Abstract
Osteoarthritis affects the whole joint structure with progressive changes in cartilage, menisci, ligaments and subchondral bone, and synovial inflammation. Biomarkers are being developed to quantify joint remodelling and disease progression. This article was prepared following a working meeting of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis convened to discuss the value of biochemical markers of matrix metabolism in drug development in osteoarthritis. The best candidates are generally molecules or molecular fragments present in cartilage, bone or synovium and may be specific to one type of joint tissue or common to them all. Many currently investigated biomarkers are associated with collagen metabolism in cartilage or bone, or aggrecan metabolism in cartilage. Other biomarkers are related to non-collagenous proteins, inflammation and/or fibrosis. Biomarkers in osteoarthritis can be categorised using the burden of disease, investigative, prognostic, efficacy of intervention, diagnostic and safety classification. There are a number of promising candidates, notably urinary C-terminal telopeptide of collagen type II and serum cartilage oligomeric protein, although none is sufficiently discriminating to differentiate between individual patients and controls (diagnostic) or between patients with different disease severities (burden of disease), predict prognosis in individuals with or without osteoarthritis (prognostic) or perform so consistently that it could function as a surrogate outcome in clinical trials (efficacy of intervention). Future avenues for research include exploration of underlying mechanisms of disease and development of new biomarkers; technological development; the ‘omics’ (genomics, metabolomics, proteomics and lipidomics); design of aggregate scores combining a panel of biomarkers and/or imaging markers into single diagnostic algorithms; and investigation into the relationship between biomarkers and prognosis.
Keywords: Osteoarthritis, Outcomes research, Inflammation
Introduction
Osteoarthritis manifests as alteration of the whole joint structure, including progressive degradation of cartilage, menisci and ligaments, synovial inflammation and changes to the subchondral bone.1 The diagnosis of osteoarthritis is currently based on radiographic criteria (eg, joint space width) and clinical symptoms (eg, pain and loss of function).1 The evaluation of new disease-modifying osteoarthritis drugs (DMOADs) is performed on the same basis, since the regulatory bodies currently require evidence for an impact on radiographic joint space narrowing (JSN) and an impact on symptoms.2 3 However, the limitations of radiography (eg, technical issues, precision and sensitivity)4 have led to research into alternative parameters for monitoring osteoarthritis that could serve as biomarkers in drug development. The National Institutes of Health (NIH) defines a biomarker as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.’5
Imaging markers, from magnetic resonance and ultrasound, may be useful biomarkers in the evaluation of osteoarthritis and in drug development in the field.4 6 7 A promising outcome is the use of quantitative MRI to assess changes in cartilage volume or thickness. However, widespread use of MRI is limited by cost, availability and the absence of a validated international score. These imaging markers are beyond the scope of this review and the use of MRI is covered in a separate European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO).8
Another attractive alternative is the measurement of biochemical markers in blood, urine or synovial fluid samples, which could reflect dynamic and quantitative changes in joint remodelling and therefore disease progression. In the setting of osteoarthritis, a biochemical marker could be either an effector molecule (ie, an operator of joint damage), the result of joint damage, or both, as in the case of cartilage extracellular matrix fragments, such an hyaluronan, that serve as both biomarkers and stimuli of the innate immune chronic wound healing response in the osteoarthritic joint.9 Such biomarkers may be useful in early phase evaluation of the efficacy and safety of DMOADs and may also find applications in the diagnosis of disease, the assessment of severity and the risk of progression and the monitoring of health status in the general population.5 Insofar as current diagnostic methods in osteoarthritis combine radiographic and clinical signs, the disease is definitively diagnosed only when destruction of joint tissue is irreversible. Hence, an important characteristic of a new biochemical marker should also be that it can detect early osteoarthritis. Candidate biomarkers in osteoarthritis should also have proven validity, reproducibility and predictive value, and there should be ample information on how they relate to processes in the joint and clinical endpoints (such as structural damage, pain or dysfunction and/or joint replacement). Despite much active research into biomarkers in osteoarthritis,10–16 no single biomarker stands out as the gold standard or is sufficiently well validated and recognised for systematic use in drug development. In the light of this situation, the ESCEO convened a working meeting in October 2012 with a group of experts in the field to discuss the value of biomarkers in drug development in osteoarthritis, with a focus on the potential avenues for future research. This article is a summary of these discussions, and the manuscript was revised by the participants of the meeting, as well as additional invited authors, who provided further substantial input.
Methods
Relevant articles, reviews and abstracts were identified through a PubMed/MEDLINE and EMBASE search of English language articles published between 1994 and September 2012. The initial search strategy included the terms: osteoarthritis, biomarker, biological marker, CTX-I, CTX-II, CPII, C2/C, NTX-I, PINP, OC, COMP, CS846, PIIANP, HA, PIIINP, leptin, adiponectin, visfatin, leptin-receptor, adiponectin receptor, interleukin, C-terminal and N-terminal telopeptides of collagen I, aminoterminal propeptide of type I procollagen, osteocalcin, cartilage oligomeric matrix protein, chondroitin sulphate 846, soluble receptor for advanced glycation endproducts (sRAGE), RAGE, type IIA collagen N-propeptide, matrix metalloproteinase, cartilage glycoprotein 39 (YKL-40), uric acid, prostaglandin, bone turnover, drug effect, cartilage articular/drug effect, outcome assessment and treatment outcome. The initial search yielded 149 items. Separate subsearches were also performed using a cross-search of the above terms combined, as well as the reference lists of the selected articles and the presentations made during the ESCEO meeting. Overall, 66 relevant items were selected by the authors according to their quality and pertinence for discussion by the ESCEO working group.
