Abstract
Disturbed body energy balance can lead to obesity and obesity-driven diseases such as Type 2 diabetes, which have reached an epidemic level. Evidence indicates that obesity induced inflammation is a major cause of insulin resistance and Type 2 diabetes. Environmental factors, such as nutrients, affect body energy balance through epigenetic or chromatin-based mechanisms. As a bromodomain and external domain family transcription regulator, Brd2 regulates expression of many genes through interpretation of chromatin codes, and participates in the regulation of body energy balance and immune function. In the severely obese state, Brd2 knockdown in mice prevented obesity-induced inflammatory responses, protected animals from Type 2 diabetes, and thus uncoupled obesity from diabetes. Brd2 provides an important model for investigation of the function of transcription regulators and the development of obesity and diabetes; it also provides a possible target to treat obesity and diabetes through modulation of the function of a chromatin code reader.
Keywords: Type 2 diabetes, obesity, insulin resistance, epigenetics, bromodomain, bariatric surgery, maternal transmission
I. INTRODUCTION: THE PROBLEM OF OBESITY AND ITS COMPLICATIONS
Over the course of recorded history, many different diseases and risks have threatened human survival on a wide scale, including epidemics of infectious organisms, war, starvation, famine, sudden climate change and ethnic cleansing. As recently as the end of the Second World War, a chronic lack of calories in Europe and Japan was widely acknowledged as a major contributor to poor health, elevated infant mortality, increased disease susceptibility (particularly to tuberculosis) and its attendant consequences of economic and political instability. The end of that war brought the benefits of modern peace: a dramatic transformation of the international economy, accompanied by huge increases in industrial and agricultural output, vast international trade in manufactured goods, raw materials and foods (both processed and unprocessed) and a pronounced shift from manual to sedentary labor. These tremendous structural and economic changes, combined with increased life expectancy and the rise of agribusiness, advertising and a hugely successful international food industry, have contributed to the transformation of obesity from a quaint feature of royal families in former times to an alarming, international public health worry that affects all socioeconomic classes at present. Thus, of the serious and widespread menaces to public health in human history, obesity is the newest challenge.
Obesity is defined as a body mass index (BMI) of ≥30, calculated as body weight in kilograms divided by the square of height in centimeters. Lean individuals are defined as BMI < 25 and individuals with intermediate BMI (≥25 to < 30) are classified as overweight. Morbid obesity is defined as BMI ≥ 40 and super obesity is defined as BMI ≥ 50. These higher categories of obesity used to be extremely rare, and they still are relatively rare. However, as the US population distribution shifts to higher mean BMI, these extreme forms of obesity are increasing in prevalence at a greater rate than the mean. According to the most recent statistics from the US Centers for Disease Control (CDC, 2010 data), all US states now report at least 20% prevalence of obesity among adults. Mississippi, West Virginia and Alabama currently report the highest rates (34.0, 32.5% and 32.2%, respectively), but several other states are not far behind. Worldwide, 1.7 billion people are classified as overweight (Haslam and James, 2009). These statistics pose fundamental and novel problems for health maintenance and delivery systems.
The speed with which overweight and obesity have become a public health crisis is breathtaking http://www.cdc.gov/obesity/data/adult.html. For a useful meta-analysis, see Finucane et al. (2011). Obesity in the US is associated with a dramatic increase in the prevalence of obesity-associated diseases, particularly cardiovascular disease and Type 2 diabetes, as well as hypertension, stroke, metabolic syndrome and insulin resistance, non-alcoholic fatty liver disease and some forms of cancer, notably breast cancer in post-menopausal women and adult colorectal cancer in both men and women. About 90% of Type 2 diabetes is attributable to excess weight (Hossain et al., 2007). The incidence of Type 2 diabetes in particular has now reached epidemic proportions in the US and internationally, affecting about 12% of US adults and more than 25% of those over the age of 65. Type 2 diabetes is thought to be responsible for 4.6 million deaths worldwide each year. An oft-cited early estimate of the incidence of Type 2 diabetes was: 366 million worldwide by 2030 (Wild et al., 2004). However, more recent estimates have pegged the anticipated number of diabetic individuals to be 439 million by 2030 (Shaw et al., 2010), almost entirely due to obesity-driven metabolic complications. There is reason to worry that this prediction will soon have to be revised upward yet again.
Unless reversed, the rapidly worsening problem of obesity predicts an epidemic of co-morbidities that will strain or break many health care delivery systems, both in the US and internationally. In the absence of national agreement about the proper role of US health care delivery systems with controlled costs, or political will to deliver well-designed solutions, the US economy is uniquely vulnerable to this expected impact. Thus, obesity poses a critical challenge of overarching importance for US public health, particularly pediatric health: many American children are already being swept along with this national wave of progressive metabolic dysfunction in obesity. For the purpose of this report, some of the genes and epigenetic forces that drive obesity and metabolic dysfunction will be examined.
II. COMPLEX POLYGENIC INTERACTIONS WITH THE ENVIRONMENT AND EPIGENETICS IN OBESITY
We live in an era of genetic determinism. Major effort has been invested to identify ‘obesity-associated genes’ with the translational goal of isolating the ‘drivers’ from the ‘passengers’, much the same way that oncogenes have been identified, in order to develop novel therapeutic targets. Evidence for a genetic component that influences risk for obesity controlling for environment has been developed through studies of adoption and twins (Maes et al., 1997, Silventoinen et al., 2010). Genetic factors appear to explain as much as 90% of the variance in BMI among certain monozygotic twins. Furthermore, several recent genome-wide association studies have established convergent validity in the discovery of genes that are strongly associated with obesity throughout the human lifespan. The first robust association was reported for FTO (fat mass and obesity associated) (Frayling et al., 2007). This association was joined by insulin-induced gene 2 (INSIG2), melanocortin 4 receptor (MC4R), transmembrane protein 18 (TMEM18), glucosamine-6-phosphate deaminase 2 (GNPDA2), neuronal growth regulator 1 (NEGR1), brain-derived neurotrophic factor (BDNF) and potassium channel tetramerization domain containing 15 (KCTD15), as associated with pediatric obesity (Zhao et al., 2009; den Hoed et al., 2010). Intensive research in molecular genetics continues on other well-established loci of obesity-associated genes, including those that encode the uncoupling proteins (Ricquier et al., 1991; Oppert et al., 1994), peroxisome proliferator-activated receptor (PPAR)γ (Vidal-Puig et al., 1997; Lefebvre et al., 1998), low density lipoprotein receptor (Morris et al., 1994), hormone-sensitive lipase (Stich et al., 1997; Klannemark et al., 1998), beta adrenergic receptors (Reynisdottir et al., 1994; Walston et al., 1995) and inflammatory cytokine genes such as tumor necrosis factor (TNF)-α (Saghizadeh et al., 1996) and interleukin-6 (Kern, 1997; Kern et al., 2001). The hope of these studies has been that, through identification and characterization of candidate genes for obesity and metabolic syndrome, potent new drugs will be developed that specifically target these proteins or pathways and will have therapeutic value for pre-diabetic or diabetic obese patients.
