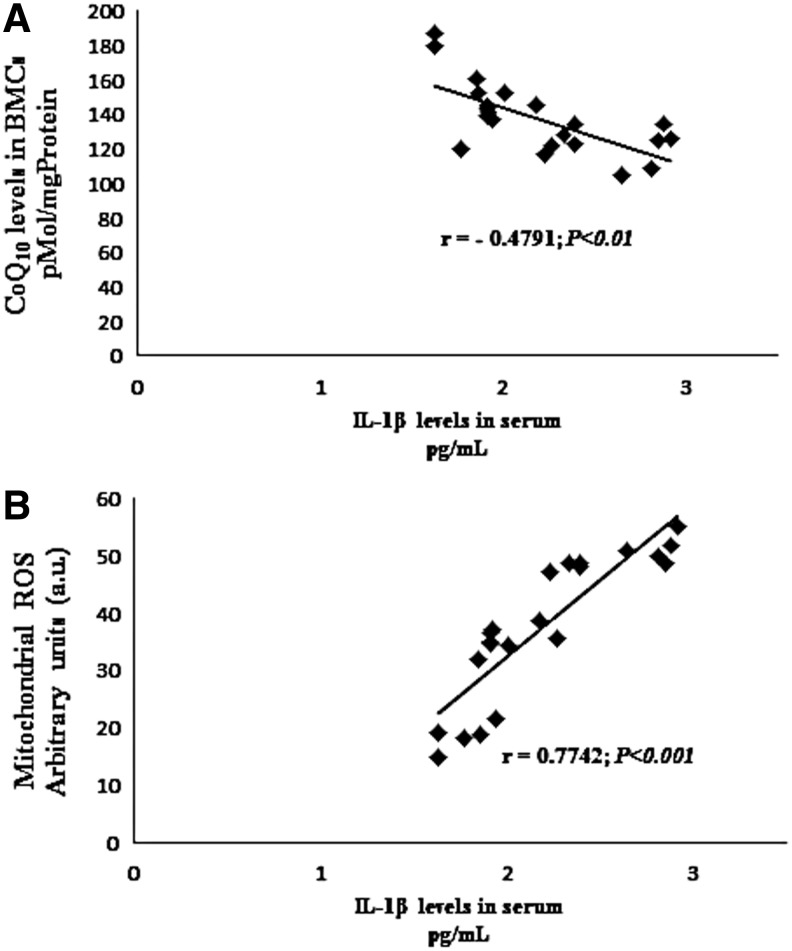
The postinflammatory induction of mitochondrial biogenesis supports metabolic function and cell viability while helping to control inflammation (19). Because mitochondrial biogenesis genes were downregulated in BMCs from FM patients, we analyzed the activation of inflammasome-related proteins. We found increased NLRP3 and caspase-1 gene expression, increased NLRP3 protein expression levels, and caspase-1 cleavage suggesting inflammosome activation (Fig. 3A, B). We have not found activation of caspase 3, so caspase-1 activation was not secondary to activation of cell death pathways in their cell populations. BMCs treated with lipopolysaccharide (LPS) from Escherichia coli were used as a positive control of caspase-1 cleavage. Interestingly, it has been described that oxidized mitochondrial DNA (mtDNA) is a potent activator of the NLRP3 inflammasome (25). Furthermore, other proteins involved in inflammasome and inflammation activation, as IL-1β and IL-18, were increased in serum from FM patients (Fig. 3D, E). BMCs isolated and cultured from FM patients shown increased levels of the IL-1β protein showing that these cells are actively producing the cytokines, and interestingly, BMCs cultured with CoQ10 30 μM showed after 24 h an important reduction of IL-1β and caspase 1 protein levels (Fig. 3F). IL-1β serum levels in FM patients showed a significant negative correlation (p<0.05) with CoQ10 levels and a significant positive correlation (p<0.001) with mitochondrial ROS levels (Fig. 4A, B). The incubation of BMCs isolated from patients with CoQ10 induced a reduction of the IL-1β protein. Furthermore, IL-1β serum levels showed a high positive correlation with pain scale scores of FM patients (Fig. 5A). These data suggest that high IL1-β and IL-18 levels may have a role in the pathophysiology of FM. Interestingly, IL1-β and IL-18 have been described to be involved in the increased sensibility of the sensory receptors, which means they directly cause or at least modulate pain (12, 28).
FIG. 3.
Inflammasome activation in BMCs and proinflammatory cytokines in serum from FM patients. (A) NLRP3 protein levels, caspase 1 cleavage, and caspase 3 cleavage were analyzed by western blotting. We include a positive control of caspase 1 and 3 activation using lipopolysaccharides. Protein levels were determined by densitometric analysis (IOD, integrated optical intensity) of three different western blots and normalized to the GADPH signal, using BMCs from four representative FM patients, compared with a pool of five healthy age- and sex-matched control subjects. (B, C) NLRP3 and caspase 1 cleavage transcript expression levels were determined by real-time quantitative RT-PCR. n=20 for control and n=30 for FM groups, respectively. Data represent the mean±SD of three separate experiments.*p<0.05 between control and FM patients. (D, E) IL-1β and IL-18 levels in serum from control and FM patients were determined by ELISA as described in Material and Methods section. n=20 for control and n=30 for FM groups, respectively. (F) IL-1β protein levels in several patients and IL-1β and caspase 1 activation in patients before and after CoQ10 treatment in BMCs isolated from FM patients and cultured. Data represent the mean±SD of three separate experiments. *p<0.001 between control and FM patients.