Candidate biomarkers in osteoarthritis
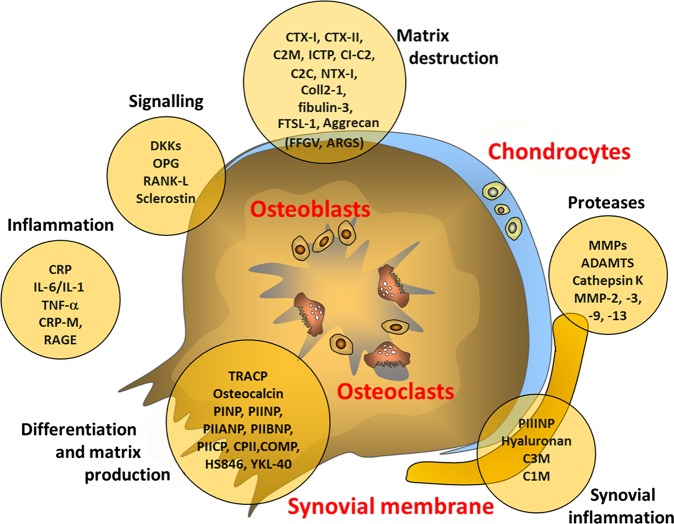
The best candidates for biomarkers in osteoarthritis are most likely to be structural molecules or fragments linked to cartilage, bone or synovium and may be specific to one type of joint tissue or common to them all. They may represent tissue degradation or tissue synthesis and may be measured in synovial fluid, blood or urine. Box 1 and figure 1 outline a selection of the various biomarkers that are currently being tested in osteoarthritis.10–20 Figure 1 highlights the different compartments of the joint and potential biomarkers, all of which require careful investigation. The cartilage, bone and synovial tissues all have different potential biomarkers for investigation, each of which has their own strengths and weaknesses. Discussion of each of the individual markers can be found elsewhere in the literature.18–20 Many are associated with the metabolism of collagen in cartilage (type II collagen) or subchondral bone (type I collagen) or the metabolism of aggrecan in cartilage. Together, type II collagen and aggrecan constitute the most abundant proteins in the cartilage matrix, making them promising targets for research. In addition, there are biomarkers related to a range of non-collagenous proteins that have a role in other metabolic pathways in the joint, including glycoproteins, proteoglycans, metalloproteinases and advanced glycation endproducts, as well as hyaluronan, which is a constituent of both cartilage and synovium. Finally, there are a number of biomarkers associated with other processes, such as inflammation or fibrosis.
Box 1. Selected biomarkers currently being investigated for the evaluation of osteoarthritis.10–20.
Biomarkers related to collagen metabolism
C-terminal telopeptide of collagen type II (CTX-II)
Type II collagen α chains collagenase neoepitope (α-CTX-II)
Type II collagen propeptides (PIINP, PIIANP, PIIBNP, PIICP, CPII)
Pyridinoline and Glc-Gal-PYD
Type II collagen cleavage product (C2C)
Collagen type II-specific neoepitope (C2M)
C-terminal telopeptide of collagen type I (CTX-I, α-CTX-I)
N-terminal telopeptide of collagen type I (NTX-I)
Aminoterminal propeptide of collagen type I (PINP)
Types I and II collagen cleavage neoepitope (C1,C2)
Biomarkers related to aggrecan metabolism
Core protein fragments (aggrecan neoepitopes, ARGS and FFGV fragments)
Chondroitin sulfate epitope 846 and monoclonal antibody 3B3(–)
Keratan sulfate
Biomarkers related to other non-collagenous proteins
Cartilage oligomeric matrix proteins (COMP and its deamidated form D-COMP)
Fibulin (peptides of fibulin 3, Fib3-1, Fib3-2)
Follistatin-like protein 1 (FSTL-1)
Hyaluronan (hyaluronic acid)
Matrix metalloproteinases (MMP-1, MMP-3, MMP-9, MMP-13 and TIMPs)
YKL-40 (cartilage glycoprotein 39)
Soluble receptor for advanced glycation endproducts (sRAGE)
Biomarkers related to other processes
Inflammatory biomarkers: hs-CRP, IL-1β and IL-6 and COX-2
Factors indicating fibrosis and complement proteins
Adipokines (adiponectin, leptin, visfatin)
Soluble receptor for leptin (sOB-Rb)
Cellular interactions in bone (periostin)
Wnt inhibitors (DKKs and SOST)
Uric acid
COMP, cartilage oligomeric protein; COX-2, cyclo-oxygenase-2; Glc-Gal-PYD, glucosyl–galactosyl–pyridinoline; hs-CRP, high sensitivity C reactive protein; IL, interleukin; MMP, matrix metalloproteinase; PIIANP, N-propeptide IIA of type II collagen; PIIBNP, N-propeptide IIB of type II collagen; PIICP, C-propeptide of collagen type II; PIINP, N-propeptide II of type II collagen; SOST, sclerostin; TIMP, tissue inhibitor of matrix metalloproteinase.
Figure 1.
Sources of possible biomarkers in osteoarthritis.10–20 C2C, cleavage of type II collagen; C2M, collagen type II-specific neoepitope; C3M, collagen type III-specific neoepitope; Coll 2-1, 9-amino acid peptide of type II collagen (nitrated form Coll 2-1 NO2); COMP, cartilage oligomeric protein; CPII, type II collagen propeptide; CRP, C reactive protein; CTX, C-terminal telopeptide of collagen; DKK, wnt inhibitor; FSTL-1, follistatin-like protein 1; ICTP, type I collagen-derived cross-linked carboxy-terminal telopeptide; IL, interleukin; MMP, matrix metalloproteinase; NTX, N-terminal telopeptide of collagen OPG, osteoprotegerin; PIIANP, N-propeptide IIA of type II collagen; PIIBNP, N-propeptide IIB of type II collagen; PIICP, C-propeptide of collagen type II; PINP, N-propeptide of type I collagen; PIINP, N-propeptide of type II collagen; RANK-L, receptor activator of nuclear factor κB ligand; RAGE, receptor for advanced glycation endproducts; TNF, tumour necrosis factor.
