Abstract
Introduction
Tivozanib is a novel tyrosine kinase inhibitor (TKI) which inhibits vascular endothelial growth factor (VEGF) receptors-1, -2, and -3 at nanomolar concentrations.
Areas Covered
A comprehensive MEDLINE and American Society of Clinical Oncology abstract search was performed to gather all relevant clinical and translational data related to tivozanib. We discuss preclinical studies associated with tivozanib, and the results of aphase I assessment in advanced solid tumors. We highlight combination studies with tivozanib, including pairings of tivozanib with cytotoxic therapy in patients with colorectal cancer and breast cancer. A randomized discontinuation phase II study and a randomized phase III study assessing the activity of tivozanib in metastatic renal cell carcinoma (mRCC) are described in detail.
Expert Opinion
Tivozanib will face the challenge of entering an already crowded therapeutic space in mRCC – emerging combination studies and biomarker assessments may distinguish this agent amongst other VEGF-TKIs. The current review will outline the development pathway of tivozanib to date, and offer lessons learned and future opportunities.
Keywords: Tivozanib, AV-951, Phase I, renal cell carcinoma, breast cancer, gastrointestinal
1.0 INTRODUCTION
Since the seminal work of Judah Folkman, the scientific community has recognized the role of angiogenesis in solid tumors1. Although various moieties may modulate tumor angiogenesis, vascular endothelial growth factor (VEGF) has been noted to play a key role2-4. Furthermore, abrogation of VEGF-mediated signaling has been consistently demonstrated to have an antitumor effect5-6. Various strategies have been developed to combat VEGF-mediated signaling. The monoclonal antibody bevacizumab has affinity for VEGF ligand and has been approved for a wide variety of malignancies (including metastatic renal cell carcinoma [mRCC], glioblastoma multiforme, non-small cell lung cancer [NSCLC], and metastatic colorectal cancer [mCRC])7-10. Ramucirumab, a monoclonal antibody directed at VEGF receptor-2 (VEGFR2), is currently being assessed in pivotal phase III trials in the setting of gastric cancer, breast cancer, hepatocellular carcinoma (HCC), NSCLC and mCRC11-15.
In contrast to VEGF ligand inhibition with monoclonal antibodies, equally vigorous efforts have been made to determine the role of small molecules that inhibit the tyrosine kinase domain of VEGF receptor. These so-called VEGF-tyrosine kinase inhibitors (VEGF-TKIs) have found wide application, ranging from gastrointestinal stromal tumor (GIST) to medullary thyroid cancer to HCC16-18. Clear cell mRCC is a disease particularly susceptible to this mode of angiogenesis inhibition, likely due to aberrations in the von Hippel Lindau (VHL) gene that prevent degradation of hypoxia inducible factor (HIF; a VEGF transcription factor)19-20. A total of four VEGF-TKIs have been approved for the treatment of mRCC on the basis of positive phase III studies: (1) sorafenib, (2) sunitinib, (3) pazopanib, and (4) axitinib21-24. With this relative wealth of available agents with somewhat overlapping indications, a major dilemma for investigators is determining the appropriate sequence in which to use each. Before this issue is resolved, however, other VEGF-TKIs will likely be introduced into the therapeutic repertoire for mRCC. One such agent is tivozanib (AV-951) – positive data from a phase III trial comparing tivozanib and sorafenib was recently reported.
In the current review, the clinical development of tivozanib will be outlined across a broad spectrum of malignancies. Commentary will focus on the anticipated trajectory of this agent both in mRCC and other diseases. This trajectory can serve as a paradigm for other targeted agents that are competing within crowded therapeutic spaces, such as HER2-directed therapies in breast cancer, ALK- and EGFR-directed therapies in lung cancer, and BRAF-directed therapies in melanoma.
1.1 CHEMISTRY & PHARMACOKINETICS
Tivozanib (MW: 454.86; IUPAC: 1-[2-chloro-4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-3-(5-methyl-1,2-oxazol-3-yl)urea) bears structural resemblance to other VEGF-TKIs, although the half maximal inhibitory concentration (IC50) for receptors mediating angiogenesis appears to be much lower25. In Table 2, both the chemical structure and VEGFR1-3 IC50 values for tivozanib are compared to those of sorafenib (as previously noted, the two have been recently compared in a large, phase III effort in mRCC)26. Significant growth inhibition with tivozanib was noted with modest doses (1 mg/kg) in athymic rate xenograft models of breast, colon, lung and prostate cancer. Xenografts derived from the Calu-6 cell line, a human lung cancer cell line that endogenously expresses renin, were treated with tivozanib and further assessed using DCE-MRI. Accompanying a decrease in tumor size was a marked increase in vascular permeability, which was reversible after tivozanib withdrawal.
Table 2.