FIG. 4.
Correlation of CoQ10 (A), and mitochondrial ROS levels (B) in BMCs from FM patients and IL-1β levels. n=30 for fibromyalgia groups. The correlation was established by calculating correlation coefficients.
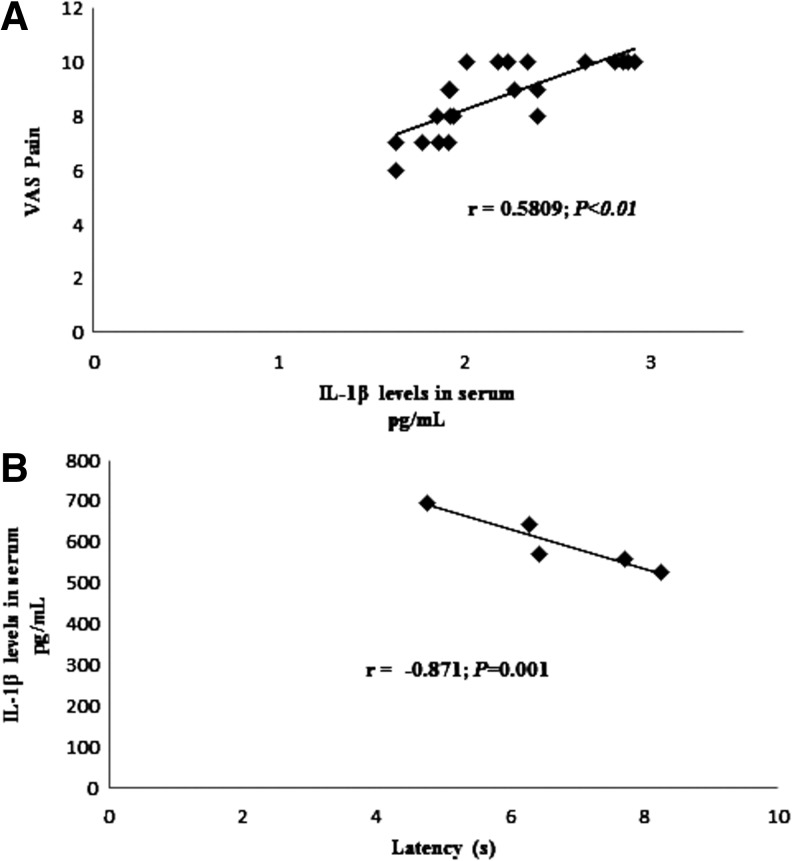
FIG. 5.
Association of IL-1β serum levels and pain scores in FM patients (A) and the mouse model of CoQ10 deficiency (B). n=30 for fibromyalgia groups and n=5 for mice. The strength of the association was established by calculating correlation coefficients.
CoQ10 deficiency induce inflammasome activation in FM
To verify the role of CoQ10 in the inflammatory process in FM, we induced CoQ10 deficiency in BMCs from five healthy controls by inhibiting endogenous CoQ10 biosynthesis with 1 mM p-aminobenzoate (PABA) for 24 h. CoQ10 deficiency was also induced in mice by the subchronic treatment with PABA (twice daily doses of 20 mg/kg/day). The inhibition of CoQ10 (Fig. 6A) in BMCs had a remarkable effect on cellular bioenergetics, inducing a decrease of 57% of intracellular ATP levels, which were restored after 10 μM CoQ10 treatment (Fig. 6B). CoQ10 deficiency in BMCs also induced increased DNA oxidation (OGG1) and inflammasome activation demonstrated by increased expression levels of NLRP3 and caspase-1 cleavage (Fig. 6C, D), associated with a significant increase of IL-1β and IL-18 levels in the culture medium (Fig. 6E, F). Interestingly, 10 μM of CoQ10 supplementation induced a significant reduction in DNA oxidation, inflammosome activation, and IL-1β and IL-18 levels in the culture medium of CoQ10-deficient BMCs (Fig. 3C–F).
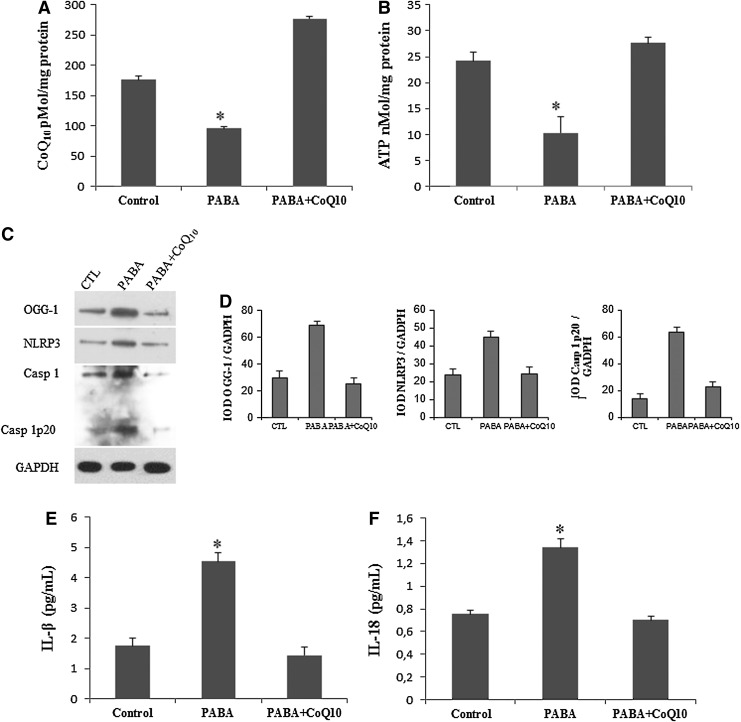
FIG. 6.