Although more than 100 genes have been identified that influence body weight (Leibel et al., 2008), the usual patterns and rules of Mendelian inheritance have proven inadequate to understand the inheritance of predisposition to metabolic syndrome and Type 2 diabetes. At least eighteen genes have been directly associated with Type 2 diabetes (Florez et al., 2008; Ridderstråle and Groop, 2009). This fact indicates that, unlike inborn errors of metabolism, such as Tay-Sachs disease, for which a straightforward causal connection exists between a mutant allele and a well characterized dysfunction (Schneck et al., 1970), the genetic mechanisms that promote increased risks in obesity for Type 2 diabetes, cardiometabolic diseases and obesity-associated cancers are not so straightforward.
It is also clear that the interaction of genes alone is insufficient to explain the contemporary problem of obesity. In view of the new international environment of widespread over-nutrition, it also seems important to ask how genes respond to weight gain or weight loss, not just how specific genes make us fat or thin. Dietary caloric excess that is totally misaligned with physical energy expenditure characterizes this environment. Epidemiologists and US public health officials have taken note of critical features of the current American environment, particularly easy access to cheap calories, the profligate use of sugar sweeteners like ‘high fructose corn syrup’ in the food industry (Popkin and Nielsen, 2003; Popkin, 2007; Duffey and Popkin, 2008) and widespread overconsumption of fried, fatty, ‘fast’ foods.
Ten thousand years of human evolution have tended to produce a lean physical phenotype. Indeed, severe pediatric obesity of genetic origin is observed in less than 0.01% of the population (Farooqi et al., 2006). Thus, the dramatically increased prevalence of overweight, obese and morbidly obese phenotypes in the last decades of the twentieth century and first decade of the twenty-first must be attributable primarily to environmental and consumption changes, that is, ‘diet-induced obesity’. Strong evidence for the role of the environment when genetic factors are relatively well controlled was obtained from studies of the Pima Indians, an aboriginal population that lives in the desert southwest of the United States and in an area of northern Mexico with a similar climate. The genetic variation between the two populations is relatively small. Pima Indians in the US are on average 25 kg heavier than Pima Indians in Mexico (Ravussin, 1995), with corresponding differences in cardiometabolic risk. These differences have been attributed primarily to diet. Alternatively, population patterns of obesity may be attributed in part to epigenetic shifts, not to mutation in human DNA, because the time scale on which these changes in public health have occurred are far too rapid to be due primarily to evolution.
There is new interest in the maternal-fetal environment and an effort to understand a potential role for epigenetic mechanisms in the transmission of metabolic risk factors. The epigenetic modifications of nucleosomal histones and DNA that underlie these phenomena are poorly understood. These modifications include DNA methylation and histone acetylation or phosphorylation and are critical for proper control of the response of promoters to signal transduction in the differentiated cells of the adult (Bernstein et al., 2007; Li et al., 2007), as well as for embryonic and fetal development (Bernstein et al., 2006). Indeed, some of the most interesting work on epigenetic and developmental mechanisms has been conducted in rat dams fed a high fat diet. Investigators have identified alterations in homeobox gene expression patterns, such as HoxA10, that likely influence stem cell fate choice in osteogenic and adipogenic cell lineages (Chen et al., 2012). These alterations of transcriptional programming depend critically on patterns of acetylation and methylation of histone lysines in nucleosomal chromatin at key target genes. This concept suggests that patterns of obesity and metabolic dysfunction may indeed run in families, but not in the same way or subject to the same mechanisms as patterns of familial cancers.
One famous and well-studied case that strongly supports an epigenetic mechanism for cardiometabolic risk is the Dutch ‘Hunger Winter’ of 1944 to 1945. This wartime event, when severe starvation affected the Netherlands, revealed in detail for the first time that epigenetic mechanisms play a critical role in human BMI and insulin sensitivity. Specifically, maternal hunger created by cold, harsh living conditions and caloric deprivation of pregnant mothers was linked to insulin resistance, obesity, an atherogenic lipid profile and elevated cardiovascular risk in the surviving children as they aged (Kyle and Pichard, 2006). Of particular interest, children born to the same mothers in times of less severe deprivation did not show the same risk patterns. More recent work has shown that infants born to obese, overweight and Type 2 diabetic mothers display increased adiposity and elevated risk for later metabolic disease (Heerwagen et al., 2010). In addition, a UK study found that males who were most underweight at birth were 7 times more likely to develop metabolic dysfunction and Type 2 diabetes later in life than males who were heaviest at birth (Hales et al., 1991). There is strong support for an inverse relationship between birth weight and hypertension in the adult, for both men and women, in which low birth weight predicts risk among the highest weight adults (Barker et al., 1989). These observations suggest that maternal uterine environments vary in ways that create stable and lasting consequences for the metabolic patterns of offspring exposed to that environment during gestation (Barker, 1995). Many of these patterns have been ascribed to epigenetic modification of key genes that regulate energy metabolism. Such mechanisms have been and continue to be explored and validated in rodent models (Levin and Govek 1998; Samuelsson et al., 2008; Nathanielsz et al., 2007; Shankar et al., 2008). Thus, new research focusing on the specific modifications of nucleosomal histones in chromatin and DNA is worthy of attention.
Interesting work has shown that manipulation of DNA methylation pathways by metabolic supplementation can alter the intergenerational, epigenetic patterns of obesity (Waterland et al., 2008), which directly addresses mechanisms that may be relevant to the Hunger Winter offspring. Such approaches, if validated with animal models and in clinical trials, offer the possibility of a ‘personalized medicine’ approach to obesity therapeutics (Martinez et al., 2012). Innovative clinical investigations to measure how altered maternal uterine environment during gestation affects DNA methylation patterns have already been reported (Cooper et al., 2012).
Although chromatin modifications are well studied in embryogenesis and cancer (Sharma et al., 2010), much less is known about their role in metabolic diseases. Intense research is now focused on how DNA sequence-specific transcription factors such as PPARγ and transcription co-regulators such as PGC-1α (Puigserver et al., 1998) interact with histones and nucleosome remodelers (Pedersen et al., 2001) to alter chromatin and reprogram gene expression networks (Jaenisch and Bird, 2003). These transcriptional co-regulator functions are also important in inflammation (Orjalo et al., 2009; Freund et al., 2010), which plays a critical role in the development of insulin resistance in obesity (Bastard et al., 2006; Shoelson et al., 2007).
Histone acetylation is also being considered as a relevant target for epigenetic manipulation to affect energy metabolism. For example, genome-wide patterns of histone H3 acetylation at lysine 9 and 18 have been reported in INS-1 cells, a rat model for the pancreatic β-cell, upon exposure to peptide incretin hormones (Kim et al., 2009). These modifications alter transcriptional co-regulator function with likely coordinate reprogramming of transcriptional networks that respond to glucose signal transduction in the β-cell. This approach may have translational significance for obese, insulin resistant patients, because these peptide hormones potentiate glucose-stimulated insulin secretion in the islet, among other effects. On the other hand, increased histone acetylation has been widely appreciated for decades as a mark of transcriptional activation at numerous loci. Absent a more specific, promoter-defined or β-cell-specific signature of histone acetylation that is unique and resolvable from the general somatic pattern, these insights have relatively modest utility and present no new therapeutic targets for Type 2 diabetes. The emerging practicality of manipulation of chromatin-controlled transcriptional programs for therapeutic benefit is potentially useful in view of the new availability of a class of bromodomain protein-directed, small molecule inhibitors (Filippakopoulos et al., 2010; Nicodeme et al., 2010; Muller, Filippakopoulos et al., 2010; Belkina and Denis, 2012). If specific, functional targets of histone acetylation could be identified, small molecule therapeutics for aberrant histone acetylation/transcriptional co-activation may develop an experimental basis for animal model experiments. However, the field has not yet reached this stage of development.
