Abstract
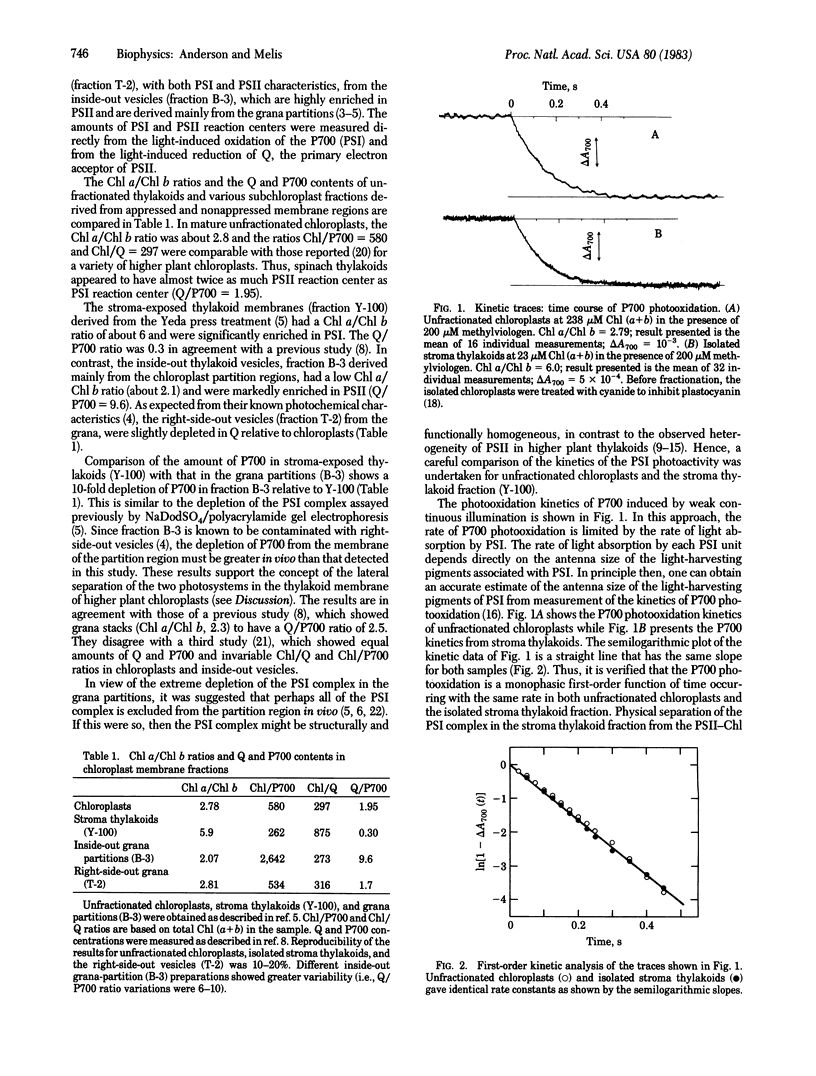
The stoichiometric amounts and the photoactivity kinetics of photosystem I (PSI) and of the α and β components of photosystem II (PSIIα and PSIIβ) were compared in spinach chloroplast membrane (thylakoid) fractions derived from appressed and nonappressed regions. Stroma-exposed thylakoid fractions from the nonappressed regions were isolated by differential centrifugation following a mechanical press treatment of the chloroplasts. Thylakoid vesicles derived mainly from the appressed membranes of grana were isolated by the aqueous polymer two-phase partition method. Stroma-exposed thylakoids were found to have a chlorophyll a/chlorophyll b ratio of 6.0 and a PSIIβ/PSI reaction center ratio of 0.3. Kinetic analysis of system II photoactivity revealed the absence of PSIIα from stroma-exposed thylakoids. The photoactivity of system I in stroma-exposed thylakoids showed a single kinetic component identical to that of unfractionated chloroplasts, suggesting that PSI does not receive excitation energy from the PSII-chlorophyll ab light-harvesting complex. Thus, stroma-exposed thylakoids are significantly enriched in both PSI and PSIIβ. Inside-out vesicles from the appressed membranes of grana-partition regions had a chlorophyll a/chlorophyll b ratio of 2.0 and a PSII/PSI reaction center ratio of 10.0. The photoactivity of system II showed the membranes of the grana-partition regions to be significantly enriched in PSIIα. We conclude that PSIIα is exclusively located in the membranes of the grana partitions while PSIIβ and PSI are located in stroma-exposed thylakoids. The low PSI reaction center (P700) content of vesicles derived from grana partitions and the kinetic homogeneity of the PSI complex suggest total exclusion of P700 as a functional component in the membrane of the grana-partition region.