CoQ10 deficiency induction activates inflammasome complex in BMCs. (A, B) CoQ10 deficiency and decreased ATP levels induced by 1 mM p-aminobenzoate (PABA) treatment for 24 h in BMCs from five healthy volunteers. (C) Protein expression levels of OGG-1 and NLRP3 and induction of caspase 1 cleavage analyzed by western blotting in a homogenate cell pool of BMCs from five controls. (D) Protein expression levels were determined by densitometric analysis (IOD, integrated optical intensity) of three different western blots and normalized to the GADPH signal. (E, F) IL-1β and IL-18 levels in the culture media of BMCs incubated with PABA for 24 h and analyzed by ELISA as described in Material and Methods section. Data represent the mean±SD of three separate experiments. *p<0.001 between control and PABA, and between PABA and CoQ10.
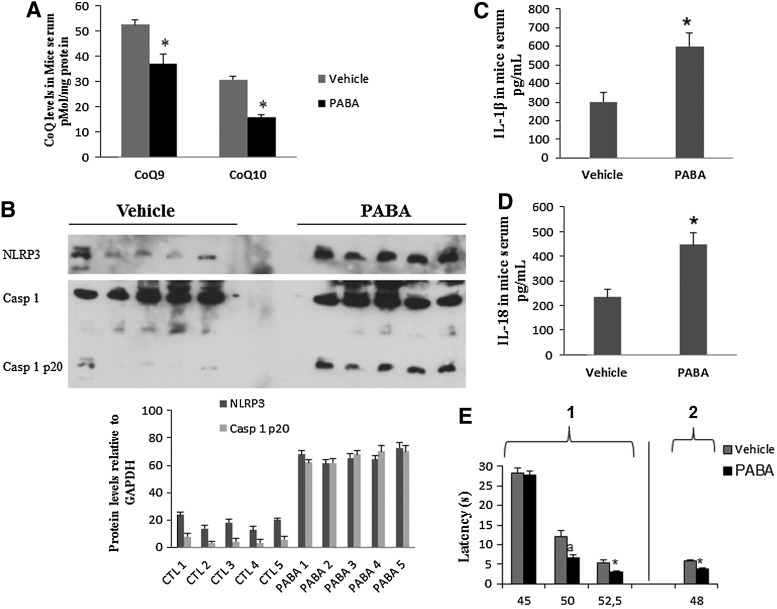
In mice, when compared with vehicle-treated controls, PABA treatment reduced CoQ9 and CoQ10 levels in BMCs by 30% and 49%, respectively, p<0.001 (Fig. 7A). This effect was accompanied by increased expression levels of NLRP3, induction of caspase-1 cleavage in BMCs (Fig. 7B), and increased IL-1β and IL-18 levels in serum (Fig. 7C, D). Single injections of PABA in mice did not evoke any differential effect between vehicle- and drug-injected mice regarding thermal pain (data not shown). However, PABA administration during five consecutive days produced a marked algesia in the hot plate test when thermal stimuli were up of 45°C (hot plate test: for 50°C, latencies were 12.06±1.64 and 6.67±0.61 s for vehicle- and PABA-injected mice, respectively [p=0.013]; for 52.5°C, latencies were 5.2±0.88 and 3.03±0.23 s for vehicle- and PABA-injected mice, respectively [p=0.041]), and for tail flick (latencies were 5.95±0.13 and 3.64±0.26 s for vehicle- and PABA-injected mice, respectively [p<0.001]) (Fig. 7E). Interestingly, IL-1β serum levels showed high positive correlations with pain scale scores in mice (Fig. 5B), similar to those observed in FM patients. Routine serum biochemical parameters were not affected by the subchronic administration of PABA in mice (data not shown).
FIG. 7.
Inflammasome activation induced by CoQ10 deficiency induction in mice. (A). CoQ levels were measured in BMCs isolated from mice treated with vehicle or PABA for 15 days (n=5 per group). *p<0.05; between vehicle and PABA-treated mice. (B) NLRP3 protein expression levels and caspase 1 cleavage were analyzed by western blotting. Protein levels were determined by densitometric analysis (IOD, integrated optical intensity) of three different western blots and normalized to GADPH signal, using BMCs isolated from mice treated with the vehicle or PABA. (C, D) IL-1β and IL-18 in serum levels from mice treated with the vehicle or PABA-treated were determined by ELISA as described in Material and Methods section. *p<0.001 between vehicle and PABA-treated mice. (E) Pain sensitivity in vehicle- and PABA-treated mice was evaluated in the hot plate test (1) at 45°C–52.5°C±0.5°C and with the tail flick test (2) at 45°C±0.5°C. *p<0.05; ap<0.001.