Comparison of key chemical and structural properties of tivozanib and sorafenib.27, 48 Sorafenib served as a comparator to tivozanib in the phase III TIVO-1 study in mRCC.
| Characteristic | Tivozanib | Sorafenib |
|---|---|---|
| MW | 454.863 | 464.824 |
| VEFR1 IC50 | 0.21 nmol/L | 26 nmol/L |
| VEFR2 IC50 | 0.16 nmol/L | 90 nmol/L |
| VEFR3 IC50 | 0.24 nmol/L | 100 nmol/L |
| 2-D Structure |
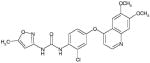
|
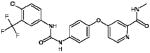
|
| 3-D Structure |
|
|
The substantial growth inhibition observed with tivozanib across preclinical models led to a phase I study in advanced solid tumors27. Key eligibility criteria included a life expectancy of at least 3 months and an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Patients with symptomatic brain metastasis were excluded, as were patients with evidence of severe cardiovascular disease (i.e., uncontrollable hypertension or symptomatic heart failure). A starting dose of 2.0 mg was selected based on the no observed adverse effect level (NOAEL) in primates. Extensive pharmacokinetic (PK) sampling was performed, and pharmacodynamic (PD) studies were also performed to assess the effect of tivozanib therapy on serum VEGF and soluble VEGFR2 (sVEGFR2).
A total of 41 patients were enrolled into the study. mCRC (n=10), mRCC (n=9), and pancreatic cancer (n=6) represented the most common tumor types represented in the study. Two patients in the 2.0 mg daily cohort experienced dose-limiting toxicities (DLTs), specifically, grade 3 asymptomatic proteinuria in one patient and grade 3 ataxia in another. No DLTs were observed in 6 patients treated at 1.0 mg daily, and therefore, an intermediate dose of 1.5 mg daily was explored. DLTs observed at this cohort included grade 3 and 4 transaminitis, uncontrollable hypertension, grade 3 fatigue, and grade 3 dyspnea. Given these toxicities, an expansion cohort including 12 patients treated at 1.0 mg daily was assessed.
With respect to efficacy, two patients with mRCC had partial responses (one confirmed, one unconfirmed), while the majority of patients on the study (55.2%) had stable disease as a best response. Eight patients (20%) received therapy for more than 9 months. PK analyses accompanying the study showed a mean half life of 4.7 days (range, 1.3 to 9.7 days). The time to achieve maximum serum concentration (tmax) was between 2 and 24 hours. Other correlative studies showed that although VEGF-A levels rose consistently at the start of tivozanib therapy in a dose-dependent manner, these levels normalized after 14 days off therapy. In contrast, sVEGFR2 levels were reduced with increasing doses of tivozanib with the most marked decrease noted towards the end of the cycle (i.e., a larger decrease was observed at day 27 of therapy versus day 14). Dynamic contrast-enhanced MRI (DCE-MRI) was performed in a small subset of patients enrolled in the study (n=8). Consistent with preclinical observations, a decrease in tumor vessel density was observed over time.
1.2 CLINICAL EFFICACY IN DISEASE-SPECIFIC STUDIES
1.2.1 Colorectal Cancer
In the setting of colorectal cancer, a phase Ib study was conducted to assess the activity of tivozanib in combination with 5-flurouracil/oxaliplatin (FOLFOX) chemotherapy28. A regimen of modified FOLFOX6 (mFOLFOX6), which incorporates an 85 mg/m2 dose of oxaliplatin, was administered on days 1 and 15 of a 28-day cycle. Escalating doses of tivozanib were administered on a 3-week on, 1-week off cycle. To supplement PK analyses, a single dose of tivozanib was given 5 days preceding therapy with tivozanib.
A total of 30 patients were ultimately enrolled in the study, with a median age of 58 years29. In total, 9 patients received tivozanib at a dose of 0.5 mg daily, 3 patients received 1.0 mg daily and 18 patients received 1.5 mg daily. The median duration of treatment was 5.2 months, and 30.8% of patients achieved a response with this combination. An additional 36% of patients had stable disease as a best response. With respect to safety, there were four DLTs observed. In dose escalation cohorts, a DLT of uncontrolled hypertension was observed. In an expansion cohort including an additional 10 patients, three DLTs were observed; specifically, two episodes of reversible transaminitis and one episode of uncontrolled hypertension. PK analyses from this phase I experience did not suggest any appreciable interaction between mFOLFOX6 and tivozanib – levels of tivozanib were akin to those observed with monotherapy.
The appreciable safety and efficacy associated with the combination of mFOLFOX6 and tivozanib have culminated in a larger, randomized phase II effort (Figure 1)30. In this study, patients will be randomized to receive either mFOLFOX6 with tivozanib or bevacizumab. The primary endpoint of the study is investigator determined PFS, with secondary endpoints including OS, response, and health-related quality of life (HRQoL). The study also aims to explore a variety of serum biomarkers, including VEGF-A, VEGF-C, and VEGF-D and will assess a 42-gene signature (discussed subsequently in the “Expert Opinion” section).
Figure 1.