Activators of PPARγ function, such as pioglitazone, also appear to play a role in transcriptional programming controlled by PPARγ in part through alteration of histone modifications. In the β-cell, increased methylation of histone H3 at lysine 4 in the promoter regions of Ins1, Ins2 and Glut2 genes is maintained through the dimethyl-lysine regulating complex Set7/9 methyltransferase and is required for proper transcription of these loci (Deering et al., 2009). Pioglitazone treatment of obese db/db (leptin receptor-deficient) mice or high fat diet-fed wild type mice, which is well known to improve metabolism, dramatically improves in vivo transcription of Ins1, Ins2 and Glut2 genes. This improvement is associated with increased histone acetylation of these key target genes (Evans-Molina et al., 2009). Similar enrichment in methylation of histone H3 at lysine 4 has been reported in β-cell lines and islets, and parallel increases transcription of these same genes (Francis et al., 2005). In normal human pancreatic islets, histone lysine methylation patterns have been identified that define transcriptionally ‘primed’ as well as transcriptionally active promoters (Bhandare et al., 2010), but this vein of research is relatively underdeveloped, compared to the study of histone lysine methylation in cancer. Additional experiments to test mechanistic hypotheses are critically needed to deepen understanding of how manipulation of chromatin ‘readers’ and ‘writers’, and DNA methylation enzymes, might be mobilized therapeutically (Belkina and Denis, 2012) to improve β-cell dysfunction. Most of the relevant work to date has been no more than correlative.
III. THE ‘BRD2 LO’ MOUSE MODEL OF ‘METABOLICALLY HEALTHY’ OBESITY
How are these engines of histone modification (the ‘writers’ of the epigenome) and the transcriptional machines that respond to signal transduction to interpret these modifications (the ‘readers’ of the epigenome) to be studied and understood in their regulation of diverse transcriptional networks? Animal models that enable manipulation of chromatin and epigenetic mechanisms will obviously become increasingly important as investigators seek to understand the relevant genes and signal transduction pathways at work in obesity. In particular, one model with an epigenetic basis for obesity has received attention recently and offers a novel interpretive tool: the Brd2-deficient model for ‘metabolically healthy’ obesity. In both humans and mice, the BRD2 gene encodes an unusual transcriptional co-regulator that contains double bromodomains (Haynes et al., 1992; Jeanmougin et al., 1997; Winston and Allis, 1999; Horn and Peterson, 2001). Brd2 belongs to the BET family of transcriptional co-regulators defined by two tandem, mutually related bromodomains at the amino-terminus of the protein that bind to acetylated lysines in nucleosomal chromatin (Kanno et al., 2004; Nakamura et al., 2007), particularly acetyl histone H4 (Umehara et al., 2010) and transcriptionally couple histone acetylation to gene activation (LeRoy et al., 2008). The dual bromodomains in this family are followed by an ‘extraterminal’ domain that is involved in protein-protein interactions (Lin et al., 2008; Rahman et al., 2011), thus the ‘BET’ family name. The bromodomain is the only protein structural motif (Dhalluin et al., 1999) that is capable of ‘reading’ sites of histone acetylation in nucleosomal chromatin (Sanchez and Zhou, 2009). Brd2 studies have been important because they have revealed remarkable and unexpected roles for chromatin regulation in energy metabolism.
Gene targeting of the Brd2 locus in mice
Targeted disruption of the Brd2 gene, which is located in the class II Major Histocompatibility Complex (MHC) near Tnf, causes extreme obesity with hyperinsulinemia, but also hypoglycemia, hyperadiponectinemia and improved glucose tolerance quite distinct from other animal models of obesity (Wang et al., 2009). Brd2 had no previously known link to obesity, insulin sensitivity or energy metabolism (Belkina and Denis, 2010; Denis et al., 2010). The gene was disrupted in mouse embryonic stem cells by insertion of a lacZ cassette that encodes β-galactosidase. The cells were engineered by BayGenomics at the University of California, Davis, which is an arm of the Mutant Mouse Regional Resource Centers, and which receives support from the National Institutes of Health [http://www.mmrrc.org/catalog/overview_BG.php]. Two types of embryonic stem cells were developed, one with a lacZ insertion in the coding region of the gene (designated RRE050) and one with a lacZ insertion in the promoter region (designated RRT234). These latter cells were used to develop the brd2 lo mice (Wang et al., 2009). The phenotype could not have been predicted (Wang et al., 2009), yet holds out the possibility of a more unified, epigenetically-based mechanism that underlies several human co-morbidities associated with obesity and inflammation (Denis, 2010).
Systemic, protective phenotypes in brd2 lo mice
Despite severe obesity approaching 100g, brd2 lo animals show improved whole-body insulin sensitivity and do not develop insulin resistance or glucose intolerance. They show better whole-body insulin sensitivity than wild type, control animals on the C57Bl6/J background, despite dramatic obesity (Jornayvaz et al., forthcoming). Several whole-body mechanisms likely contribute to this protection (Wang et al., 2009). For example, although brd2 lo animals, both males and females, consume slightly more chow than age- and sex-matched wild type controls, they burn slightly more calories as heat in isothermal housing. The animals carry more interscapular brown adipose tissue and their white adipose tissue expresses higher levels of uncoupling proteins, which provides a mitochondrial mechanism for increased heat production. This phenotype is known to be protective of metabolism in obesity. In addition, brd2 lo mice show a respiratory exchange ratio of oxygen consumption and carbon dioxide production that is suggestive of fat metabolism at all times of day and night, fed state or fasting state, rather than a shift between carbohydrate metabolism expected for the fed state to fat metabolism expected for the fasted state (Wang et al., 2009). Continuous β-oxidation of fatty acid as the primary energy source might be expected to be metabolically protective, although the mitochondrial basis for this preferred mechanism of energy metabolism is not understood in these mice. Finally, production of high molecular weight adiponectin, an insulin-sensitizing adipokine, was elevated almost to the serum levels seen in adiponectin transgenic mice on the ob/ob (leptin deficient) background (Kim et al., 2007). Yet it is important to point out here that the adiponectin promoter has not been directly genetically manipulated in the source embryonic stem cells; this result suggests that Brd2 co-regulator function is ordinarily required for co-repression of adiponectin transcription. This hypothesis has not yet been tested experimentally.
The brd2 lo phenotype protects adipose tissue
Remarkably, knocked-down expression of Brd2 in the 3T3-L1 differentiation model also protects cultured adipocytes from TNF-α-induced insulin resistance in vitro, probably by uncoupling TNF receptor signaling from transcription (Wang et al., 2009). The effect of reduced Brd2 levels also phenocopies the action of thiazolidinedione drugs (TZDs), such as rosiglitazone and pioglitazone, which are used as insulin-sensitizers. Brd2 co-activator function opposes the action of PPARγ; Brd2 associates with PPARγ protein complexes and knockdown of Brd2 strongly stimulates PPARγ-dependent transcription and adipogenesis in 3T3-L1 cells (Wang et al., 2009).