Oral CoQ10 supplementation in FM patients reduces inflammasome activation
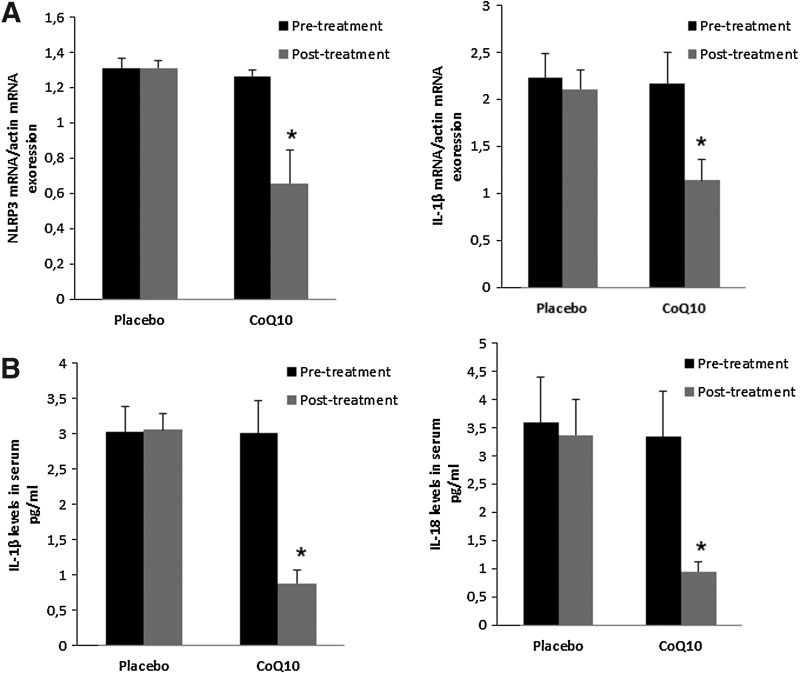
A key obstacle to the successful treatment of FM is the limited effectiveness of the currently available pharmacological therapies. About half of all treated patients experienced only a mild reduction in their symptoms, indicating that many FM patients are in need of alternative therapies (26). In several cases, CoQ10 has shown to be effective in the treatment of FM (6). Identification of pathophysiological mechanisms targeted by CoQ10 treatment in FM is a key research goal. In this respect, we conducted a placebo-controlled, double-blinded trial with 20 patients, to evaluate the effect of CoQ10 in inflammasome gene expression and inflammasome serum markers of FM patients. After CoQ10 supplementation, NLRP3 and IL-1β gene were downregulated. CoQ10 levels from BMCs were restored after CoQ10 treatment (data not shown). According to this, IL-1β and IL-18 serum levels were significantly reduced respect to placebo. (Fig. 8A–E).
FIG. 8.
NLRP3 and IL-1β gene expression and IL-1β and IL-18 levels in FM patients pre- and post-treatment with oral CoQ10 versus placebo. (A) Relative gene expressions of NLRP3 and IL-1β (mean±SE) determined by quantitative PCR in BMCs from FM patients. (B) Serum IL-1β and IL-18 levels in FM patients were measured as described in Material and Methods section. Data represent the mean±SD of three separate experiments. *p<0.01 between FM patients after and before oral treatment.
Discussion
Inhibition of complex I or III of the mitochondrial respiratory chain has been shown to induce ROS production and NLRP3 inflammasome activation (31). In BMCs from FM patients, we detected mitochondrial respiratory chain dysfunction, CoQ10 deficiency, and increased mitochondrial ROS production and OGG-1 expression levels (a marker of oxidized DNA), both activators of inflammasome and inflammation (14, 31). Inflammasome has emerged recently as an unexpected sensor for metabolic danger and stress. Indeed, it has been implicated in the development of major diseases such as gout, type 2 diabetes, and obesity-induced insulin resistance. Moreover, the NLRP3 inflammasome is increasingly suspected of playing a major role in other human pathologies such as cancer, asbestosis, and Alzheimer's disease (15). FM patients have shown increased activation of inflammasome in BMCs and increased serum levels of proinflammatory cytokines, such as IL-1β and IL-18. Interestingly, compensatory activation of mitochondrial biogenesis genes was impaired in BMCs from FM patients. In response to mitochondrial dysfunction and inflammation, cells remove damaged mitochondria by autophagy and, as a compensatory mechanism, induce mitochondrial biogenesis and upregulate antioxidant and counter-inflammatory defense genes (19). Mitophagy in FM patients has been reported (9). However, it has been described that compensatory mechanisms are impaired in BMCs from FM patients that leads to reduced mitochondrial mass, reduced antioxidants levels (1, 2, 18), and an inflammatory damage predisposition. Inflammation has been shown to be associated with FM symptoms by high positive correlations between IL-1β and IL-18 serum levels and pain scores suggesting an inflammatory component in the induction of pain. Despite this, there are discrepancies regarding the pathological role of IL-1β serum levels in FM (4, 22, 24, 29). Feng and coworkers have shown the implication of MEFV, which encodes pyrin, a major regulator of the inflammasome platform controlling caspase-1 activation and IL- 1β (10); so, this preliminary data from Feng et al., are according to our results. Respect to inflammatory cytokine involvement in FM pathogenesis, we have recently demonstrated a mitochondrial dysfunction-dependent increase of tumor necrosis factor (TNF)-alpha serum levels in FM patients, which was reduced by CoQ10 treatment (8).