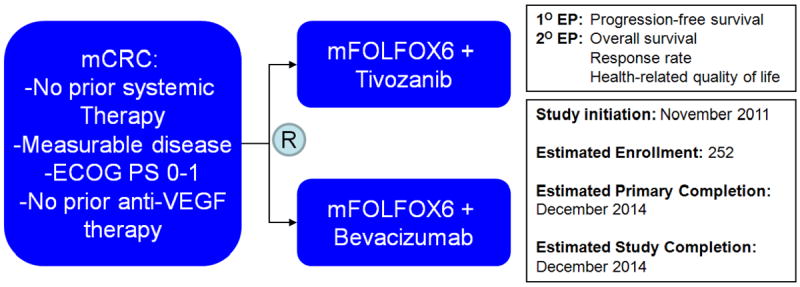
Schema for a randomized, phase II studies assessing tivozanib therapy in mCRC.
A second ongoing effort will explore the combination of capecitabine and tivozanib in patients with all solid tumor types, but will include an expansion cohort in two malignancies – namely, colorectal cancer and breast cancer31. The primary objective of the study is to determine the safety of the combination. If a maximally tolerated dose (MTD) is not reached in a general population of solid tumor patients, then a recommended phase II dose will be determined and an expansion cohort including breast and colorectal cancer patients will be assessed.
1.2.2 Breast Cancer
Assessment of tivozanib in breast cancer thus far is limited to a phase I dose-finding study in combination with paclitaxel32. The primary objective of the study was to determine the safety and tolerability of tivozanib in combination with paclitaxel in patients with metastatic breast cancer and to evaluate the PK profile of this combination. Patients with metastatic breast cancer with an ECOG performance status 0-2 and ≤ 4 prior lines of therapy were enrolled. Notably, patients were allowed to receive one prior taxane and there was no limit to the number of prior biologic or endocrinologic treatments, although prior treatment with a VEGF-TKI was not allowed. Key exclusion criteria included evidence of brain metastasis or pre-existing neuropathy. The treatment regimen included weekly paclitaxel (90 mg/m2 intravenous, 3 out of 4 weeks) and escalating doses of tivozanib therapy (0.5 mg, 1.0 mg, and 1.5 mg daily).
Ultimately, a total of 18 patients were included in the study. The median age of the cohort was 48, with 56% and 22% of patients demonstrating hormone receptor- and HER2-positivity. All patients had received at least one prior taxane, with 61% of patients having received taxane therapy in the adjuvant setting. Notably, over half of patients (56%) had received prior bevacizumab. Two dose-limiting toxicities (DLTs) were encountered in the study. Namely, one patient treated at the first dose level (tivozanib at 0.5 mg/day) experienced grade 1 palpitations, while another patient treated at the third dose level (tivozanib at 1.5 mg/day) developed grade 2 asymptomatic pneumoperitoneum. Otherwise, fatigue and alopecia were the most commonly reported treatment-emergent adverse events.
The median duration of tivozanib therapy was 5.4 months. Amongst 13 patients evaluable for response, partial responses were observed in 5 patients (38%), while stable disease was observed in 7 patients (54%). Although detailed PK data were not available for the patients enrolled, it was suggested that the PK parameters for tivozanib in combination with paclitaxel were similar to those associated with tivozanib monotherapy. Despite the appreciable efficacy and safety observed with this combination, it is not clear that any larger efforts are currently underway to explore the combination of paclitaxel and tivozanib in breast cancer. Outside of the aforementioned phase I study assessing capecitabine and tivozanib with an expansion cohort in breast and colorectal cancer, it is unclear whether other trials are planned to assess the activity of tivozanib in breast cancer31.
1.2.3 Renal Cell Carcinoma
The randomized phase II discontinuation study of tivozanib in mRCC provided key insights related to the drugs activity in this disease (Figure 2)33. Eligibility in the study was limited to mRCC patients with up to one prior therapy (other than a VEGF-directed agent) and a Karnofsky performance status of ≥ 70%. Patients were not allowed to enroll if they had evidence of brain metastasis or had evidence of substantial cardiovascular disease (i.e., uncontrollable hypertension or clinically significant heart failure). In accordance with the randomized phase II design, patients received open-label tivozniab at 1.5 mg oral daily (3 weeks on, 1 week off) for a total of 16 weeks. A the end of the 16-week interval, those patients with ≥ 25% tumor shrinkage proceeded on open-label tivozanib therapy, while those patients with ≥ 25% tumor growth (or progression as otherwise defined by RECIST) discontinued protocol treatment. Those patients falling between these cutoffs were randomized in a 1:1 double-blind fashion to receive either tivozanib or placebo for the next 12 weeks, and all patients were unblinded at that point. Those patients without evidence of progression or intolerable adverse events with tivozanib were allowed to proceed on tivozanib therapy.
Figure 2.