Insulin resistant obesity is an inflammatory disease
Insulin resistance in the context of obesity is associated with a chronic state of subclinical inflammation (Weisberg et al., 2003; Xu et al., 2003; Bastard et al., 2006; Shoelson et al., 2006), including increased serum concentrations of C-reactive protein, interleukin-6, interleukin-8 and TNF-α in patients and different animal models of obesity (Kahn et al., 2006). In insulin resistant obesity, production of TNF-α in liver, fat and muscle by infiltrating, pro-inflammatory (M1) adipose tissue macrophages promotes insulin resistance directly (Hotamisligil et al., 1993). Here, M1 macrophages are distinguished from the more remodeling-purposed, alternatively polarized ‘M2’ type of macrophage. Commonly, pro-inflammatory macrophages infiltrate white adipose tissue in patients and different animal models of obesity (Kahn et al., 2006), forming characteristic, histologically identifiable patterns of leukocytes called ‘crown-like structures’ (Cancello et al., 2005; Cinti et al., 2005, Apovian et al., 2008) that surround stressed, dead and dying adipocytes, and are closely associated with metabolic risk. These structures have recently been shown also to contain B cells (McDonnell et al., 2012), and both T cells and B cells engage in crosstalk with peripheral blood monocytes to define the pro-inflammatory and anti-inflammatory balance of cytokines in the obese, insulin resistant adult (Jagannathan et al., 2009; 2010). To a first approximation, the greater the pro-inflammatory balance, the greater the metabolic dysfunction and the more advanced the disease. However, much work remains to be done to understand the specific T cell, B cell and macrophage subtypes that are required for disease progression, the anti-inflammatory and homeostatic mechanisms that oppose aggravated chronic inflammation, and the kinetics of entry onto and departure from the metabolic ‘theatres of action’ of each of these cell types: the central and peripheral adipose depots, the liver, the pancreas and the blood.
Metabolically protective phenotypes in the brd2 lo immune system
The brd2 lo mice exhibit broad-spectrum protection against the inflammatory complications of obesity (Wang et al., 2009). Inflammatory responses are mildly deficient in these animals (Belkina et al., 2010), which likely contributes to their protection against metabolic dysfunction. These observations lead to novel hypotheses about the interactions and crosstalk between adipocytes, macrophages and T cells that control the inflammatory state of adipose tissue. White adipose tissue shows reduced inflammation: bone marrow-derived macrophages underproduce pro-inflammatory cytokines (Belkina et al., 2010; Belkina et al., forthcoming), T cell migration is ablated (Denis et al., unpublished observations) and regulatory T cells are expanded (Denis et al., unpublished observations). Furthermore, because polarization of macrophages to the anti-inflammatory, pro-remodeling (M2) state requires PPARγ (Odegaard et al., 2007), increased PPARγ activity in brd2 lo macrophages and elevated serum adiponectin (Ohashi et al., 2009) is likely to promote M2 polarization, also protecting against inflammation-driven insulin resistance. By transcriptionally uncoupling obesity from insulin resistance, the mild hypo-inflammatory phenotype of the brd2 lo model focuses attention on T cell and adipose tissue macrophage mechanisms that likely protect ‘metabolically healthy’ obese individuals and might be harnessed to protect the larger population of obese patients with insulin resistance. Consistent with the inflammatory mechanism, a report in Nature Genetics has identified human single nucleotide polymorphisms (SNPs) in the BRD2 locus that are significantly associated with rheumatoid arthritis, which is driven by autoimmune and inflammatory processes (Mahdi et al., 2009). Furthermore, BRD2 variants may be associated with BMI in Pima Indians (Muller et al., 2011). Upregulation of pro-inflammatory cytokine genes has long been appreciated to promote insulin resistance and glucose intolerance, which precede serious metabolic dysregulation that leads to Type 2 diabetes (Pickup and Crook, 1998).
Furthermore, reduced inflammation protects against insulin resistance and characterizes human ‘metabolically healthy’ obese populations with attenuated glucose intolerance, dyslipidemia, hyperuricemia and hypertension (Bonora et al., 1991; Wildman et al., 2008). Therefore, control of adipose tissue-infiltrating inflammatory cells and their chromatin-regulated gene expression holds promise for insulin resistance patients. In obesity, macrophages, T cells and adipocytes produce pro-inflammatory and anti-inflammatory cytokines, chemokines and adipokines. Given that these factors synergize with, antagonize and regulate each other, there are many unanswered questions about what happens during the adipocyte-macrophage-T cell interactions (Kintscher et al., 2008), and how and when insulin resistance develops. We have proposed that these animals offer a novel model for human ‘metabolically healthy’ obesity. The model motivates interesting and informative hypotheses to test the links among leukocyte migration, pro-inflammatory cytokine production and insulin resistance in obesity.
IV. WHO ARE ‘METABOLICALLY HEALTHY’ OBESE HUMANS?
In most humans, the main effect of increasing BMI is a non-linear deterioration in metabolic and cardiovascular health. This process has been described as a gradual clustering of traits that include visceral obesity (as distinct from elevated BMI), insulin resistance, dyslipidemia, hypercholesterolemia and hypertension. In the clinic, insulin resistance status is often determined by the HOmeostatic Metabolic Assessment (HOMA), which is assessed using the formula: fasting insulin (μU/mL) x fasting glucose (mg/dL)/405 (Matthews et al., 1985; Ditschuneit et al., 1999). As a stringent measure of insulin resistance, a value of HOMA > 2 is often applied. According to the revised Adults Treatment Panel III (2001), a metabolic syndrome diagnosis is rendered when three or more of these five criteria are fulfilled: fasting plasma glucose concentration of at least 100 mg/dL, waist circumference > 88 cm, serum high density lipoprotein concentration < 50 mg/dL, blood pressure of at least 130/85 mmHg and serum triglyceride concentration of at least 150 mg/dL (Grundy et al., 2005). The International Diabetes Federation/American Heart Association/National Heart Lung and Blood Institute unified criteria are similar and require three of the above criteria, including the above cut points for fasting glucose, triglycerides and blood pressure (Alberti et al., 2009) with the added criteria of high density lipoprotein cholesterol level <40 mg/dl in men and <50 mg/dl in women, as well as waist circumference >94 cm in men and >80 cm in women, or drug treatment for diabetes, elevated triglycerides, low levels of high density lipoprotein or hypertension. Nevertheless, there is no universally accepted definition of metabolic syndrome. There has been disagreement in the literature about the optimal criteria to use for metabolic dysfunction in obesity because risk in certain human populations, such as specific ethnic groups, for certain diseases such as cardiovascular disease, may be better predicted by some criteria, such as glucose intolerance, than by others, such as metabolic syndrome. Oral glucose tolerance tests and criteria for impaired fasting glucose and impaired glucose tolerance have been used to supplement the diagnosis of metabolic syndrome, but discussion of this literature is beyond the scope of this report.
However, it is pertinent that certain obese individuals are ‘metabolically healthy’ and enjoy reduced risk for cardiovascular disease and Type 2 diabetes (Sims, 2001; Succurro et al., 2008; Wildman et al., 2008; Klöting et al., 2010). ‘Metabolically healthy’ obese adults have been defined as abdominally obese (BMI ≥ 30) but lacking metabolic syndrome (Meigs et al., 2006). Such individuals comprise about 25% of the adult obese population in the US, however, this prevalence depends on inclusion criteria, with some reported disagreement in prevalence, from a minimum of 11% of obese subjects in an Italian study (Calori et al., 2011) to 47.9% of obese subjects in a Korean study (Lee, 2009). The ‘metabolically healthy’ obese phenotype is best conceptualized as a continuous distribution of preserved insulin sensitivity as a function of increasing BMI (Blüher, 2010). Some of these individuals show protective, elevated levels of adiponectin (Aguilar-Salinas et al., 2008) and maintain normal glucose tolerance despite startlingly high BMI.