According to our results, the negative correlation between IL-1β and IL-18 levels and CoQ10 levels and positive correlation with mitochondrial ROS levels and pain scale scores supports the hypothesis that inflammation in FM is dependent on mitochondrial dysfunction. To our knowledge, this is the first report describing inflammasome activation and its association with increased levels of proinflammatory cytokines in FM patients. Furthermore, this study has nominated several new parameters that could be used as diagnostic biomarkers in FM. Their further validation (alone or in combination) could help in creating objective diagnostic testing in FM. However, despite our results about inflammasome in FM, we think that inflammasome contributes in the pathophysiology of this disease accompanied by other inflammasome nondependent inflammatory events, such as IL-6, IL-8, or TNF-alpha (4, 22, 24). These inflammatory parameters have been associated through NF-kappaB. Immunohistochemical studies in FM tissues revealed a stronger expression of NF-kappaB in muscle, and IL-6, IL-8, and TNF-alpha are NF-kappaB-dependent proinflammatory cytokines (8).
In summary, we have shown (in vitro and in vivo) the involvement of CoQ10 deficiency in the pathological process of inflammasome activation and release of proinflammatory cytokines. Since pathological processes can be reversed by CoQ10 supplementation, we propose that CoQ10 could be a suitable therapy for FM patients. Larger controlled clinical trials are needed to provide data on the effectiveness of CoQ10 in FM.
Materials and Methods
Ethical statements
Written informed consent and the approval of the ethics committee of the University of Seville were obtained, according to the principles of the Declaration of Helsinki. The study was conducted in compliance with the International Conference on Harmonization Good Clinical Practice (ICH-GCP) guidelines and registered controlled-trials.com (ISRCTN 21164124).
Pain studies in mice were performed in accordance with the European Union guidelines (86/609/EU) and Spanish regulations for the use of laboratory animals in chronic experiments (BOE 67/8509-12, 1988). All experiments were approved by the local institutional animal care committee.
Patients
Briefly, 30 patients from the register of the Andalusian Federation of Fibromyalgia (ALBA ANDALUCÍA) and 20 healthy matched controls were enrolled on the study, having previously obtained informed consent and the approval of the local ethics committee. The inclusion criterion was FM, based on current American College of Rheumatology (ACR) diagnostic criteria (29), and diagnosed 2 to 3 years ago. The clinical characteristics of each group are shown in Table 1. Exclusion criteria were acute infectious disease within the previous 3 weeks; past or present neurological, psychiatric, metabolic, autoimmune, allergy-related, dermal, or chronic inflammatory disease; undesired habits (e.g., smoking, alcohol); medical conditions that required glucocorticoid treatment, analgesics, or antidepressant drugs; past or current substance abuse or dependence; pregnancy or current breastfeeding. Twenty healthy volunteers (5 males, 15 females) were included in the study and matched with the recruited female FM patients for age range, gender, ethnicity, and demographic features (completion of at least 9 years of education and member of the middle socioeconomic class). Healthy controls had no signs or symptoms of FM and had not taken any medication for at least 3 weeks before commencing the study. None of the patients or controls had taken any drug or vitamin/nutritional supplements during the 3 weeks before blood sample collection. All patients and controls followed a standard balanced diet (carbohydrate 50%–60%, protein 10%–20%, and fat 20%–30%) for 3 weeks before blood collection, as established by a diet program. Clinical data were obtained by physical examination and the subjects were evaluated using the fibromyalgia impact questionnaire (FIQ) and the visual analogues scale (VAS). Tender points were identified by digital pressure at the 18 locations recommended by ACR, which included a minimum of 11 out of 18. Heparinized and coagulated blood samples were collected from patients and controls after 12 h of fasting, centrifuged at 3800 g for 5 min, and the plasma and serum was stored at −80°C until testing. Serum biochemical parameters were assayed by routine analytical methods. Routine laboratory tests yielded normal results for glucose, uric acid, creatine kinase, aspartate aminotransferase, alanine aminotransferase, cholesterol, and triglycerides (Table 2)
Table 1.
Anthropometric and Symptomatic Parameters in Healthy Volunteers and Fibromyalgia Patients
| Parameter | Controls | FM Patients |
|---|---|---|
| Age (years) | 45.5±6.1 | 46.1±8 |
| Tender points | — | 14.5±1.8 |
| Disease duration (years) | — | 8.1±3.3 |
| Sex (male/female) | 5\15 | 5\25 |
| BMI (kg/m2) | 23.2±2.5 | 22.9±1.2 |
| FIQ total score, range 0–80 | 2.7±1.5 | 56.6±8.3a |
| Pain | 0.6±0.2 | 6.9±2.1b |
| Fatigue | 1.2±0.5 | 7.1±1.2a |
| Morning tiredness | 1±0.3 | 5.5±1b |
| Stiffness | 0.4±0.1 | 6.2±2.2b |
| Anxiety | 1±0.5 | 5.8±1.2b |
| Depression | 0.2±0.6 | 5.6±1.2a |
| VAS pain total score 0–10 | 0.7±0.2 | 7.5±2.1a |
n=20 and 30 for control and fibromyalgia groups, respectively.
p<0.001.
p<0.01.