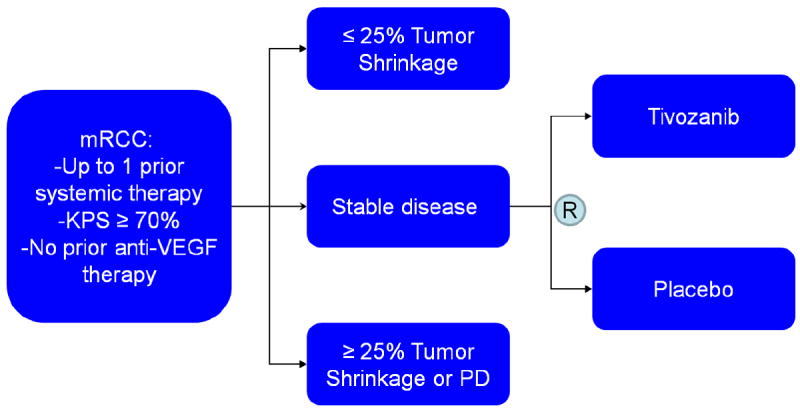
Schema for a randomized, phase II discontinuation study in mRCC.
The primary objective of the study was do determine the safety and efficacy of tivozanib, the latter defined by the objective response rate (ORR) after the first 16 weeks of open-label therapy33. A secondary objective was a comparison of PFS in subgroups within the randomized discontinuation phase. Ultimately, a total of 272 patients with mRCC were enrolled. The majority of patients had clear cell histology (83%), had received prior nephrectomy (73%) and was classified intermediate risk by Memorial Sloan-Kettering Cancer Center (MSKCC) risk criteria (60%).
After the 16 week open label phase, 18% and 66% of patients achieving objective response and stable disease, respectively33. The median PFS amongst all treated patients was 11.7 months, with censoring performed at the time of allocation to placebo. This value was considerably higher (14.7 months) in the subset of patients who had undergone prior nephrectomy and had clear cell histology – this observation accounts for the refined eligibility noted in the subsequently described phase III effort34. Within the randomized discontinuation phase, PFS was significantly higher amongst those patients who received tivozanib (10.3 months vs 3.3 months, P=0.01). The most common non-hematologic adverse events noted with tivozanib were hypertension, dysphonia, diarrhea and asthenia. Grade 3/4 events occurred at a frequency of less than 10%, with the exception of hypertension (12%) and GGT elevation (17%).
Combination studies of tivozanib have also been performed. A phase I study combining tivozanib and temsirolimus in patients with mRCC reached full doses of both agents (tivozanib: 1.5 mg oral daily; temsirolimus: 25 mg intravenous weekly)35. No grade 4 drug-related events or dose-limiting toxicities were observed, and the most common adverse events were diarrhea, fatigue, decreased appetite and stomatitis. Thus far, tivozanib also appears to be easily combinable with cytotoxic therapies, as well. As highlighted in the “Expert Opinion” section, the combinability of tivozanib with mTOR inhibitors is unique amongst VEGF-TKIs.
1.3 Phase III Assessment
The phase III TIVO-1 trial was initiated on the basis of the compelling data for tivozanib monotherapy in the aforementioned randomized, phase II study in mRCC (Figure 3)34. Eligibility for the study included clear cell histology with measurable disease and ECOG performance status between 0-1. Furthermore, participants were required to have had prior nephrectomy. Although participants may have had 1 prior therapy, prior use of VEGF- or mTOR-directed agents were not permitted. Patients were stratified by geographic region, the number of prior therapies, and the number of metastatic lesions.
Figure 3.
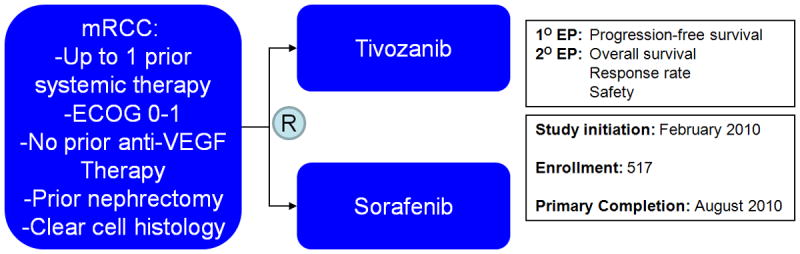
Schema for the randomized, phase III TIVO-1 study in patients with mRCC.
In a 1:1 fashion, patients were randomized to receive either tivozanib at 1.5 mg/day orally, 3 weeks on, 1 week off, or sorafenib at 400 mg oral twice daily34. The primary objective of the study was to determine superiority of tivozanib as compared to sorafenib in terms of PFS, with secondary objectives including assessment of response rate, safety and survival. The primary endpoint was independently assessed by a blinded third party. With a planned sample size of 500 patients, it was suggested that the study would have 90% power to detect an improvement in PFS from 6.7 months with sorafenib to 9.7 months with tivozanib (α=0.05, 2-sided).
Ultimately, a total of 517 patients were enrolled between February and August of 2010 at a total of 76 sites with the majority (approximately 90%) derived from European sites34. Patient characteristics were similar on both study arms with an identical median age (59 for both) and proportion of patients with no prior therapy (70% for both). Notably, there was a higher proportion of patients receiving sorafenib with ECOG performance status of 0 (54%, as compared to 45% for tivozanib; P<0.05).