Factors that couple obesity to insulin resistance and metabolic syndrome are of great medical interest, because they underlie the etiology of obesity-driven Type 2 diabetes. Thus, the ‘metabolically healthy’ obese individual is likely to provide a goldmine of information. The study of this population for genes and pathways that couple obesity to insulin resistance has the potential to identify novel, ‘druggable’ targets to help unhealthy obese patients avoid the worst co-morbidities of their condition. Significantly, the ‘metabolically healthy’ obese phenotype is associated with a reduced inflammatory profile (Romano et al., 2003; Karelis et al., 2005). Thus, it is likely that inflammatory functions of the innate and adaptive immune system are essential to link obesity to insulin resistance, cardiometabolic risk and Type 2 diabetes. Greater detail concerning the immune cell subtypes, their cytokine production profiles and kinetics of mobilization in the insulin resistant obese subject, and how these differ in critical ways form the ‘metabolically healthy’ obese subject is urgently required.
V. OTHER ANIMAL MODELS OF ‘METABOLICALLY HEALTHY’ OBESITY
Additional mechanistic understanding of how this population of humans is protected from obesity-driven co-morbidity will be achieved through hypothesis building and testing in animal models. Beyond the example of brd2 lo mice, there are other, fundamentally different types of animal models available, some that are primarily immunological, while others are adipose tissue-directed. These different molecular and cellular perturbations or deficiencies will enable more precise mechanistic exploration of the relevant pathways that couple obesity to insulin resistance.
1. Low-inflammatory models include
Interleukin-1 Receptor 1 knockout. A reduced inflammatory profile, particularly lower levels of TNF and interleukin-6, appears to protect these animals from high fat diet-induced insulin resistance and glucose intolerance (McGillicuddy et al., 2011).
Inducible nitric oxide synthase (iNOS) knockout: Deficient polarization of macrophages as a result of knockout of Nos2 protects against obesity-induced skeletal muscle insulin resistance, and this is associated with improved phosphoinositide 3-kinase/Akt activity (Perreault and Marette, 2001)
Ablation of TNF: Antibody against TNF improves insulin resistance in obesity (Hotamisligil et al., 1993), and mice deficient in TNF signaling are protected from insulin resistance in obesity (Uysal et al., 1997).
TWEAK knockout: TNF-related weak inducer of apoptosis (TWEAK), a cytokine of the TNF superfamily, is important for tissue remodeling after injury (Burkly et al., 2007) but also for remodeling of adipose tissue to accommodate increased storage in obesity (Li et al., 2009). The TWEAK pathway is activated in obese Type 2 diabetic patients (Chacón et al., 2006). TWEAK deficiency (Campbell, 2006) shifts macrophage polarization to the alternatively-activated ‘low-inflammatory’ phenotype, increases collagen turnover and decreases JNK activation in gonadal adipose tissue, conferring metabolic protection in obesity (M. S. Obin, Tufts University School of Medicine, personal communication).
IκBα superrepressor: Expression in the liver of the repressor of NF-κB signaling protects against high fat diet-induced and low level NF-κB-induced insulin resistance (Cai et al., 2005).
2. Adipose tissue models include
Collagen 6 knockout: Increased capability of adipose depots to remodel and accept increased storage in obesity appears to reduce adipocyte stress and apoptosis (Khan et al., 2009). This increased capacity depends on loss of collagen 6, and improves fasting glucose and glucose tolerance perhaps by relaxing physical, steric constraints on adipose depots.
Adiponectin transgenic: Severe obesity with insulin resistance on an ob/ob (leptin deficient) background can be ameliorated in a dramatic fashion by transgenic expression of adiponectin (Kim et al., 2007), a factor that sensitizes cells to insulin signaling.
Such mouse models will be useful to reveal different kinds of metabolic protection and how crosstalk may also protect organ systems from co-morbidities (Denis, 2010).
VI. DEREPRESSION OF INSULIN TRANSCRIPTION IN THE ‘BRD2 LO’ ENVIRONMENT
A deeper understanding of the mechanisms that control maternal-fetal transmission of increased risk for Type 2 diabetes is critical at this stage of the obesity epidemic. There is significant evidence, obtained initially from studies of the Pima Indians, that mothers with Type 2 diabetes can confer elevated diabetes risk to offspring (Dabelea et al., 2000; Dabelea and Pettitt, 2001; Dabelea 2007; Dabelea et al., 2008). The development of Type 2 diabetes is dependent on both the gradually decreasing metabolic health of the obese individual as insulin resistance and inflammation increases, as well as strain on β-cell production of insulin that eventually leads to β-cell failure. The question of how epigenetic mechanisms influence the distinct but related risks to declining insulin action and insulin production has not been well studied.
Increased β-cell proliferation and differentiation, as well as increased insulin transcription and release from pancreatic β-cells in vivo, undoubtedly protects obese brd2 lo animals from progression to glucose intolerance and β-cell failure. Islets show no signs of apoptosis or stress but are expanded from an early age, likely as an early perturbation to islet homeostasis that is directly attributable to Brd2 reduction, not to insulin resistance in the periphery (Wang et al., 2009).
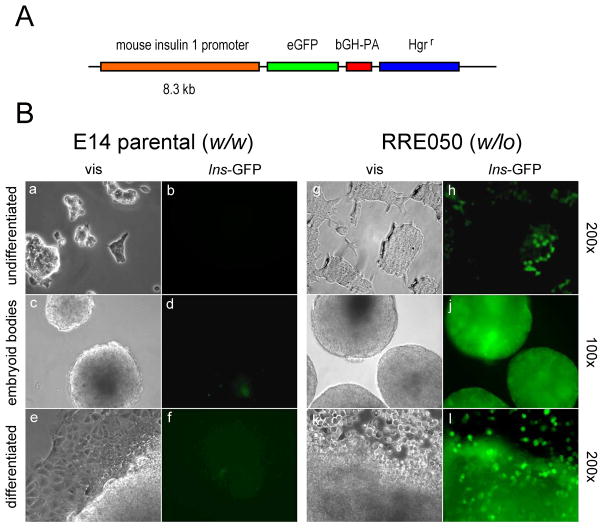
We explored the potential significance of reduced Brd2 expression during development. We wanted to know if reduced Brd2 expression in the embryonic stem cells of RRE050 or RRT234 origin (Wang et al., 2009) had altered biology relevant to energy metabolism. Based on published results of increased insulin 1 gene transcription in β-cell lines, we hypothesized that insulin transcription would be potentiated in the RRE050 or RRT234 cells. Accordingly, we transfected RRE050 embryonic stem cells with an eGFP reporter construct for the Ins1 promoter, obtained as a generous gift from Dr Manami Hara, then selected the cells under hygromycin as shown in Figure 1.
Figure 1. Reduced expression of Brd2 potentiates insulin transcription in embryonic stem cells.