Keywords: photosynthesis, chloroplast structure, chloroplast function, reaction center, membrane differentiation
Full text
PDF
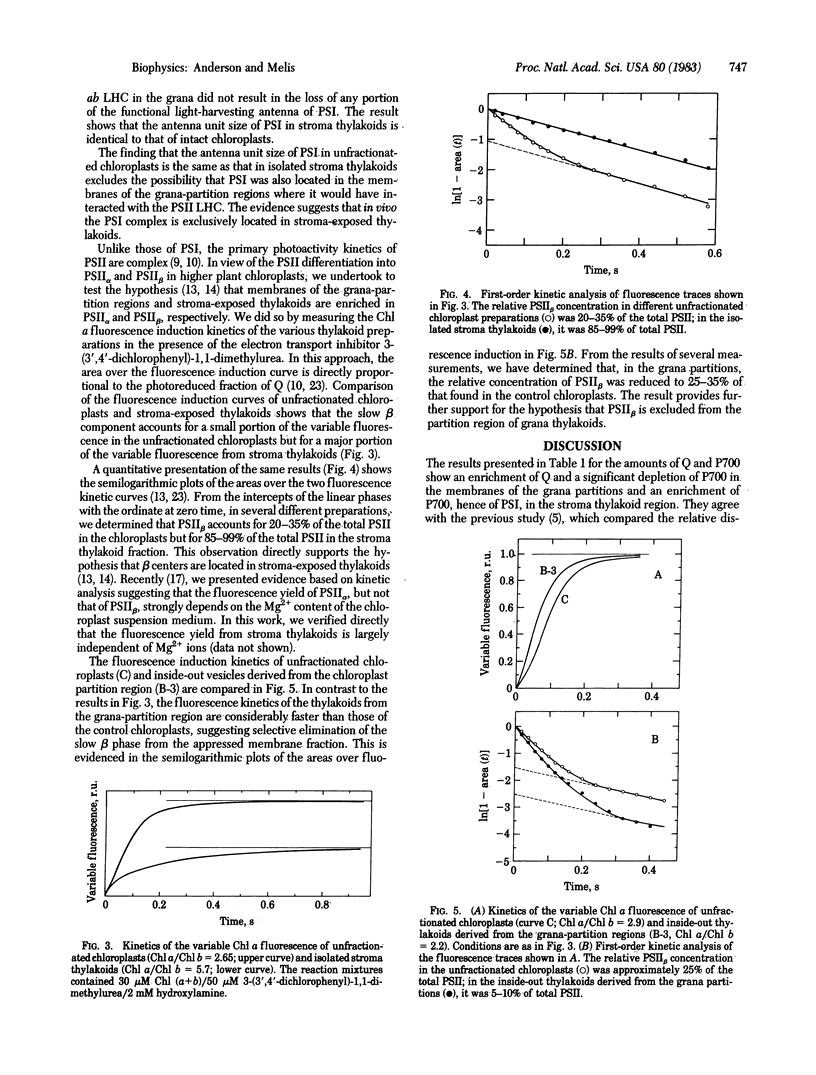


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund H. E., Andersson B., Albertsson P. A. Isolation of photosystem II enriched membrane vesicles from spinach chloroplasts by phase partition. Biochim Biophys Acta. 1976 Dec 6;449(3):525–535. doi: 10.1016/0005-2728(76)90161-4. [DOI] [PubMed] [Google Scholar]
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Andersson B., Anderson J. M. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1980 Dec 3;593(2):427–440. doi: 10.1016/0005-2728(80)90078-x. [DOI] [PubMed] [Google Scholar]
- Armond P. A., Arntzen C. J. Localization and Characterization of Photosystem II in Grana and Stroma Lamellae. Plant Physiol. 1977 Mar;59(3):398–404. doi: 10.1104/pp.59.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Chain R. K. Regulation of ferredoxin-catalyzed photosynthetic phosphorylations. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4961–4965. doi: 10.1073/pnas.72.12.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G. M., Bhatnagar D., Dilley R. A. Proton release in photosynthetic water oxidation: evidence for proton movement in a restricted domain. Biochemistry. 1981 Apr 14;20(8):2307–2315. doi: 10.1021/bi00511a037. [DOI] [PubMed] [Google Scholar]
- Baker G. M., Bhatnagar D., Dilley R. A. Site-specific interaction of ATPase-pumped protons with photosystem II in chloroplast thylakoid membranes. J Bioenerg Biomembr. 1982 Aug;14(4):249–264. doi: 10.1007/BF00751019. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Croze E. Characterization of two quenchers of chlorophyll fluorescence with different midpoint oxidation-reduction potentials in chloroplasts. Biochim Biophys Acta. 1979 Jan 11;545(1):188–201. doi: 10.1016/0005-2728(79)90125-7. [DOI] [PubMed] [Google Scholar]
- Izawa S., Kraayenhof R., Ruuge E. K., Devault D. The site of KCN inhibition in the photosynthetic electron transport pathway. Biochim Biophys Acta. 1973 Sep 26;314(3):328–339. doi: 10.1016/0005-2728(73)90117-5. [DOI] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Homann P. H. A selective effect of Mg2+ on the photochemistry at one type of reaction center in photosystem II of chloroplasts. Arch Biochem Biophys. 1978 Oct;190(2):523–530. doi: 10.1016/0003-9861(78)90306-5. [DOI] [PubMed] [Google Scholar]
- Melis A., Homann P. H. Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol. 1976 May;23(5):343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Melis A. Kinetic analysis of P-700 photoconversion: effect of secondary electron donation and plastocyanin inhibition. Arch Biochem Biophys. 1982 Sep;217(2):536–545. doi: 10.1016/0003-9861(82)90535-5. [DOI] [PubMed] [Google Scholar]
- Melis A. Oxidation-reduction potential dependence of the two kinetic components in chloroplast system II primary photochemistry. FEBS Lett. 1978 Nov 15;95(2):202–206. doi: 10.1016/0014-5793(78)80993-4. [DOI] [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Staehelin L. A. Analysis of the thylakoid outer surface. Coupling factor is limited to unstacked membrane regions. J Cell Biol. 1976 Jan;68(1):30–47. doi: 10.1083/jcb.68.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Thielen A. P., van Gorkom H. J. Quantum efficiency and antenna size of photosystems II alpha, II beta and I in tobacco chloroplasts. Biochim Biophys Acta. 1981 Mar 12;635(1):111–120. doi: 10.1016/0005-2728(81)90012-8. [DOI] [PubMed] [Google Scholar]
- Wang R. T., Myers J. On the state 1-state 2 phenomenon in photosynthesis. Biochim Biophys Acta. 1974 Apr 23;347(1):134–140. doi: 10.1016/0005-2728(74)90206-0. [DOI] [PubMed] [Google Scholar]