Potential value of biomarkers in osteoarthritis
Biomarkers in osteoarthritis can be categorised using the five-point burden of disease, investigative, prognostic, efficacy of intervention and diagnostic (BIPED) classification scheme, which was developed by the Osteoarthritis Biomarkers Network with the aim of providing a common framework for communication in the field.21 Thus, a ‘diagnostic’ biomarker can distinguish between individuals with and without osteoarthritis with good positive and negative likelihood ratios (sensitivity and specificity) and area under the curve in the receiving operator curve. A ‘burden of disease’ biomarker assesses disease severity in individuals with osteoarthritis, while a ‘prognostic’ biomarker predicts the onset of osteoarthritis in those without the disease or the progression of osteoarthritis in those with existing disease. Prognostic biomarkers may be used to determine risk in those without overt disease, clinical outcomes in patients with signs or symptoms of osteoarthritis or the efficacy of potential treatments (ie, in drug development). Biomarkers for the ‘efficacy of intervention’ are those that can be applied in randomised controlled trials to assess the short-term or long-term changes associated with pharmacological treatments. Finally, BIPED classifies biomarkers as ‘investigative’ if there is insufficient evidence to classify promising candidates into one of the other categories. A sixth category was added later to include the notion of ‘safety’, updating the acronym to burden of disease, investigative, prognostic, efficacy of intervention, diagnostic, and safety (BIPEDS).10 22 Safety is an important consideration for more invasive investigations (eg, exposure to drugs, radiation or contrast agents).10 22 23 Similar frameworks for the categorisation of biomarkers exist in other fields of medicine.5 24 The aim of the BIPEDS classification, designed specifically for osteoarthritis but also applicable to other diseases, was to help capture information in the early stages of development of the disease to inform the design of future clinical trials and research in osteoarthritis.21
Classification (using BIPED) was applied in a systematic review of the literature in biomarkers in osteoarthritis, which identified 84 relevant publications covering 26 different biomarkers published up to 2010.20 22 A systematic review of the literature is beyond the scope of this ESCEO paper, but for each of the different categories of biomarkers we provide a few illustrative examples below.
Diagnostic biomarkers
Diagnostic biomarkers ideally identify patients at the stage of osteoarthritis where treatment may be most effective. Although there have been a number of promising studies in this direction, no single biomarker stands out for use in diagnosis. A cross-sectional study in 67 patients with knee osteoarthritis and 67 healthy controls tested the clinical performance of a range of 10 biomarkers sampled in serum and urine. Most of the biomarkers of cartilage and synovium were significantly different from controls in the osteoarthritis patients (p<0.05 for eight of the biomarkers tested). Urinary C-terminal telopeptide of collagen type II (CTX-II), urinary Glc-Gal-Pyd and serum N-propeptide II of type II collagen (PIINP), all of which were increased in patients with osteoarthritis, were noted as potentially useful biomarkers for the presence of osteoarthritis since they also correlated with joint surface area.25 However, there was a substantial overlap for all of the biomarkers tested between the patients and controls. A study in 142 patients with osteoarthritis and 145 healthy controls supported the diagnostic potential of urinary CTX-II, although it also reported that an aggregate score combining CTX-II with imaging parameters had the best diagnostic potential.26 Serum levels of cartilage oligomeric protein (COMP) and type II collagen cleavage product (C2C) have also been shown to correlate with knee degeneration in patients with symptomatic knee osteoarthritis.27
Other candidates for diagnostic biomarkers have been identified, with results for follistatin-like protein-1 (FSTL-1) in 42 osteoarthritis patients compared with six controls and also for peptides of fibulin 3 (Fib3), Fib3-1 and Fib3-2.28 In another study, the cartilage synthesis product N-propeptide IIA of type II collagen (PIIANP) was reported to be decreased in 43 patients with knee osteoarthritis compared with 88 healthy controls.29 The presence of aggrecan neoepitopes was also shown to be associated with the presence of osteoarthritis.30 Future possible candidate diagnostic biomarkers include sRAGE, the plasma levels of which were significantly lower in 36 patients with knee osteoarthritis than in 15 healthy controls (p<0.01),31 and the presence of the glycoprotein YKL-40 in synovium or cartilage, which is known to correlate with the presence of osteoarthritis and disease severity32 and was demonstrated to be elevated in patients with osteoarthritis versus healthy controls.33 Factors indicating fibrosis and complement proteins also play a role in the pathogenesis of osteoarthritis and may prove to be useful diagnostic biomarkers.34
Burden of disease biomarkers
The degradation product urinary CTX-II correlates well with the presence of osteophytes upon radiography in most joints affected by osteoarthritis and may be a sensitive quantitative biomarker for the severity of osteoarthritis.35 36 α-CTX-II has been shown to reflect accelerated bone turnover in knee osteoarthritis.37
As regards biomarkers unrelated to collagen, studies in 141 subjects with knee osteoarthritis showed that levels of synovial fluid aggrecan fragments can differentiate between mild and moderate osteoarthritis in terms of radiographic JSN, as well as pain and function (patient-reported outcomes).38 COMP is also promising in the evaluation of the severity of knee osteoarthritis,39 40 as is the level of uric acid in synovial fluid.41
The relationship between the levels of three biomarkers (urinary CTX-II, serum COMP and serum hyaluronan) and radiographic features in various joints (hand, hip, knee and lumbar spine) was explored in a study in 461 women originally recruited on the basis of hand osteoarthritis.36 The results showed differences between the biomarkers in terms of correlations with radiographic indices of osteoarthritis. Although there were positive correlations between the number of joints affected by osteophytes and CTX-II (R2=0.51), COMP (R2=0.47) and hyaluronan (R2=0.51), there was no correlation (upon adjustment for the presence of osteophytes) between the number of joints with JSN for CTX-II (R2=0.01) or hyaluronan (R2=0.05) and a negative correlation for COMP (R2=0.69).36 The native form of COMP (without post-translational deamidation of the amino-terminus) was found to be more specific for knee osteoarthritis, while the deamidated form of COMP (D-COMP) was found to be more specific for hip osteoarthritis than for knee.42 This indicates that some molecules may be more powerful biomarkers in specific joints.43 On the other hand, the high correlation between serum COMP and age and the observed high heritability of the marker43 44 imply that the marker may have an innate, systemic value independent of the joint site with osteoarthritis, which could hamper the association.