(A) Schematic of mouse insulin 1 promoter that drives expression of eGFP (eGFP). Promoter elements are also shown: bovine growth hormone poly A sequence (bGH-PA) and hygromycin resistance (Hgrr). (B) Visual (vis) and fluorescence (Ins-GFP) micrographs of ES cells transfected with the construct in (A) and permitted to undergo differentiation. Undifferentiated cells were cultured on gelatin-coated tissue culture plastic in the presence of Leukemia Inhibitory Factor (LIF) (undifferentiated), then LIF was withdrawn to permit the formation of embryoid bodies for two weeks (embryoid bodies), and culture was continued for an additional two weeks to permit additional differentiation (differentiated). Magnification is shown at the right hand edge of the Figure. The parental ES cells (E14 parental; w/w) were compared to Brd2 KO ES cells (RRE050; w/lo) as described in Wang et al 2009, at the same stage of differentiation.
Remarkably, this result shows that reduced levels of Brd2 potentiate transcription of the insulin gene extremely early in mouse development, even before the embryonic stem cell has lost totipotent characteristics during the course of in vitro differentiation. One potential implication of these data is that targeted inhibition of Brd2 or genetic modification of embryonic stem cells could provide a therapeutic strategy for β-cell failure through regeneration of β-cell mass, or for gene therapy for Type 1 diabetes. Epigenetically based therapeutics for metabolic dysfunction are therefore feasible, although detailed mechanistic studies are obviously now required.
VII. TRANSLATIONAL IMPLICATIONS OF EPIGENETIC REPROGRAMMING: CONCLUSIONS
Apart from the maternal-fetal transmission of increased cardiometabolic risk in the Hunger Winter case, other processes with a potential epigenetic component are likely at work in obesity. For example, weight regain after bariatric surgery has emerged as a worrisome problem for clinicians (Magro et al., 2008). Over the long term, a significant fraction of bariatric patients (20.4% for morbidly obese patients and 34.9% for super obese patients in one study) (Christou et al., 2006) regain significant weight. For the fraction of patients for whom regain is unrelated to surgical failure, it is possible that epigenetic factors play a role in the difficulty with maintaining healthy weight in the ten year period that follows surgery. Likewise, diet and lifestyle modification for the less-morbidly obese also shows only mixed success.
Important, outstanding questions remain. It is unclear whether there is an epigenetic component to the ‘metabolically healthy’ obese phenotype, either in humans or in mice. Studies to measure a maternal genetic contribution to Brd2-regulated metabolism have yet to be performed in brd2 lo mice. There is some evidence for maternally-defined DNA methylation patterns in brd2 lo mice (Fangnian Wang, unpublished observations), but additional experiments are required to address this issue. Because Brd2 is homologous to a known ‘maternal effect’ gene in Drosophila, called female sterile (1) homeotic (Digan et al., 1986; Beck et al., 1992; Denis and Green, 1996), we have proposed that Brd2-regulated metabolism may have a maternal effect in humans and mice (Denis, 2010; Belkina and Denis, 2012). Finally, we speculate that epigenetic modifications that attenuate expression of the BRD2 locus, nearby TNF, or other genes that encode pro-inflammatory factors, will be present in ‘metabolically healthy’ obese patients, but modifications that exacerbate expression of such genes will be present in at-risk, ‘pro-inflammatory’ obese populations. In support of this idea, recent work identifies increased inflammatory signatures in the uterine environment of obese female rats, which leads to weight gain and metabolic consequences for offspring (Shankar et al., 2011). The details of maternal effect or epigenetic contribution to body composition and fat distribution in humans are not well understood and will require further investigation in clinical studies and animal model systems.
More urgently, based on our published and preliminary data and other published data discussed here, we speculate that surgical, dietary and exercise interventions may be insufficient to ensure sustained weight loss for obese, morbidly obese and super obese patients if key genes important for energy metabolism have become epigenetically modified or reprogrammed in a way that resists whole-body return to healthy BMI. Such a mechanism has not been studied in patients or animal models after weight loss, but offers a compelling possible explanation for the persistent failure of these therapeutic interventions among many patients. Epigenetic ‘correction’ might be possible with drugs that directly affect ‘writers’ of histone methylation and acetylation marks such as histone deacetylase inhibitors, or ‘readers’, such as the small molecule BET protein inhibitors. Re-randomization of certain epigenetic marks of histone acetylation, methylation, or of DNA methylation, might help patients to lose weight by encouraging key tissues of the body to ‘forget’ the unfortunate metabolic history of chronic obesity. Thus, metabolic ‘set-point’ could be reset to a healthy orexigenic state and BMI would be easier to normalize after weight loss. Without this kind of drug intervention to correct the epigenetic status quo of chronic obesity, successful and sustained, long-term weight loss, even after bariatric surgery, may well be impossible for most patients.
Acknowledgments
We thank Dr. Minami Hara of the University of Chicago for the generous gift of a mouse insulin promoter-eGFP reporter construct. We also thank Caroline Apovian, Barbara Corkey, Barbara Nikolajczyk and Martin Obin for useful discussions and valuable suggestions. GVD is supported by the National Institutes of Health (R56 DK090455), the Boston University Clinical and Translational Science Institute (UL1-TR000157) and two NIH-supported Centers: the Boston Area Diabetes and Endocrinology Research Center (P30 DK057521; PI: Joseph Avruch) and the Boston Nutrition Obesity Research Center (P30 DK046200; PI: Susan K. Fried). GVD is currently the Chair of Basic Science Section of The Obesity Society, and gratefully acknowledges the Society for its support and promotion of scientific interactions and collaborations.