BMI, body mass index; FIQ, fibromyalgia impact questionnaire; VAS, visual analogical scale.
To evaluate the therapeutic role of CoQ10 in inflammasome activation in FM, we conducted a placebo-controlled, double-blinded trial with 20 patients, to evaluate the effect of CoQ10 in inflammasome gene expressions of FM patients and inflammasome serum markers. The study protocol was registered (ISRCTN 21164124) and reviewed and approved by the Ethics Committee of the University of Sevilla. All study participants provided written informed consent before initiation of the study. This study was conducted in compliance with the Declaration of Helsinki, and all International Conference on Harmonisation Good Clinical Practice Guidelines.
Intervention
Subjects who met the enrolment criteria were randomized in a double-blind fashion, according to a 1:1 ratio, to one of the two treatment groups (CoQ10 or placebo). All subjects receiving CoQ10 (purchased from Pharma Nord) were given, in soft gel capsules for 40 days (300 mg/day CoQ10 divided into three doses), and all subjects receiving placebo were given a matching placebo. The duration of the treatment and dose of CoQ10 were selected accordingly to the preliminary studies (6, 8).
Isolation of BMCs
Peripheral BMCs (lymphocytes and monocytes) from all patients were purified from heparinized blood by isopycnic centrifugation using Histopaque-1119 and Histopaque-1077 (Sigma Chemical Co.). BMCs were cultured at 37°C in a 5% CO2 atmosphere in the RPMI-1640 medium supplemented with L-glutamine, an antibiotic/antimycotic solution (Sigma Chemical Co.), and 10% fetal bovine serum. The number and subgroup distribution of BMCs (monocytes and lymphocytes) in FM patients were in the normal range (data not shown). We use in all experiments, BMCs from all patients except in western blot where we use BMCs from several representative patients. In several experiments, BMCs from FM patients were cultured with CoQ10 30 μM during 24 h.
A positive control of activated caspase 1 was induced in BMC control by LPS 1 μg/ml LPS for 2 h.
Partial CoQ10 deficiency was induced by inhibiting endogenous biosynthesis in control BMCs by treatment with PABA (Sigma), a competitive inhibitor of polyprenyl-4-hydroxybenzoate transferase (27). To achieve this, cells were cultured for 24 h in the presence of 1 mM PABA and PABA+10 μM CoQ10.
IL-1β and IL-18 levels
IL-1β (GenWay) and IL-18 (Biosensis) levels in serum or culture media were assayed in duplicates by commercial ELISA kits.
Mitochondrial enzyme activities
Activities of NADH:coenzyme Q1 oxidoreductase (complex I), succinate deshydrogenase (complex II), cytochrome c oxidase (complex IV), ubiquinol:cytochrome c oxidoreductase (complex III), succinate:cytochrome c reductase (complex II+complex III), and citrate synthase (CS) were determined in homogenate extracts using previously described spectrophotometric methods (23). Results are expressed as Units/CS (mean±SD). Proteins of homogenates were analyzed by the Lowry procedure (13).
ATP levels
ATP levels were determined by a bioluminescence assay using an ATP determination kit from Invitrogen-Molecular Probes according to the instructions of the manufacturer.
Oxidative stress
Mitochondrial ROS generation in BMCs was assessed by MitoSOX™ red, a red mitochondrial superoxide indicator. MitoSOX Red is a novel fluorogenic dye recently developed and validated for highly selective detection of superoxide in the mitochondria of live cells (17). The MitoSOX Red reagent is live-cell permeant and is rapidly and selectively targeted to the mitochondria. Once in the mitochondria, the MitoSOX Red reagent is oxidized by superoxide and exhibits red fluorescence.
DNA repair enzyme 8-oxoguanine DNA glycolase-1 (OGG-1; Novus Biologicals, Inc.) levels were analyzed by western blotting.
The 8-oxoG levels in BMCs from patients were determined using a commercial EIA kit from Cayman Chemical.
Immunoblotting
Western blotting was performed using standard methods. After protein transfer, the membrane was incubated with various primary antibodies diluted 1:1000, and then with the corresponding secondary antibody coupled to horseradish peroxidase at a 1:10000 dilution. Specific protein complexes were identified using the Immun Star HRP substrate kit (Biorad Laboratories, Inc.).
Quantification of CoQ levels
The CoQ content in BMCs was analyzed by high-performance liquid chromatography (HPLC) (Beckmann 166–126 HPLC) with ultraviolet detection (275 nm), as described previously (15).
Reagents
PABA and LPS were purchased from Sigma Chemical Co. Monoclonal antibodies specific for oxidative phosphorylation, complex I (39 kDa subunit), and complex III (core 1 subunit) were purchased from Invitrogen/Molecular Probes. The anti-GAPDH monoclonal antibody from Calbiochem-Merck Chemicals Ltd. The anti-NLRP3 antibody from Adipogen. Anti-active caspase-1 was obtained from Cell Signaling Technology. A cocktail of protease inhibitors (complete cocktail) was purchased from Boehringer Mannheim. The Immun Star HRP substrate kit was from Bio-Rad Laboratories, Inc.