By independent review, there was a statistically significant improvement in PFS with tivozanib therapy (11.9 months vs 9.1 months; P=0.042), and this difference was slightly higher in those patients who had received no prior therapy (12.7 vs 9.1 months, P=0.037)34. A more pronounced difference in PFS was noted by investigator assessment (14.7 months vs 9.6 months, P=0.003). Subset analyses based on demographic or clinicopathologic characteristics showed benefit across subgroups, including distribution by MSKCC risk stratification. With respect to secondary endpoints, there was a higher proportion of objective responses with tivozanib as compared to sorafenib (33% vs 23%, P=0.014).
Fewer patients receiving tivozanib (as compared to sorafenib) required dose interruptions (18% vs 35; P<0.001) or dose reductions (12% vs 43%, P<0.001) due to adverse events34. The extent of drug discontinuation on account of treatment-related adverse events was similar on both arms (4% with tivozanib vs 5% with sorafenib). Hypertension, diarrhea and dysphonia were the most common adverse events noted with tivozanib. In comparing grade 3/4 toxicities, hypertension was more frequent with tivozanib (26% vs 17%), while hand-foot syndrome was more common with sorafenib (17% vs 2%).
The development of hypertension was noted to be a clinical biomarker predictive of efficacy, a phenomenon observed amongst other VEGF-TKIs for Mrcc34, 36. Those patients with a diastolic blood pressure greater than 90 mmHg had an improved PFS with tivozanib (18.3 months vs 9.1 months). Similarly, a systolic pressure greater than 150 mmHg developed while on study conferred a benefit in the same endpoint (16.7 months vs 9.0 months).
1.4 Expert Opinion
While meeting its primary endpoint in the TIVO-1 study of improvement in progression free survival when compared to an active comparator, in a disease with many similar targeted agents, the impact of this treatment on the therapeutic index for patients remains to be demonstrated. A recent press release offered a first look at survival data from the study and suggested a 1-year OS rate of 81% with sorafenib as compared to 77% with tivozanib37. Although the same press release appropriate points out that many patients receiving sorafenib crossed over to receive tivozanib (approximately 53%), while only 17% of patients receiving sorafenib went on to receive another therapy. These data highlight the importance of a longer follow up time required for the demonstration of a survival benefit, the confounding challenges in clinical research when a cross over design is implemented, and again suggest that sequential therapy in mRCC has an important role in patients management.
The issue of overall survival is a contentious one in mRCC, and much of the murkiness centers in datasets that still have yet to be formally presented. Another example is the so-called INTORSECT trial, a phase III effort comparing sorafenib to the intravenous mTOR inhibitor temsirolimus. Similar to TIVO-1, the study was powered to demonstrate an improvement in PFS with temsirolimus as compared to sorafenib38. Although the study did not meet this primary endpoint, there was a significant improvement in OS (a secondary endpoint) with sorafenib therapy. Until now, the academic community has questioned the role of PFS as a surrogate for OS – loose correlations have been derived in existing datasets.39 Formal presentation of TIVO-1 and INTORSECT will likely be accompanied by stirring debates regarding the utility of PFS as a primary endpoint. These conversations could cause a paradigm shift in the regulatory pathways for new agents in the untreated patient populations for mRCC. Although foreign in the setting of mRCC, this paradigm shift is not new to all malignancies. A prime example is the debacle surrounding the approval (and subsequent withdrawal of approval) of bevacizumab therapy for breast cancer40. Moving forward, clinical trial designs should have the foresight to better control for factors (i.e., crossover, secondary therapies, etc.) that may affect what is arguably the most fundamental endpoint in oncology – overall survival.
Regulatory issues aside, tivozanib therapy (as with other VEGF-TKIs) may find specific application in subsets of patients that demonstrate certain predictive laboratory-based biomarkers. While clinical biomarkers such as hypertension are useful in defining prognosis post hoc, certain laboratory biomarkers may be collected at baseline and define patients that may garner the greatest benefit from tivozanib therapy. As one example, a 42-gene signature was derived through daily treatment of HER2-negative murine breast tumor isolates with tivozanib41. RNA microarrays were generated and correlated with the degree of response based on histopathologic analysis, ultimately yielding 42 candidate genes associated with tivozanib resistance. This gene signature will be assessed in a phase II study42. Briefly, mRCC patients similar to those enrolled in the TIVO-1 study (i.e., with prior nephrectomy, 0-1 prior therapies excluding prior VEGF- and mTOR-inhibitors) will be treated with tivozanab at 1.5 mg oral daily (3 weeks off, 1 week off). The primary endpoint of the study is to demonstrate a correlation between blood biomarkers (VEGF and hepatocyte growth-factor, HGF) and tissue biomarkers (the aforementioned gene expression profile, CD68, VEGF, and HGF) with response, PFS, and toxicity. The study has already completed enrollment of 100 patients. Although several of these markers have been studied exhaustively in the context of other targeted therapies, the 42-gene signature could provide a more specific method to discern patients who may render benefit specifically from tivozanib therapy43-44. While larger validation studies may be necessary before such a response signature can be clinically implemented, studies such as these provide a key first step towards personalizing cancer therapy.