ABBREVIATIONS
- BET
bromodomain and extraterminal domain
- BMI
body mass index
- HOMA
homeostatic metabolic assessment
- MHC
major histocompatibility complex
- PPAR
peroxisome proliferator-activated receptor
- SNP
single nucleotide polymorphisms
- TNF
tumor necrosis factor
- TWEAK
TNF-related weak inducer of apoptosis
- TZD
thiazolidinedione
References
- Aguilar-Salinas CA, García EG, Robles L, Riaño D, Ruiz-Gomez DG, García-Ulloa AC, Melgarejo MA, Zamora M, Guillen-Pineda LE, Mehta R, Canizales-Quinteros S, Tusie Luna MT, Gómez-Pérez FJ. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–4079. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. Br Med J. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J. 1989;298:654–657. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Beck S, Hanson I, Kelly A, Pappin DJC, Trowsdale J. A homologue of the Drosophila female sterile homeotic (fsh) gene in the class II region of the human MHC. DNA Seq. 1992;2:203–210. doi: 10.3109/10425179209020804. [DOI] [PubMed] [Google Scholar]
- Belkina AC, Denis GV. Obesity genes and insulin resistance. Curr Opin Endocrinol Diabetes Obes. 2010;17:472–477. doi: 10.1097/MED.0b013e32833c5c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkina AC, Blanton W, Wang F, Liu H, Denis GV. Whole body Brd2 deficiency protects obese mice from insulin resistance by creating a low inflammatory environment. Obesity. 2010;18:S58. [Google Scholar]
- Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12:465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Bhandare R, Schug J, Le Lay J, Fox A, Smirnova O, Liu C, Naji A, Kaestner KH. Genome-wide analysis of histone modifications in human pancreatic islets. Genome Res. 2010;20:428–433. doi: 10.1101/gr.102038.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- Bonora E, Willeit J, Kiechl S, Oberhollenzer F, Egger G, Bonadonna R, Muggeo M. U-shaped and J-shaped relationships between serum insulin and coronary heart disease in the general population. The Bruneck Study. Diabetes Care. 1991;21:221–230. doi: 10.2337/diacare.21.2.221. [DOI] [PubMed] [Google Scholar]
- Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: Role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16. doi: 10.1016/j.cyto.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G, Perseghin G. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–215. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Burkly LC, Gao H, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C. Proinflammatory effects of Tweak/Fn14 interactions in glomerular mesangial cells. J Immunol. 2006;176:1889–1898. doi: 10.4049/jimmunol.176.3.1889. [DOI] [PubMed] [Google Scholar]
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- Chacón MR, Richart C, Gómez JM, Megía A, Vilarrasa N, Fernández-Real JM, García-España A, Miranda M, Masdevall C, Ricard W, Caubet E, Soler J, Vendrell J. Expression of TWEAK and its receptor Fn14 in human subcutaneous adipose tissue. Relationship with other inflammatory cytokines in obesity. Cytokine. 2006;33:129–137. doi: 10.1016/j.cyto.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Chen JR, Zhang J, Lazarenko OP, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J. 2012;26:1131–1141. doi: 10.1096/fj.11-197822. [DOI] [PubMed] [Google Scholar]
- Christou NV, Look D, MacLean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Cooper WN, Khulan B, Owens S, Elks CE, Seidel V, Prentice AM, Belteki G, Ong KK, Affara NA, Constância M, Dunger DB. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J. 2012;26:1782–1790. doi: 10.1096/fj.11-192708. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Pettitt DJ. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001;14:1085–1091. doi: 10.1515/jpem-2001-0803. [DOI] [PubMed] [Google Scholar]
- Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30 (Suppl 2):S169–S174. doi: 10.2337/dc07-s211. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB, Jr, Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering TG, Ogihara T, Trace AP, Maier B, Mirmira RG. Methyltransferase Set7/9 maintains transcription and euchromatin structure at islet-enriched genes. Diabetes. 2008;58:185–193. doi: 10.2337/db08-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hoed M, Ekelund U, Brage S, Grontved A, Zhao JH, Sharp SJ, Ong KK, Wareham NJ, Loos RJ. Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes. 2010;59:2980–2988. doi: 10.2337/db10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis GV, Green MR. A novel, mitogen-activated nuclear kinase is related to a Drosophila developmental regulator. Genes Dev. 1996;10:261–271. doi: 10.1101/gad.10.3.261. [DOI] [PubMed] [Google Scholar]
- Denis GV. Bromodomain coactivators in cancer, obesity, type 2 diabetes and inflammation. Discov Med. 2010;10:489–499. [PMC free article] [PubMed] [Google Scholar]
- Denis GV, Nikolajczyk BN, Schnitzler GR. An emerging role for bromodomain-containing proteins in chromatin regulation and transcriptional control of adipogenesis. FEBS Lett. 2010;584:3260–3268. doi: 10.1016/j.febslet.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- Digan ME, Haynes SR, Mozer BA, Dawid IB, Forquignon F, Gans M. Genetic and molecular analysis of fs(1)h, a maternal effect homeotic gene in Drosophila. Dev Biol. 1986;114:161–169. doi: 10.1016/0012-1606(86)90392-1. [DOI] [PubMed] [Google Scholar]
- Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- Duffey KJ, Popkin BM. High-fructose corn syrup: is this what’s for dinner? Am J Clin Nutr. 2008;88:1722S–1732S. doi: 10.3945/ajcn.2008.25825C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, Mirmira RG. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol Cell Biol. 2009;29:2053–2067. doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi S, O’Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- Florez J. The genetics in Type 2 diabetes: A realistic appraisal in 2008. J Clin Endocrinol Metab. 2008;93:4633–4642. doi: 10.1210/jc.2008-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J, Chakrabarti SK, Garmey JC, Mirmira RG. Pdx-1 links histone H3-Lys-4 methylation to RNA polymerase II elongation during activation of insulin transcription. J Biol Chem. 2005;280:36244–36253. doi: 10.1074/jbc.M505741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart Lung and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. Erratum in: Circulation 112, e297, e298 (2005) [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. Br Med J. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2009;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Haynes SR, Dollard C, Winston F, Beck S, Trowsdale J, Dawid IB. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucl Acids Res. 1992;20:2603. doi: 10.1093/nar/20.10.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299:R711–722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn PJ, Peterson CL. The bromodomain: a regulator of ATP-dependent chromatin remodeling? Front Biosci. 2001;6:D1019–1023. doi: 10.2741/horn. [DOI] [PubMed] [Google Scholar]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, Kantarci A, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmougin F, Wurtz JM, Le Douarin B, Chambon P, Losson R. The bromodomain revisited. Trends Biochem Sci. 1997;22:151–153. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- Kanno T, Kanno Y, Siegel RM, Jang MK, Lenardo MJ, Ozato K. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol Cell. 2004;13:33–43. doi: 10.1016/s1097-2765(03)00482-9. [DOI] [PubMed] [Google Scholar]
- Klannemark M, Orho M, Langin D, Laurell H, Holm C, Reynisdottir S, Arner P, Groop L. The putative role of the hormone-sensitive lipase gene in the pathogenesis of Type II diabetes mellitus and abdominal obesity. Diabetologia. 1998;41:1516–1522. doi: 10.1007/s001250051099. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Haffner SM, O’Neill MC, Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, Lachin JM, Viberti GC ADOPT Study Group. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- Kern PA. Potential role of TNFalpha and lipoprotein lipase as candidate genes for obesity. J Nutr. 1997;127:1917S–1922S. doi: 10.1093/jn/127.9.1917S. [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Nian C, McIntosh CH. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 modulate beta-cell chromatin structure. J Biol Chem. 2009;284:12896–12904. doi: 10.1074/jbc.M809046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006;9:388–394. doi: 10.1097/01.mco.0000232898.74415.42. [DOI] [PubMed] [Google Scholar]
- Lee K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: characteristics and health behaviors. Asia Pac J Clin Nutr. 2009;18:280–284. [PubMed] [Google Scholar]
