Abstract
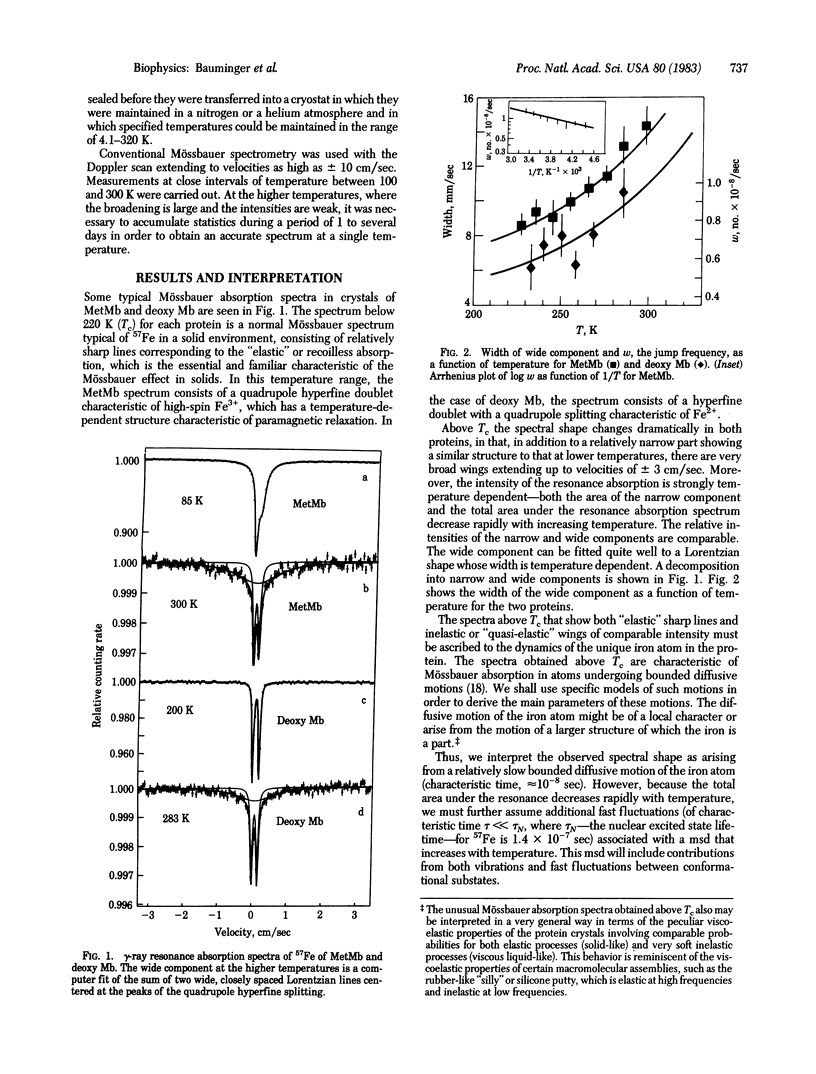
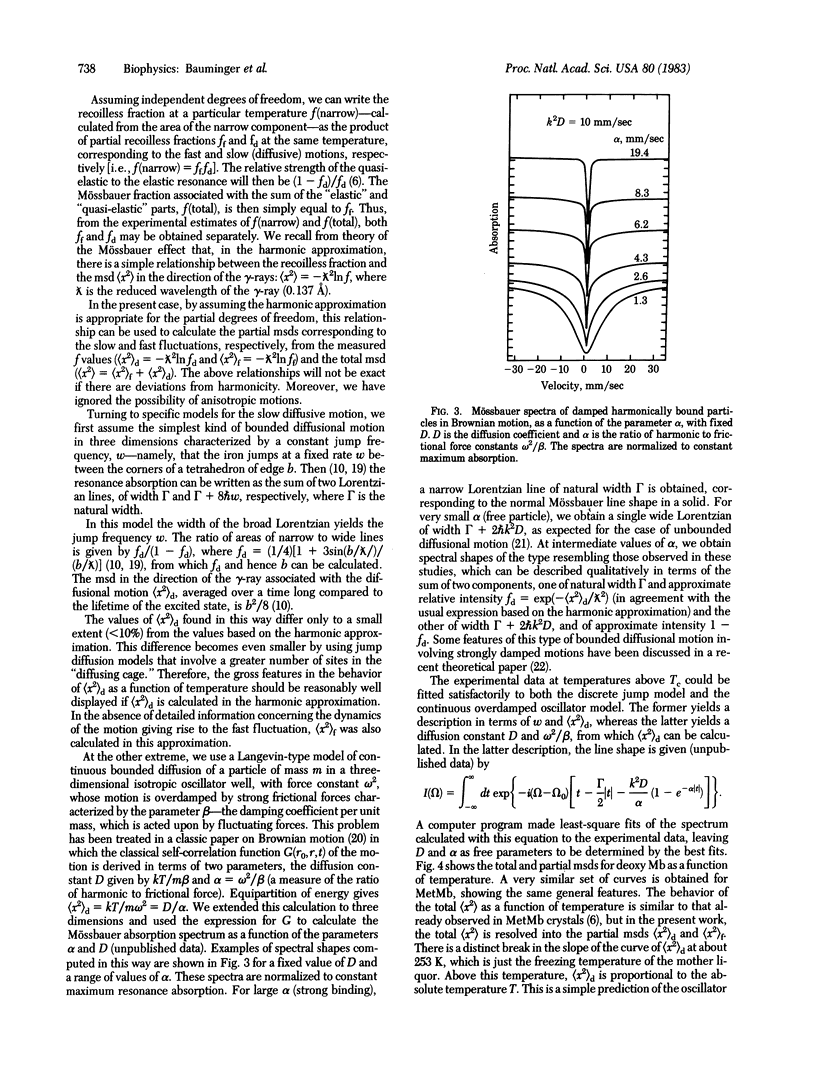
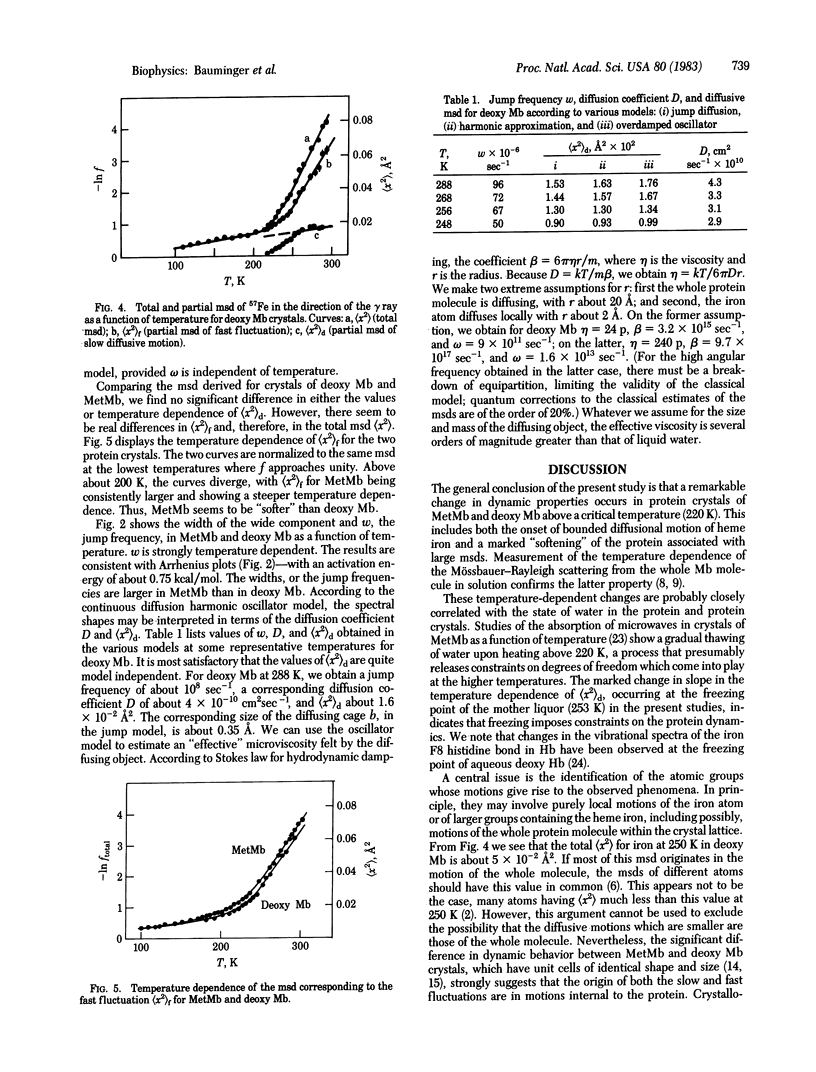
The 57Fe gamma-ray resonance absorption spectra have been measured in crystals of metmyoglobin and deoxymyoglobin over a wide range of temperatures. Above a critical temperature common to both proteins (220 K), the dynamics of heme iron display a dramatic change, in that two kinds of thermal fluctuations come into play--a fast fluctuation associated with a steep decrease of the total fluctuation of characteristic time 10(-8) sec, associated with bounded diffusive motion. By using both discrete jump and continuous diffusion models, the latter based on the Brownian motion of an overdamped harmonic oscillator, the essential parameters of the iron motion (mean square displacement and jump frequency or diffusion constant) can be derived as a function of temperature. Thus, for deoxy Mb at 288 K, the mean square displacement for the fast fluctuation is about 6 X 10(-2) A2 and for the diffusive motion is 1.6 X 10(-2) A2; the diffusion constant is 4 X 10(-10) cm2/sec. The diffusive process is associated with an activation energy of about 0.75 kcal/mol. Although the same general kinds of phenomena are observed in crystals of MetMb and deoxy Mb, significant differences in behavior are found, which suggest that the main dynamical phenomenon observed reflects internal large-scale