Another pathway for tivozanib development may be through larger assessments in combination studies. Many attempts to combine VEGF-TKIs with cytotoxic therapies or other targeted agents have been problematic. For instance, a phase I clinical trial combining sunitinib with bevacizumab in patients with mRCC demonstrated a substantial response rate (52%), but also resulted in wieldy toxicities such as microangiopathic hemolytic anemia and grade 4 hemorrhage45. An even more aggressive effort to combine sunitinib with carboplatin, paclitaxel and bevacizumab in NSCLC was discontinued due to similar adverse events46. Efforts to combine VEGF-TKIs with mTOR inhibitors have also been marred by toxicity. For instance, a phase I study exploring the combination of sunitinib with temsirolimus was stopped early at low doses of both agents due to primarily hematologic adverse events47. The previously cited studies for tivozanib suggest that the agent may be combinable with both cytotoxic therapies (specifically, capecitabine, paclitaxel, 5-FU, and oxaliplatin) and with mTOR inhibitors (temsirolimus). Amongst the three disease states in which tivozanib has been the most intensively studied (breast cancer, mCRC, and mRCC), earning a place in the first- or second-line setting as monotherapy will be challenging. Novel combinations may uniquely position tivozanib amongst other agents in this therapeutic space.
While the regulatory fate of tivozanib awaits an FDA decision, the oncology community stands to learn a great deal from the trajectory the agent has followed thus far. First and foremost, endpoints (both primary and secondary) should be carefully selected, and factors that modulate these endpoints should be accounted for to the greatest extent possible. In view of the preliminary reports of inferior 1-year survival with tivozanib in the TIVO-1 trial, it is likely that efforts will be made to report analyses with censoring of patients who crossed over to tivozanib therapy. Even if this analysis yields no difference in survival between sorafenib and tivozanib, does it overcome the findings of an intention-to-treat analysis? Assuming tivozanib does gain regulatory approval in mRCC, it has yet another hurdle to overcome – namely, it is the fifth VEGF-TKI to be added to the therapeutic arsenal for this disease. The strategies that have been proposed herein (i.e., developing new combinations, identifying predictive biomarkers) are a means through which tivozanib can distinguish itself from other available agents, especially given the low likelihood that comparative trials will be developed to juxtapose tivozanib against its other “competitors” (i.e., sunitinib, pazopanib and axitinib). While the dilemmas that tivozanib has thus far encountered in its lifecycle are burdensome, they are by no means unique. There is a rapidly developing pipeline of targeted therapies built around identical targets (i.e., HER2, JAK2, BRAF, etc.). Lessons learned from tivozanib may aid in the future clinical implementation of these agents.
Table 1.
Drug summary box.
| Drug name | Tivozanib |
| Phase | III |
| Indication | Advanced RCC (pending) |
| Pharmacology Description/Mechanism of Action | Multi-targeted receptor tyrosine kinase inhibitor with high specificity for VEGFR-1, -2, and -3 |
| Route of Administration | Oral |
| Chemical Formula | C22H19ClN4O5 |
| Pivotal Trial | Phase III TIVO-1 study in mRCC comparing sorafenib and tivozanib monotherapy in patients with 0-1 prior lines of therapy (excluding VEGF- or mTOR-directed therapies) |
Acknowledgments
Dr. Pal’s efforts are supported by the NIH Loan Repayment Plan (LRP) and NIH K12 2K12CA001727-16A1.
Footnotes
Declaration of interests
The first author and senior author are consultants for Aveo, who are the licensee for Tivozanib. None of the other authors have any competing interests to declare.
References
- 1*.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285(21):1182–6. doi: 10.1056/NEJM197111182852108. A key article that highlights the biologic relevance of tumor angiogenesis, and paves the way for future therapeutic avenues. [DOI] [PubMed] [Google Scholar]
- 2.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-Regulation of Vascular Endothelial Growth Factor and Its Cognate Receptors in a Rat Glioma Model of Tumor Angiogenesis. Cancer Research. 1993;53(23):5822–27. [PubMed] [Google Scholar]
- 3.Toi M, Inada K, Suzuki H, Tominaga T. Tumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expression. Breast Cancer Res Treat. 1995;36(2):193–204. doi: 10.1007/BF00666040. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127–37. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 5.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–44. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 6.Asano M, Yukita A, Matsumoto T, Kondo S, Suzuki H. Inhibition of Tumor Growth and Metastasis by an Immunoneutralizing Monoclonal Antibody to Human Vascular Endothelial Growth Factor/Vascular Permeability Factor121. Cancer Research. 1995;55(22):5296–301. [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine. 2006 Dec 14;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8. [August 8, 2012];FDA Approval for Bevacizumab. Available at http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab.