- Lefebvre AM, Laville M, Vega N, Riou JP, van Gaal L, Auwerx J, Vidal H. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes. 1998;47:98–103. doi: 10.2337/diab.47.1.98. [DOI] [PubMed] [Google Scholar]
- Leibel RL. Energy in, energy out, and the effects of obesity-related genes. N Engl J Med. 2008;359:2603–2604. doi: 10.1056/NEJMe0808660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Li H, Mittal A, Paul PK, Kumar M, Srivastava DS, Tyagi SC, Kumar A. Tumor necrosis factor-related weak inducer of apoptosis augments matrix metalloproteinase 9 (MMP-9) production in skeletal muscle through the activation of Nuclear Factor-κB-inducing kinase and p38 mitogen-activated protein kinase. J Biol Chem. 2009;284:4439–4450. doi: 10.1074/jbc.M805546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Umehara T, Inoue M, Saito K, Kigawa T, Jang MK, Ozato K, Yokoyama S, Padmanabhan B, Güntert P. Solution structure of the extraterminal domain of the bromodomain-containing protein BRD4. Protein Sci. 2008;17:2174–2179. doi: 10.1110/ps.037580.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–651. doi: 10.1007/s11695-007-9265-1. [DOI] [PubMed] [Google Scholar]
- Mahdi H, Fisher BA, Källberg H, Plant D, Malmström V, Rönnelid J, Charles P, Ding B, Alfredsson L, Padyukov L, Symmons DP, Venables PJ, Klareskog L, Lundberg K. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41:1319–1324. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- Martínez JA, Cordero P, Campión J, Milagro FI. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc Nutr Soc. 2012;71:276–283. doi: 10.1017/S0029665112000055. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McDonnell ME, Ganley-Leal LM, Mehta A, Bigornia SJ, Mott M, Rehman Q, Farb MG, Hess DT, Joseph L, Gokce N, Apovian CM. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity (Silver Spring) 2012;20:1372–1378. doi: 10.1038/oby.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy FC, Harford KA, Reynolds CM, Oliver E, Claessens M, Mills KHG, Roche HM. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60:1688–1698. doi: 10.2337/db10-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Zee RY, Robinson BG. Significant relationships of plasma lipids and body mass index with polymorphisms at the linked low-density-lipoprotein receptor gene and insulin receptor gene loci (19p13.2) in essential hypertensive patients. Clin Sci (Lond) 1994;86:583–592. doi: 10.1042/cs0860583. [DOI] [PubMed] [Google Scholar]
- Muller Y, Abdussamad M, Hanson R, Knowler WC, Bogardus C, Baier L. Variants in/near BRD2 are associated with body mass index in Pima Indians. Diabetes. 2011;60 (Suppl 1):A1400. [Google Scholar]
- Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Umehara T, Nakano K, Jang MK, Shirouzu M, Morita S, Uda-Tochio H, Hamana H, Terada T, Adachi N, Matsumoto T, Tanaka A, Horikoshi M, Ozato K, Padmanabhan B, Yokoyama S. Crystal structure of the human BRD2 bromodomain: insights into dimerization and recognition of acetylated histone H4. J Biol Chem. 2007;282:4193–4201. doi: 10.1074/jbc.M605971200. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Poston L, Taylor PD. In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Clin Perinatol. 2007;34:515–526. doi: 10.1016/j.clp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, White J, Kirilovsky J, Rice CM, Lora JM, Prinjha RK, Lee K, Tarakhovsky A. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Amstrup Pedersen A, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization towards an anti-inflammatory phenotype. J Biol Chem. 2009;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppert JM, Vohl MC, Chagnon M, Dionne FT, Cassard-Doulcier AM, Ricquier D, Pérusse L, Bouchard C. DNA polymorphism in the uncoupling protein (UCP) gene and human body fat. Int J Obes Relat Metab Disord. 1994;18:526–531. [PubMed] [Google Scholar]
- Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims EAH. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- Pedersen TA, Kowenz-Leutz E, Leutz A, Nerlov C. Cooperation between C/EBPalpha TBP/TFIIB and SWI/SNF recruiting domains is required for adipocyte differentiation. Genes Dev. 2001;15:3208–3216. doi: 10.1101/gad.209901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obes Res. 2003;11:1325–1332. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The world is fat. Sci Am. 2007;297:88–95. doi: 10.1038/scientificamerican0907-88. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, Howley PM. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol Cell Biol. 2011;31:2641–2652. doi: 10.1128/MCB.01341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E. Metabolic differences and the development of obesity. Metabolism. 1995;9 (Suppl 3):12–14. doi: 10.1016/0026-0495(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest. 1994;93:2590–2599. doi: 10.1172/JCI117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricquier D, Casteilla L, Bouillaud F. Molecular studies of the uncoupling protein. FASEB J. 1991;5:2237–2242. doi: 10.1096/fasebj.5.9.1860614. [DOI] [PubMed] [Google Scholar]
- Ridderstråle M, Groop L. Genetic dissection of type 2 diabetes. Mol Cell Endocrinol. 2009;297:10–17. doi: 10.1016/j.mce.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Romano M, Guagnano MT, Pacini G, Vigneri S, Falco A, Marinopiccoli M, Manigrasso MR, Basili S, Davì G. Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. J Clin Endocrinol Metab. 2003;88:5321–5326. doi: 10.1210/jc.2003-030508. [DOI] [PubMed] [Google Scholar]
- Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Sanchez R, Zhou MM. The role of human bromodomains in chromatin biology and gene transcription. Curr Opin Drug Discovery Dev. 2009;12:659–665. [PMC free article] [PubMed] [Google Scholar]
- Schneck L, Valenti C, Amsterdam D, Friedland J, Adachi M, Volk BW. Prenatal diagnosis of Tay-Sachs disease. Lancet. 1970;1:582–584. doi: 10.1016/s0140-6736(70)91624-7. [DOI] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:R528–R538. doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- Shankar K, Zhong Y, Kang P, Lau F, Blackburn ML, Chen JR, Borengasser SJ, Ronis MJ, Badger TM. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology. 2011;152:4158–4170. doi: 10.1210/en.2010-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation and insulin resistance. Gastroenterol. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Rokholm B, Kaprio J, Sørensen TI. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int J Obes (Lond) 2010;34:29–40. doi: 10.1038/ijo.2009.177. [DOI] [PubMed] [Google Scholar]
- Stich V, Harant I, De Glisezinski I, Crampes F, Berlan M, Kunesova M, Hainer V, Dauzats M, Rivière D, Garrigues M, Holm C, Lafontan M, Langin D. Adipose tissue lipolysis and hormone-sensitive lipase expression during very-low-calorie diet in obese female identical twins. J Clin Endocrinol Metab. 1997;82:739–744. doi: 10.1210/jcem.82.3.3793. [DOI] [PubMed] [Google Scholar]
- Succurro E, Marini MA, Frontoni S, Hribal ML, Andreozzi F, Lauro R, Perticone F, Sesti G. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring) 2008;16:1881–1886. doi: 10.1038/oby.2008.308. [DOI] [PubMed] [Google Scholar]
- Umehara T, Nakamura Y, Jang MK, Nakano K, Tanaka A, Ozato K, Padmanabhan B, Yokoyama S. Structural basis for acetylated histone H4 recognition by the human BRD2 bromodomain. J Biol Chem. 2010;285:7610–7618. doi: 10.1074/jbc.M109.062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, Werman A, Pories WJ, Caro JF, Flier JS. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. 1997;99:2416–2422. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Silver K, Bogardus C, Knowler WC, Celi FS, Austin S, Manning B, Strosberg AD, Stern MP, Raben N, et al. Time of onset of non-insulin-dependent diabetes mellitus and genetic variation in the beta 3-adrenergic-receptor gene. N Engl J Med. 1995;333:343–347. doi: 10.1056/NEJM199508103330603. [DOI] [PubMed] [Google Scholar]
- Wang F, Liu H, Blanton WP, Belkina A, LeBrasseur NK, Denis GV. Brd2 disruption in mice causes severe obesity without type 2 diabetes. Biochem J. 2009;425:71–83. doi: 10.1042/BJ20090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes. 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- Winston F, Allis CD. The bromodomain: a chromatin-targeting module? Nature Struct Biol. 1999;6:601–604. doi: 10.1038/10640. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bradfield JP, Li M, Wang K, Zhang H, Kim CE, Annaiah K, Glessner JT, Thomas K, Garris M, Frackelton EC, Otieno FG, Shaner JL, Smith RM, Chiavacci RM, Berkowitz RI, Hakonarson H, Grant SF. The role of obesity-associated loci identified in genome-wide association studies in the determination of pediatric BMI. Obesity (Silver Spring) 2009;17:2254–2257. doi: 10.1038/oby.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]