A number of studies have revealed links between adipokines (adiponectin, leptin and visfatin) and joint disease. While these proinflammatory cytokines are generally produced by white adipose tissue, they are also expressed by osteoblasts, synoviocytes and chondrocytes45–47 and have been described as important elements in joint inflammation and extracellular matrix degradation.45 48 49 High levels of adiponectin and leptin have been measured in synovial fluid of patients with osteoarthritis and were reported to correlate with the severity of osteoarthritis.50 51
Prognostic biomarkers
Elevated levels of urinary CTX-II have been demonstrated to be associated with radiographic progression.26 52 In a study in 1235 men and women participating in the Rotterdam study, those with urinary CTX-II levels in the highest quartile were at six times higher risk for radiographic progression of knee osteoarthritis (OR 6.0, 95% CI 1.2 to 30.8) and more than eight times higher risk for progression of hip osteoarthritis (OR, 8.4, 95% CI 1.0 to 72.9).52 These results were independent of known clinical risk factors for osteoarthritis, such as age, sex and body mass index. Similarly, CTX-II was shown to predict cartilage loss by MRI, and in addition to MRI modalities, to provide an OR of more than 10 for identification of progressors.26 53 In this context, and as mentioned above, data suggest that CTX-II may be more a marker of bone turnover than cartilage breakdown,54 although further research is necessary to assess whether this association is based on confounding of bone turnover with the osteoarthritis process or is actually causally related due to cross-reactivity of the epitope. There have also been promising results for prognosis with serum COMP and urinary CTX-II in a cohort of patients with knee osteoarthritis.55 In a community-based cohort of 800 individuals, high levels of serum COMP and hyaluronan were reported to predict incident knee osteoarthritis and symptoms over a follow-up period of 6 years (HR for incident JSN with 1 unit higher ln(COMP), 1.82 (95% CI 1.15 to 2.89) and with 1 unit higher ln(hyaluronan), 1.46 (95% CI 1.14 to 1.87)).56 Finally, a study in 161 patients with knee osteoarthritis showed that levels of the matrix metalloproteinases MMP-1 and MMP-3 were predictive of cartilage volume loss as evaluated by quantitative MRI over 2 years (both p<0.05).57 Insofar as the relationship between cartilage biomarkers and cartilage volume loss is not linear across populations, biomarkers may prove useful to select subgroups among which osteoarthritis progresses at different rates.58
As regards preradiographic osteoarthritis, collagen type II-specific neoepitope59 (C2M) and C2C have been found to be promising for the prediction of cartilage loss, especially when the ratio of the two biomarkers, which is increased with the onset of osteoarthritis, is considered.60 This suggests that panels of biomarkers may be a better choice for predicting outcomes.
Adiponectin, visfatin, leptin and leptin's soluble receptor (OB-Rb) are increased in blood samples from patients with osteoarthritis61 62 and have been correlated with disease progression in hand osteoarthritis,63 cartilage volume loss in knee osteoarthritis64 and radiographic changes in hip osteoarthritis.65
The presence of inflammatory biomarkers has also been shown to predict outcomes in osteoarthritis. Elevated high sensitivity C reactive protein (hs-CRP) predicts cartilage loss associated with osteoarthritis, as well as poorer outcomes after total knee joint replacement.57 66 67 Similar effects have also been found for interleukin 6 (IL-6).57 68 On the other hand, the specificity of inflammatory biomarkers for osteoarthritis may be limited since they are implicated in a range of inflammatory conditions and the utility and significance of hs-CRP are generally limited in osteoarthritis when body mass index is taken into account.
Efficacy of intervention
There have been a number of studies linking the efficacy of interventions in osteoarthritis to levels of urinary CTX-II.20 The use of biomarkers for efficacy of intervention has been considerably limited by the absence of proven DMOADs, and most agents used in osteoarthritis have given rather inconsistent results with various biomarkers. Strontium ranelate has recently been shown to have DMOAD properties,69 and so further analysis of these trial results may be expected to shed more light on this issue.
Investigative biomarkers
Advances in ‘omics’ research are uncovering a range of new candidate biomarkers. Although only some of the data have been validated independently, this is a field of active research and of major importance in osteoarthritis. For the genetic component of risk for osteoarthritis, a number of genetic polymorphisms related to cartilage breakdown have been identified.70 71 A large genome-wide association study identified eight novel genetic risk loci for osteoarthritis, revealing new pathways for exploration for potential biomarkers.72 Interestingly, this study also found that the risk loci were generally specific to the hip or the knee, indicating joint-specific disease pathways. Of note, genetic studies are bound to provide insight into new aetiological routes to osteoarthritis. Such insights may eventually provide new biomarkers. However, the effect sizes of the genetic markers are very small and so there is little chance that the genetic marker itself may be powerful as a biomarker. Metabolomics studies are also promising to identify novel serum biomarkers in osteoarthritis, such as the ratio between branched chain amino acids and histidine.73 74 As the number of testable metabolites moves from small numbers towards several thousands, more biomarkers are likely to emerge from these improving technologies.75 Proteomic analyses are also likely to produce novel biomarkers, for example, one study detected four new potential biomarkers that correlated with parameters of inflammation, bone remodelling, and cartilage turnover.76 Recent proteomics studies are also in line with pronounced differences between cartilage from different joint sites.77 These results also support known differences in clinical progression in a specific joint (eg, progressive hand osteoarthritis). Finally, research in lipidomics suggests altered lipid metabolism in osteoarthritis, which may also be a source of novel biomarkers.78
Discussion
None of the current biomarkers is sufficiently discriminating to aid the diagnosis of osteoarthritis or aid the prognosis of individuals with or without the disease or performs so consistently that it could function as a surrogate outcome in clinical trials as a secondary or supportive endpoint. The ESCEO group identified a number of possible avenues for future research in the field (box 2), some of which are necessary before biomarkers can enter routine clinical use for monitoring disease activity or in drug development for the measurement of response to treatment in osteoarthritis. The arrival of new DMOADs may help better define biomarkers in osteoarthritis.
Box 2. Avenues for future research in biomarkers in osteoarthritis.