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007 Oct 20;25(30):4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England journal of medicine. 2004 Jun 3;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11. [August 8, 2012]; NCT00703326: A Multicenter, Multinational, Randomized, Double-Blind, Phase III Study of IMC-1121B Plus Docetaxel Versus Placebo Plus Docetaxel in Previously Untreated Patients With HER2-Negative, Unresectable, Locally-Recurrent or Metastatic Breast Cancer. doi: 10.3816/CBC.2009.n.044. Available at http://www.clinicaltrials.gov. [DOI] [PubMed]
- 12. [August 8, 2012]; NCT01170663: A Randomized, Multicenter, Double-Blind, Placebo-Controlled Phase 3 Study of Weekly Paclitaxel With or Without Ramucirumab (IMC-1121B) Drug Product in Patients With Metastatic Gastric Adenocarcinoma, Refractory to or Progressive After First-Line Therapy With Platinum and Fluoropyrimidine. Available at http://www.clinicaltrials.gov.
- 13. [August 8, 2012]; NCT01140347: A Multicenter, Randomized, Double-Blind, Phase 3 Study of Ramucirumab (IMC-1121B) Drug Product and Best Supportive Care (BSC) Versus Placebo and BSC as Second-Line Treatment in Patients With Hepatocellular Carcinoma Following First-Line Therapy With Sorafenib (REACH) Available at http://www.clinicaltrials.gov.
- 14. [August 8, 2012]; NCT01183780: A Randomized, Double-blind, Multicenter Phase 3 Study of Irinotecan, Folinic Acid, and 5-Fluorouracil (FOLFIRI) Plus Ramucirumab or Placebo in Patients With Metastatic Colorectal Carcinoma Progressive During or Following First-Line Combination Therapy With Bevacizumab, Oxaliplatin, and a Fluoropyrimidine. Available at http://www.clinicaltrials.gov.
- 15. [August 8, 2012]; NCT01168973: A Randomized, Double-Blind, Phase 3 Study of Docetaxel and Ramucirumab Versus Docetaxel and Placebo in the Treatment of Stage IV Non-Small Cell Lung Cancer Following Disease Progression After One Prior Platinum-Based Therapy. doi: 10.4143/crt.2015.401. Available at http://www.clinicaltrials.gov. [DOI] [PMC free article] [PubMed]
- 16.Demetri GD, Huang X, Garrett CR, Schoffski P, Blackstein ME, Shah MH, et al. Novel statistical analysis of long-term survival to account for crossover in a phase III trial of sunitinib (SU) vs. placebo (PL) in advanced GIST after imatinib (IM) failure. J Clin Oncol. 2008 May 20;26(15_suppl):10524. (Meeting Abstracts) 2008. [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Rivera F, Santillan-Gomez AA, Younes MN, Kim S, Fooshee D, Zhao M, et al. The tyrosine kinase inhibitor, AZD2171, inhibits vascular endothelial growth factor receptor signaling and growth of anaplastic thyroid cancer in an orthotopic nude mouse model. Clin Cancer Res. 2007 Aug 1;13(15 Pt 1):4519–27. doi: 10.1158/1078-0432.CCR-06-2636. [DOI] [PubMed] [Google Scholar]
- 19.Skolarikos AA, Papatsoris AG, Alivizatos G, Deliveliotis C. Molecular pathogenetics of renal cancer. Am J Nephrol. 2006;26(3):218–31. doi: 10.1159/000093631. [DOI] [PubMed] [Google Scholar]
- 20**.Kim WY, Kaelin WG. Role of VHL Gene Mutation in Human Cancer. J Clin Oncol. 2004 Dec 15;22(24):4991–5004. doi: 10.1200/JCO.2004.05.061. A well written overview of the nature and relevance of VEGF and VHL mutation in RCC biology. [DOI] [PubMed] [Google Scholar]
- 21.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med. 2007 Jan 11;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009 Aug 1;27(22):3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol. 2010 Feb 20;28(6):1061–68. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 24.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011 Dec 3;378(9807):1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 25. [August 12, 2012];Tivozanib: PubChem Compound Summary. Available at http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=9911830.
- 26.Nakamura K, Taguchi E, Miura T, Yamamoto A, Takahashi K, Bichat F, et al. KRN951, a Highly Potent Inhibitor of Vascular Endothelial Growth Factor Receptor Tyrosine Kinases, Has Antitumor Activities and Affects Functional Vascular Properties. Cancer Research. 2006;66(18):9134–42. doi: 10.1158/0008-5472.CAN-05-4290. [DOI] [PubMed] [Google Scholar]
- 27**.Eskens FA, de Jonge MJ, Bhargava P, Isoe T, Cotreau MM, Esteves B, et al. Biologic and clinical activity of tivozanib (AV-951, KRN-951), a selective inhibitor of VEGF receptor-1, -2, and -3 tyrosine kinases, in a 4-week-on, 2-week-off schedule in patients with advanced solid tumors. Clin Cancer Res. 2011 Nov 15;17(22):7156–63. doi: 10.1158/1078-0432.CCR-11-0411. A key study that highlights the preclinical development of tivozanib. [DOI] [PubMed] [Google Scholar]
- 28.Eskens F, Oldenhuis CN, Bhargava P, Loos W, Esteves B, van Doorn L, et al. A phase Ib, open-label, dose-escalation study of tivozanib and FOLFOX6 in patients (pts) with advanced gastrointestinal (GI) tumors. ASCO Meeting Abstracts. 2011;29(4_suppl):549. [Google Scholar]
- 29.Eskens F, Oldenhuis CN, Loos WJ, Esteves B, van Doorn L, Cotreau MM, et al. Final results of a phase Ib study of tivozanib and FOLFOX6 in patients (pts) with advanced gastrointestinal (GI) tumors. ASCO Meeting Abstracts. 2012;30(15_suppl):4132. [Google Scholar]
- 30. [August 12, 2012]; NCT01478594: A Phase 2, Open Label, Multicenter, Randomized Trial Comparing Tivozanib in Combination With mFOLFOX6 to Bevacizumab in Combination With mFOLFOX6, In Stage IV Metastatic Colorectal Cancer (mCRC) Subjects. Available at http://www.clinicaltrials.gov.