motions of the protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artymiuk P. J., Blake C. C., Grace D. E., Oatley S. J., Phillips D. C., Sternberg M. J. Crystallographic studies of the dynamic properties of lysozyme. Nature. 1979 Aug 16;280(5723):563–568. doi: 10.1038/280563a0. [DOI] [PubMed] [Google Scholar]
- Eden D., Matthew J. B., Rosa J. J., Richards F. M. Increase in apparent compressibility of cytochrome c upon oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):815–819. doi: 10.1073/pnas.79.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Nagai K., Tsubaki M. Assignment of the Fe-Nepsilon (His F8) stretching band in the resonance Raman spectra of deoxy myoglobin. FEBS Lett. 1979 Aug 15;104(2):376–378. doi: 10.1016/0014-5793(79)80856-x. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Simon S. R. Structural changes at the heme induced by freezing hemoglobin. Science. 1981 Aug 7;213(4508):657–659. doi: 10.1126/science.7256263. [DOI] [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Mössbauer R. L., Goldanskii V. I. Dynamics of metmyoglobin crystals investigated by nuclear gamma resonance absorption. J Mol Biol. 1981 Feb 5;145(4):825–833. doi: 10.1016/0022-2836(81)90317-x. [DOI] [PubMed] [Google Scholar]
- Shaitan K. V., Rubin A. B. Teoriia éffekta Messbauéra v belkakh. Biofizika. 1980 Sep-Oct;25(5):796–802. [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]