Mechanisms of disease/development of new biomarkers
Research into the underlying mechanisms of disease to validate existing biomarkers and identify new candidates
Research into biomarkers from ‘omics’
Identification of differences in effects of biomarkers between joints (knee, hip, hand, spine, etc) to explore whether there are specific biomarkers for specific joints
Assays and technological development
Improved assays/technologies
Standardisation/calibration of biomarkers
Development of aggregate scores combining a panel of known (confirmed) biomarkers (with or without imaging markers) into single diagnostic tests
Prognosis and risk
Improvement of the definition of early osteoarthritis
Identification of biomarkers for early stages of osteoarthritis (preradiographic)
Further studies on correlations between changes in biomarkers and hard clinical endpoints (eg, joint replacement or virtual joint replacement)
Research to place biomarkers within the assessment of risk
Despite this, there are a few very promising candidates. The most promising are biomarkers of cartilage metabolism, although biomarkers of synovial tissue degradation may also prove to be useful in future. In this context, studies of the synovial fluid proteome have explored the contribution of innate immunity and the complement cascade.34 79 80 The results suggest a major contribution of synovial gene products to the osteoarthritis synovial proteome.80 A systematic review applying the BIPED classification indicated that urinary CTX-II and serum COMP appear to have the best performance of all commercially available biomarkers.20 On the other hand, the same review also highlighted a general lack of consistent evidence, differences between the populations studied (clinical trial populations vs population-based cohorts), differences in sample collection and possibly publication bias.20 Thus, future research efforts should be made to validate the existing markers and identify new candidates (box 2). Such research should involve further exploration of the underlying mechanisms of disease. The ongoing head-to-head comparison of a large panel of biomarkers (a dozen biochemical biomarkers and multiple sensitive imaging biomarkers) through the OARSI/Foundation for NIH study promises to provide much needed vital information for determining some of the best candidates for further qualification in clinical trial scenarios.81
Interestingly, the biomarkers with the most consistent evidence appear to be those at the end of the pathways of tissue destruction, which may be more specific for a particular tissue (eg, CTX-II). In this context, there is evidence that there may be differences in some biomarkers between different joints or tissues,42 43 77 and there are indications of differences in relation to distance from the surface of the joint.82
The use of biomarkers in drug development in osteoarthritis remains a subject of research. The ESCEO group noted the differences with the field of osteoporosis, for which the use of biomarkers is much better established. This is principally due to the magnitude of clinical advances in osteoporosis in the last 20 years, for which there is now a range of disease-modifying treatments, in comparison with osteoarthritis, for which DMOADs are only just beginning to appear with the need to sufficiently justify their efficacy and safety to use them in biomarker development or research.69 Another point is that the amount of bone mass involved in bone turnover of a joint is much larger than the small amount of cartilage available in the joints; this suggests that the systemic biomarker contribution due to localised high bone turnover at a specific osteoarthritic joint might be more readily detected than the contribution due to high cartilage turnover of the joint. However, in all cases, the biomarker contributions from local disease need to be distinguished from the contributions of normal bone and cartilage turnover; this is currently a primary limitation for all systemic (serum and urine) biomarker measures. For use in Phase II or dose-finding studies, biomarkers should be able to predict structural change. In Phase III, they should predict more concrete endpoints, such as surgery, or indeed play an explanatory role in terms of mechanism of action. In this context, there is still much discussion in the osteoarthritis field regarding hard endpoints, since joint replacement is affected by a number of factors unrelated to efficacy of treatment (eg, patient preference, access to healthcare, individual variations in pain and symptoms). Surrogate endpoints for joint replacement have been developed, such as virtual joint replacement,83 although consensus has not been reached in the field and the potential role of biomarkers remains to be defined. Moreover, there is currently no hard evidence that improving structure (JSN or cartilage volume loss) improves symptoms (pain and function) in osteoarthritis.
One theoretical advantage of biomarkers in all fields of medicine is the detection of the early phases of disease. The ability to detect early stage osteoarthritis would not only improve the management of patients via preventative measures and lifestyle changes, but may also facilitate efforts in drug development by improving the design of randomised clinical trials. Many biological markers appear to be sufficiently characterised for the study of progressive osteoarthritis, but few have been identified for the diagnosis of the early stage of the disease. Moreover, early osteoarthritis has not been clearly defined. This is an important avenue for future research (box 2).
The ability to predict the onset or progression of disease is an important feature of biomarker research since it will have implications in clinical medicine. In this context, clinicians need reassurance that the biomarkers truly represent clinical outcomes (ie, structural damage). Indeed, predictive value is essential to determine which patients are likely to progress rapidly and, ideally, which patients are likely to respond to treatment. A biomarker that characterises risk for generalised disease should have broad utility. A related issue is the role of biomarkers in the context of risk factors and their assessment.
There is increasing evidence that the best solution may be a panel of biomarkers, covering the range of physiological effects, or indeed a combination of tissue biomarkers with other parameters (eg, radiographic or MRI) into single diagnostic tests.43 84 An analysis of a set of 14 biomarkers in a cohort of 1002 individuals with early osteoarthritis (Cohort Hip and Cohort Knee) suggested the possibility of clusters of biomarkers related to specific pathogenic processes, for example, cartilage synthesis, synovium or inflammation.84 Recognising such underlying patterns may increase prognostic accuracy and predictive power, thus enabling better identification of at-risk patients; further research is essential (box 2).
A well-selected panel of biomarkers may also assist in identifying the patients who are most likely to respond (personalised medicine). In theory, targeting responders would reduce the cost of treatment on the population level by eliminating redundant prescriptions and improving benefits for patients. The ideal panel should include, for example, biomarkers of turnover, synthesis and degradation and tissue-specific biomarkers (cartilage, subchondral bone and inflammation). Ratios of specific biomarkers may also prove to be important.60
There are a number of major research limitations that need to be addressed in the field of biomarkers. One of these is the rarity of DMOADs, which has hindered research to find a valid biochemical marker. There are many potential errors in biomarker analysis related to sampling, biological variations (eg, seasonal, diurnal and food intake), analyte features, assay format and parameters,19 which can confound results. These should be resolved by further research in assays and technological development, as well as standardisation and calibration of known biomarkers and optimisation of data collection (box 2). Another limitation is the non-linear relationship between biomarkers and some structural parameters,58 which will complicate the development of prognostic biomarkers. In addition, the identification of patients is complicated since the pathways to a failed joint may involve many different mechanisms (eg, bone-active treatments work in some patients and anti-inflammatories in others). Another limitation is the relationship between osteoarthritis biomarkers and ‘normal’ age-related processes independent of the osteoarthritis process, which is not well defined.85
Conclusion
The conclusion of the ESCEO working group was that there is a potential role for biomarkers in drug development in osteoarthritis. However, none of the best candidate biomarkers has entered clinical use. For the moment, radiographic measurements combined with pain and function remain the regulatory endpoint in DMOAD development.2 3 Radiographic data may be supplanted by MRI markers in future, although there is still work to be done to reach a consensus on which MRI parameters are the most relevant. While biomarkers are not likely to constitute the ideal primary endpoint, they could be valuable secondary endpoints in future drug development because they provide evidence of pharmacodynamics and mechanisms of action, support the primary endpoint and can help identify subgroups. There remains a clear need for more research in the field.