Animals and drug administration
Eight-week-old male Swiss mice weighing 25–30 g were maintained on a 12-h light/12-h dark cycle. Behavioral studies were performed in accordance with the European Union guidelines (86/609/EU) and Spanish regulations for the use of laboratory animals in chronic experiments (BOE 67/8509-12, 1988). All experiments were approved by the local institutional animal care committee. PABA, a competitive inhibitor of polyprenyl-4-hydroxybenzoate transferase (Coq2p), an essential enzyme in CoQ10 biosynthesis mediating the conjugation of 4-hydroxybenzoate with the completed polyprenyl side chain (26), was dissolved in saline (vehicle) and intraperitoneally administered at a dose of 20 mg/Kg/day for 15 days. Behavioral tests were performed 5 days after the first drug administration. After testing, mice were anesthetized with CO2 and sacrificed by decapitation. Blood samples were collected for immediate biochemical analysis and BMCs were isolated. Serum samples were frozen at −80°C for further analyses.
Behavioral assays
Behavioral analyses were performed in a testing room with homogeneous noise and light levels. The testing apparatus was cleaned with 70% ethanol (Panreac Química S.A.U) between trials to eliminate any influence of animal odor on the exploratory behavior.
Pain assay
For the hot plate test, a glass cylinder (16 cm high, 16 cm in diameter) was used to constrain the mice to the heated surface of the plate. The plate surface was maintained at 45°C–52.5°C±0.5°C and the latency to commence paw licking was measured, with a cutoff of 30 s. For the tail flick test, a thermostatic water bath was maintained at a temperature of 48°C±0.5°C and the latency of the tail reflex measured, with a cutoff for responses of 15 s.
Serum markers
Biochemical parameters were determined in serum (glucose, triglyceride, cholesterol, uric acid, aspartate aminotransferase, alanine aminotransferase, and creatine kinase) using commercial kits from Randox Laboratories.
Lipid peroxidation
Lipid peroxidation in cells was determined by analyzing the accumulation of lipoperoxides using a commercial kit from Cayman Chemical. TBARS are expressed in terms of malondialdehyde (MDA) levels. In these assays, an MDA standard is used to construct a standard curve against which unknown samples can be plotted.
Real-time quantitative PCR
The expression of NLRP3 and caspase 1 gene was analyzed by SYBR Green quantitative PCR using mRNA extracts of BMCs from patients and controls. The thermal cycling conditions used were denaturation at 95°C for 20 s, alignment at 54°C for 20 s, and elongation at 72°C for 20 s, for 40 cycles. NLRP3 primers were 5′- GGAGAGACCTTTATGAGAAAGCAA -3′ (forward) and 5′- GCTGTCTTCCTGGCATATCACA -3′ (reverse), caspase 1 were 5′- CCGAAGGTGATCATCATCCA -3′ (forward) and 5′- ATAGCATCATCCTCAAACTCTTCTG -3′ (reverse), and IL-1β were 5′- TTACAGTGGCAATGAGGATGAC -3′ (forward) and 5′- GTCGGAGATTCGTAGCTGGAT-3′ (reverse). We used a second pair of beta-actin primers as an internal control: forward, 5′- CCA GAT CAT GTT TGA GAC C-3′ and reverse, 5′- ATG TCA CGC ACG ATT TCC C-3′. All reactions were performed in duplicate. Reaction mixtures, without RNA, were used as negative controls in each run.
Statistical analysis
Statistical analyses were performed using the SPSS package for Windows (SPSS). Unless otherwise indicated, data represent the mean±SEM. The unpaired Student's t test was used to evaluate the significance of differences between groups, accepting p<0.05 as the level of significance. Statistical analyses included Pearson's correlations between IL-1β and IL-18 respect to VAS, CoQ10, and mitochondrial ROS. Two-way analysis of variance was used to compare the behavioral results from animals treated with vehicle alone or with PABA. Chi-squared tests were used for statistical analysis in cases in which qualitative variables were compared.
Abbreviations Used
- 8-oxoG
7,8-dihydro-8-oxoguanine
- ACR
American College of Rheumatology
- BMCs
blood mononuclear cells
- BMI
body mass index
- CoQ10
coenzyme Q10
- CS
citrate synthase
- FIQ
fibromyalgia impact questionnaire
- FM
fibromyalgia
- HPLC
high-performance liquid chromatography
- IL-(1β,18)
interleukins
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- mtDNA
mitochondrial DNA
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- OGG-1
8-oxoguanine glycosylase
- PABA
P-aminobenzoate
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- VAS
visual analogues scale
Acknowledgments
This work has been supported by the FIS PI10/00543 grant, Fondo Europeo de Desarrollo Regional (FEDER-Unión Europea), SAS 111242 grant, Servicio Andaluz de Salud-Junta de Andalucía, Proyecto de Investigación de Excelencia de la Junta de Andalucía CTS-5725, Federación Andaluza de Fibromialgia y Fatiga Crónica (ALBA Andalucía), and FOICAM (Spanish research association). We thank Dr. Jaime Carvajal (CABD-CSIC-Universidad Pablo de Olavide) for critical reading and editing of the manuscript.