Footnotes
Correction notice: This article has been corrected since it was published Online First. Fig 1 has been corrected and minor textual changes made.
Acknowledgements: We thank Professor Tim Spector for valuable input during the preparation of this manuscript.
Contributors: All authors participated in preparing the manuscript and approved its final version.
Competing interests: M-LB: support from Fondazione Fondazione Italiana Ricerca Malattie Ossee (F.I.R.M.O.) and grants from Merck Sharpe & Dohme, Nycomed, Roche, Glaxo, Eli Lilly and Wyeth. Speaker fees from Procter and Gamble, Merck Sharpe & Dohme, Nycomed and Wyeth. OB: grant research: GlaxoSmithKline, IBSA, Merck Sharp & Dohme, Theramex, Novartis, Pfizer, Rottapharm and Servier; consulting or lecture fees: IBSA, Rottapharm, Servier; reimbursement for attending meetings: IBSA, Merck Sharp & Dohme, Novartis, Pfizer, Rottapharm, Theramex and Servier. RC: research grants: Merck, Chugai-Roche and Warner-Chilcott; consulting and/or lecture fees: Servier, Chugai-Roche, Novartis, Amgen, Pfizer, UCB and Bioiberica. CC: consulting fees, paid advisory boards, lecture fees and/or grant support from Roche, Wyeth-Ayerst, Eli Lilly, Novartis, Novo Nordisk, Proctor and Gamble, Groupe Fournier, Besins EscoVesco, MSD, Chiesi, Boehringer Mannheim, Pfizer and Servier. Chairman of Nordic Bioscience A/S and Chairman of CCBR/Synarc. JC: None. CC: consulting fees and paid advisory boards for Alliance for Better Bone Health, Glaxo Smith Kline, Roche, Merck Sharp and Dohme, Lilly, Amgen, Wyeth, Novartis, Servier and Nycomed. GG: None. JAK: consulting fees, paid advisory boards, lecture fees and/or grant support from the majority of companies concerned with skeletal metabolism. MAK: employee of Nordic Bioscience. VK: grant support from Bioiberica. WFL: speaker fees and paid advisory boards from Pfizer, Servier, Merck, Amgen, Novartis, Will Pharma, Procter & Gamble, Abbott, Roche and Lilly. ML: grant support from Cargill and paid advisory board from Tanabe Research Laboratories. JM-P: owner, ArthroLab Inc. Consulting fees from AstraZeneca, Bioibérica, Boehringer Ingelheim, Elanco, Ferring, Merck, Pfizer, Servier, TRB Chemedica and Virbac. IM: none. J-PP: owner, ArthroLab Inc. Consulting fees from AstraZeneca, Bioibérica, Boehringer Ingelheim, Elanco, Ferring, Merck, Pfizer, Servier, TRB Chemedica and Virbac. J-PR: consulting fees and lecture fees from AbbVie, Pfizer, Servier, Novartis, Lilly, Amgen, GlaxoSmithKline, Roche, UCB, Merck and Bristol Myers Squibb. J-YR: consulting fees, paid advisory boards, lecture fees and/or grant support from Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed, NPS, Theramex, UCB, Merck Sharp and Dohme, Rottapharm, IBSA, Genevrier, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Novo-Nordisk and Bristol Myers Squibb. SR-N: none. RR: consulting and lecture fees for Merck Sharp and Dohme, Eli Lilly, Amgen, Novartis, Servier, Nycomed, Nestlé and Danone. LS: none. WEVS: none.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- 2.Committee for Medicinal Products for Human Use. Guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis. 2010. http://www.ema.europa.eu (accessed 19 Sept 2012).
- 3.Food and Drug Administration. Guidance for industry. Clinical development programs for drugs, devices, and biological products intended for the treatment of osteoarthritis. 2011. http://www.fda.gov (accessed 12 Jun 2012).
- 4.Hunter DJ, Guermazi A. Imaging techniques in osteoarthritis. PM&R 2012;4:S68–74. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson AJ, Colburn WA, DeGruttola VG, et al. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 6.Iagnocco A, Perricone C, Scirocco C, et al. The interobserver reliability of ultrasound in knee osteoarthritis. Rheumatology (Oxford) 2012;51:2013–19. [DOI] [PubMed] [Google Scholar]
- 7.Eckstein F, Wirth W. Quantitative cartilage imaging in knee osteoarthritis. Arthritis 2011;2011:475684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelletier JP, Cooper C, Peterfy C, et al. What is the predictive value of MRI for the occurrence of knee replacement surgery in knee osteoarthritis? Ann Rheum Dies 2013;72:1594–604.. [DOI] [PubMed] [Google Scholar]
- 9.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Cur Open Rheumatic 2008;20:565–72. [DOI] [PubMed] [Google Scholar]
- 10.Kraus VB, Burnett B, Coindreau J, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage 2011;19:515–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousseau JC, Garnero P. OARSI Primer. Chapter 10. Biochemical markers 2010. http://primer.oarsi.org (accessed 6 Nov 2012).
- 12.Charni-Ben TN, Garnero P. Monitoring cartilage turnover. Cur Rheumatic Rep 2007;9:16–24. [DOI] [PubMed] [Google Scholar]
- 13.Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthritis Cartilage 2012;20:1451–64. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau JC, Garnero P. Biological markers in osteoarthritis. Bone 2012;51:265–77. [DOI] [PubMed] [Google Scholar]
- 15.Williams FM, Spector TD. Biomarkers in osteoarthritis. Arthritis Res Ther 2008;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatic 2007;3:346–56. [DOI] [PubMed] [Google Scholar]
- 17.Karsdal MA, Madsen SH, Christiansen C, et al. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther 2008;10:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karsdal MA, Nielsen MJ, Sand JM, et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol 2013;11:70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsdal MA, Woodworth T, Henriksen K, et al. Biochemical markers of ongoing joint damage in rheumatoid arthritis—current and future applications, limitations and opportunities. Arthritis Res Ther 2011;13:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Spil WE, Degroot J, Lems WF, et al. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage 2010;18:605–12. [DOI] [PubMed] [Google Scholar]
- 21.Bauer DC, Hunter DJ, Abramson SB, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage 2006;14:723–7. [DOI] [PubMed] [Google Scholar]
- 22.Kraus VB. Osteoarthritis year 2010 in review: biochemical markers. Osteoarthritis Cartilage 2011;19:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus VB, Nevitt M, Sandell LJ. Summary of the OA biomarkers workshop 2009—biochemical biomarkers: biology, validation, and clinical studies. Osteoarthritis Cartilage 2010;18:742–5. [DOI] [PubMed] [Google Scholar]
- 24.Jain KK. The handbook of biomarkers. New York, NY: Springer, 2010. [Google Scholar]
- 25.Garnero P, Piperno M, Gineyts E, et al. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dies 2001;60:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dam EB, Loog M, Christiansen C, et al. Identification of progressors in osteoarthritis by combining biochemical and MRI-based markers. Arthritis Res Ther 2009;11:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King KB, Lindsey CT, Dunn TC, et al. A study of the relationship between molecular biomarkers of joint degeneration and the magnetic resonance-measured characteristics of cartilage in 16 symptomatic knees. Magn Reson Imaging 2004;22:1117–23. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Li D, Xu N, et al. Follistatin-like protein 1: a serum biochemical marker reflecting the severity of joint damage in patients with osteoarthritis. Arthritis Res Ther 2011;13:R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rousseau JC, Zhu Y, Miossec P, et al. Serum levels of type IIA procollagen amino terminal propeptide (PIIANP) are decreased in patients with knee osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 2004;12:440–7. [DOI] [PubMed] [Google Scholar]
- 30.Dufield DR, Nemirovskiy OV, Jennings MG, et al. An immunoaffinity liquid chromatography-tandem mass spectrometry assay for detection of endogenous aggrecan fragments in biological fluids: use as a biomarker for aggrecanase activity and cartilage degradation. Anal Biochem 2010;406:113–23. [DOI] [PubMed] [Google Scholar]
- 31.Chayanupatkul M, Honsawek S. Soluble receptor for advanced glycation end products (sRAGE) in plasma and synovial fluid is inversely associated with disease severity of knee osteoarthritis. Clin Biochem 2010;43:1133–7. [DOI] [PubMed] [Google Scholar]
- 32.Huang K, Wu LD. YKL-40: a potential biomarker for osteoarthritis. J Int Med Res 2009;37:18–24. [DOI] [PubMed] [Google Scholar]
- 33.Johansen JS, Hvolris J, Hansen M, et al. Serum YKL-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br J Rheumatic 1996;35:553–9. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat Med 2011;17:1674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meulenbelt I, Kloppenburg M, Kroon HM, et al. Urinary CTX-II levels are associated with radiographic subtypes of osteoarthritis in hip, knee, hand, and facet joints in subject with familial osteoarthritis at multiple sites: the GARP study. Ann Rheum Dies 2006;65:360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraus VB, Kepler TB, Stabler T, et al. First qualification study of serum biomarkers as indicators of total body burden of osteoarthritis. PLoS ONE 2010;5:e9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huebner JL, Bay-Jensen AC, Leeming DJ, et al. Urinary markers,alpha CTX and CTXII,are indicative of OA severity and bone turnover. Oral communication 048. Osteoarthritis Cartilage 2010;18(Suppl 2):S29–30. [Google Scholar]
- 38.Larsson S, Englund M, Struglics A, et al. The association between changes in synovial fluid levels of ARGS-aggrecan fragments, progression of radiographic osteoarthritis and self-reported outcomes: a cohort study. Osteoarthritis Cartilage 2012;20:388–95. [DOI] [PubMed] [Google Scholar]
- 39.Clark AG, Jordan JM, Vilim V, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum 1999;42:2356–64. [DOI] [PubMed] [Google Scholar]
- 40.Wislowska M, Jablonska B. Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin Rheumatic 2005;24:278–84. [DOI] [PubMed] [Google Scholar]
- 41.Denoble AE, Huffman KM, Stabler TV, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci USA 2011;108:2088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catterall JB, Hsueh MF, Stabler TV, et al. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem 2012;287:4640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meulenbelt I, Kloppenburg M, Kroon HM, et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage 2007;15:379–85. [DOI] [PubMed] [Google Scholar]
- 44.Chen HC, Kraus VB, Li YJ, et al. Genome-wide linkage analysis of quantitative biomarker traits of osteoarthritis in a large, multigenerational extended family. Arthritis Rheum 2010;62:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lago R, Gomez R, Otero M, et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage 2008;16:1101–9. [DOI] [PubMed] [Google Scholar]
- 46.Lago F, Dieguez C, Gomez-Reino J, et al. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 2007;18:313–25. [DOI] [PubMed] [Google Scholar]
- 47.Berner HS, Lyngstadaas SP, Spahr A, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004;35:842–9. [DOI] [PubMed] [Google Scholar]
- 48.Gosset M, Berenbaum F, Salvat C, et al. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum 2008;58:1399–409. [DOI] [PubMed] [Google Scholar]
- 49.Vuolteenaho K, Koskinen A, Kukkonen M, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage–mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm 2009;2009:345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ku JH, Lee CK, Joo BS, et al. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatic 2009;28:1431–5. [DOI] [PubMed] [Google Scholar]