Author Disclosure Statement
All the authors declare that no conflicts of interest exist for any of them.
References
- 1.Altindag O. and Celik H. Total antioxidant capacity and the severity of the pain in patients with fibromyalgia. Redox Rep 11: 131–135, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, and Erdogan C. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int 25: 188–190, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Battino M, Fato R, Parenti-Castelli G, and Lenaz G. Coenzyme Q can control the efficiency of oxidative phosphorylation. Int J Tissue React 12: 137–144, 1990 [PubMed] [Google Scholar]
- 4.Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, Ciapparelli A, Dell'Osso L, and Bombardieri S. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol 25: 225–230, 2007 [PubMed] [Google Scholar]
- 5.Cordero MD, Alcocer-Gómez E, Cano-García FJ, De Miguel M, Carrión AM, Navas P, and Sánchez Alcázar JA. Clinical symptoms in fibromyalgia are better associated to lipid peroxidation levels in blood mononuclear cells rather than in plasma. PLoS One 6: e26915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordero MD, Alcocer-Gómez E, de Miguel M, Cano-García FJ, Luque CM, Fernández-Riejo P, Fernández AM, and Sánchez-Alcazar JA. (Coenzyme Q(10): a novel therapeutic approach for Fibromyalgia? case series with 5 patients. Mitochondrion 11: 623–625, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Cordero MD, De Miguel M, Moreno Fernández AM, Carmona López IM, Garrido Maraver J, Cotán D, Gómez Izquierdo L, Bonal P, Campa F, Bullon P, Navas P, and Sánchez Alcázar JA. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromialgia patients: implications in the pathogenesis of the disease. Arthritis Res Ther 12: R17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordero MD, Díaz-Parrado E, Carrión AM, Alfonsi S, Sánchez-Alcazar JA, Bullón P, Battino M, and de Miguel M. Is inflammation a mitochondrial dysfunction-dependent event in Fibromyalgia? Antioxid Redox Signal 18: 800–807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordero MD, Moreno-Fernández AM, deMiguel M, Bonal P, Campa F, Jiménez-Jiménez LM, Ruiz-Losada A, Sánchez-Domínguez B, Sánchez Alcázar JA, Salviati L, and Navas P. Coenzyme Q10 distribution in blood is altered in patients with fibromyalgia. Clin Biochem 42: 732–735, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Zhang Z, Li W, Shen X, Song W, Yang C, Chang F, Longmate J, Marek C, St Amand RP, Krontiris TG, Shively JE, and Sommer SS. Missense mutations in the MEFV gene are associated with fibromyalgia syndrome and correlate with elevated IL-1beta plasma levels. PLoS One 4: e8480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, and Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58: 26–35, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li WW, Guo TZ, Liang D, Shi X, Wei T, Kingery WS, and Clark JD. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain 147: 277–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, and Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 14.Mabley JG, Pacher P, Deb A, Wallace R, Elder RH, and Szabó C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J 19: 290–292, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Menu P. and Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol 166: 1–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montero R, Sánchez-Alcázar JA, Briones P, Hernández AR, Cordero MD, Trevisson E, Salviati L, Pineda M, García-Cazorla A, Navas P, and Artuch R. Analysis of coenzyme Q10 in muscle and fibroblasts for the diagnosis of CoQ10 deficiency syndromes. Clin Biochem 41: 697–700, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, and Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozgocmen S, Ozyurt H, Sogut S, Akyol O, Ardicoglu O, and Yildizhan H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: etiologic and therapeutic concerns. Rheumatol Int 26: 598–603, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Piantadosi CA. and Suliman HB. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochim Biophys Acta 1820: 532–541, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinzii CM, López LC, Von-Moltke J, Naini A, Krishna S, Schuelke M, Salviati L, Navas P, DiMauro S, and Hirano M. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. FASEB J 22: 1874–1885, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Hernandez A, Cordero MD, Salviati L, Artuch R, Pineda M, Briones P, Gómez Izquierdo L, Cotán D, Navas P, and Sánchez-Alcázar JA. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 5: 19–32, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Ross RL, Jones KD, Bennett RM, Ward RL, Druker BJ, and Wood LJ. (2010). Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol J 3: 9–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, and Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228: 35–51, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Salemi S, Rethage J, Wollina U, Michel BA, Gay RE, Gay S, and Sprott H. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol 30: 146–150, 2003 [PubMed] [Google Scholar]
- 25.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, and Arditi M. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 36: 401–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staud R. Pharmacological treatment of fibromyalgia syndrome: new developments. Drug 70: 1–14, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Turunen M, Olsson J, and Dallner G. Metabolism and function of coenzyme Q. Biochem Biophys Acta 1660: 171–199, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Verri WA, Jr., Cunha TM, Parada CA, Poole S, Liew FY, Ferreira SH, and Cunha FQ. Antigen-induced inflammatory mechanical hypernociception in mice is mediated by IL-18. Brain Behav Immun 21: 535–543, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wallace DJ. Is there a role for cytokine based therapies in fibromyalgia. Curr Pharm Des 12: 17–22, 2006 [PubMed] [Google Scholar]
- 30.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, and Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160–172, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Zhou R, Yazdi AS, Menu P, and Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]