- 51.Schaffler A, Ehling A, Neumann E, et al. Adipocytokines in synovial fluid. JAMA 2003;290:1709–10. [DOI] [PubMed] [Google Scholar]
- 52.Reijman M, Hazes JM, Bierma-Zeinstra SM, et al. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum 2004;50:2471–8. [DOI] [PubMed] [Google Scholar]
- 53.Dam EB, Byrjalsen I, Karsdal MA, et al. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage 2009;17:384–9. [DOI] [PubMed] [Google Scholar]
- 54.van Spil WE, Drossaers-Bakker KW, Lafeber FP. Associations of CTX-II with biochemical markers of bone turnover raise questions on its tissue origin: data from CHECK, a cohort study of early osteoarthritis. Ann Rheum Dies 2013;72:29–36. [DOI] [PubMed] [Google Scholar]
- 55.Sowers MF, Karvonen-Gutierrez CA, Yosef M, et al. Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthritis Cartilage 2009;17:1609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golightly YM, Marshall SW, Kraus VB, et al. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis Rheum 2011;63:2276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelletier JP, Raynauld JP, Caron J, et al. Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann Rheum Dies 2010;69:2095–101. [DOI] [PubMed] [Google Scholar]
- 58.Berry PA, Maciewicz RA, Wluka AE, et al. Relationship of serum markers of cartilage metabolism to imaging and clinical outcome measures of knee joint structure. Ann Rheum Dies 2010;69:1816–22. [DOI] [PubMed] [Google Scholar]
- 59.Bay-Jensen AC, Liu Q, Byrjalsen I, et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM—increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin Biochem 2011;44:423–9. [DOI] [PubMed] [Google Scholar]
- 60.Ishijima M, Watari T, Naito K, et al. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther 2011;13:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen WP, Bao JP, Feng J, et al. Increased serum concentrations of visfatin and its production by different joint tissues in patients with osteoarthritis. Clin Chem Lab Med 2010;48:1141–5. [DOI] [PubMed] [Google Scholar]
- 62.Laurberg TB, Frystyk J, Ellingsen T, et al. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid- and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. J Rheumatic 2009;36:1885–91. [DOI] [PubMed] [Google Scholar]
- 63.Yusuf E, Ioan-Facsinay A, Bijsterbosch J, et al. Association between leptin, adiponectin and resistin and long-term progression of hand osteoarthritis. Ann Rheum Dies 2011;70:1282–4. [DOI] [PubMed] [Google Scholar]
- 64.Berry PA, Jones SW, Cicuttini FM, et al. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum 2011;63:700–7. [DOI] [PubMed] [Google Scholar]
- 65.Stannus OP, Jones G, Quinn SJ, et al. The association between leptin, interleukin-6, and hip radiographic osteoarthritis in older people: a cross-sectional study. Arthritis Res Ther 2010;12:R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith JW, Martins TB, Gopez E, et al. Significance of C-reactive protein in osteoarthritis and total knee arthroplasty outcomes. Ther Adv Musculoskelet Dies 2012;4:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharif M, Shepstone L, Elson CJ, et al. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dies 2000;59:71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage 2010;18:1441–7. [DOI] [PubMed] [Google Scholar]
- 69.Reginster J-Y, Badurski J, Bellamy N, et al. Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: results of a randomised double-blind, placebo-controlled trial. Ann Rheum Dies 2013;72:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meulenbelt I, Kraus VB, Sandell LJ, et al. Summary of the OA biomarkers workshop 2. Osteoarthritis Cartilage 2011;19:1091–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valdes AM, Loughlin J, Oene MV, et al. Sex and ethnic differences in the association of ASPN, CALM1, COL2A1, COMP, and FRZB with genetic susceptibility to osteoarthritis of the knee. Arthritis Rheum 2007;56:137–46. [DOI] [PubMed] [Google Scholar]
- 72.Zeggini E, Panoutsopoulou K, Southam L, et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet 2012;380:815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhai G, Wang-Sattler R, Hart DJ, et al. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dies 2010;69:1227–31. [DOI] [PubMed] [Google Scholar]
- 74.Adams SB, Jr, Setton LA, Kensicki E, et al. Global metabolic profiling of human osteoarthritic synovium. Osteoarthritis Cartilage 2012;20:64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suhre K, Shin SY, Petersen AK, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011;477:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Seny D, Sharif M, Fillet M, et al. Discovery and biochemical characterisation of four novel biomarkers for osteoarthritis. Ann Rheum Dies 2011;70: 1144–52. [DOI] [PubMed] [Google Scholar]
- 77.Onnerfjord P, Khabut A, Reinholt FP, et al. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J Biol Chem 2012;287:18913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castro-Perez JM, Kamphorst J, Degroot J, et al. Comprehensive LC-MS E lipidomic analysis using a shotgun approach and its application to biomarker detection and identification in osteoarthritis patients. J Proteome Res 2010;9:2377–89. [DOI] [PubMed] [Google Scholar]
- 79.Gobezie R, Kho A, Krastins B, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther 2007;9:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ritter SY, Subbaiah R, Bebek G, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum 2013;65:981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunter DJ, Nevitt M, Losina E, et al. Biomarkers for osteoarthritis: current position and steps towards further validation. Rheum Dies Clin North Am 2013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charni-Ben TN, Desmarais S, Bay-Jensen AC, et al. The type II collagen fragments Helix-II and CTX-II reveal different enzymatic pathways of human cartilage collagen degradation. Osteoarthritis Cartilage 2008;16:1183–91. [DOI] [PubMed] [Google Scholar]
- 83.Manno RL, Bingham CO, III, Paternotte S, et al. OARSI-OMERACT initiative: defining thresholds for symptomatic severity and structural changes in disease modifying osteoarthritis drug (DMOAD) clinical trials. Osteoarthritis Cartilage 2012;20:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Spil WE, Jansen NW, Bijlsma JW, et al. Clusters within a wide spectrum of biochemical markers for osteoarthritis: data from CHECK, a large cohort of individuals with very early symptomatic osteoarthritis. Osteoarthritis Cartilage 2012;20:745–54. [DOI] [PubMed] [Google Scholar]
- 85.Bos SD, Beekman M, Maier AB, et al. Metabolic health in families enriched for longevity is associated with low prevalence of hand osteoarthritis and influences OA biomarker profiles. Ann Rheum Dis 2013;72:1669–74.. [DOI] [PubMed] [Google Scholar]