- 31. [August 12, 2012]; NCT01306630: A Phase 1b, Open-Label, Dose-Escalating Trial of Tivozanib (AV-951) in Combination With Capecitabine (Xeloda®) in Subjects With Advanced Solid Tumors. Available at http://www.clinicaltrials.gov.
- 32.Mayer EL, Scheulen ME, Beckman J, Richly H, Poli A, Bhargava P, et al. Combination of tivozanib (AV-951) with weekly paclitaxel for metastatic breast cancer: Results of a phase I study. ASCO Meeting Abstracts. 2011;29(15_suppl):1092. [Google Scholar]
- 33.Nosov D, Bhargava P, Esteves WB, Strahs AL, Lipatov ON, Lyulko OO, et al. Final analysis of the phase II randomized discontinuation trial (RDT) of tivozanib (AV-951) versus placebo in patients with renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2011;29(15_suppl):4550. [Google Scholar]
- 34.Motzer RJ, Nosov D, Eisen T, Bondarenko IN, Lesovoy V, Lipatov ON, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a phase III randomized, open-label, multicenter trial. ASCO Meeting Abstracts. 2012;30(15_suppl):4501. [Google Scholar]
- 35.Kabbinavar FF, Srinivas S, Hauke RJ, Amato RJ, Esteves WB, Cotreau MM, et al. Results from a phase I trial of tivozanib (AV-951) combined with temsirolimus therapy in patients (pts) with renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2011;29(15_suppl):4549. [Google Scholar]
- 36.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011 May 4;103(9):763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. [August 11, 2012];AVEO Pharmaceuticals Press Release: Aveo Reports Second Quarter 2012 Financial Results. Available at http://investor.aveopharma.com/phoenix.zhtml?c=219651&p=irol-newsArticle&ID=1721417&highlight=
- 38. [August 11, 2012];Pfizer Press Release: Pfizer Provides Topline Results From Phase 3 Study Of Torisel® As Second-Line Treatment In Advanced Renal Cell Carcinoma (RCC) Available at http://www.pfizer.com/news/press_releases/pfizer_press_release.jsp?guid=20120515006852en&source=RSS_2011&page=2.
- 39*.Halabi S, Rini BI, Stadler WM, Small EJ. Use of progression-free survival (PFS) to predict overall survival (OS) in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2010 May 20;28(15_suppl):4525. (Meeting Abstracts) A reference that provides key insight and thoughtful discussion related to relevant clinical endpoints in mRCC. [Google Scholar]
- 40.Montero AJ, Vogel C. Fighting fire with fire: rekindling the bevacizumab debate. N Engl J Med. 2012 Jan 26;366(4):374–5. doi: 10.1056/NEJMe1113368. [DOI] [PubMed] [Google Scholar]
- 41.Robinson MO, Lin J, Feng B, Sun X, Rideout W, Chiu MI, et al. Correlation of a tivozanib response biomarker identified in a preclinical model with clinical activity in a phase II study in renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2010;28(15_suppl):e13564. [Google Scholar]
- 42.Hutson TE, Rathmell K, Hudes GR, Kabbinavar F, Vogelzang NJ, Knox JJ, et al. A phase II biomarker assessment of tivozanib in oncology (BATON) trial in patients (pts) with advanced renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2012;30(15_suppl):TPS4686. [Google Scholar]
- 43.DePrimo S, Bello C, Smeraglia J, Baum C, Spinella D, Rini B, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. Journal of Translational Medicine. 2007;5(1):32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilias-Khan NA, Khakoo AY, Tannir NM. A clinical and biological profile to predict risk of development of hypertension in patients with non-clear cell renal cell carcinoma treated with sunitinib. J Clin Oncol. 2010 May 20;28(15_suppl):4601. (Meeting Abstracts) 2010. [Google Scholar]
- 45.Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009 Mar 20;27(9):1432–9. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Socinski MA, Scappaticci FA, Samant M, Kolb MM, Kozloff MF. Safety and efficacy of combining sunitinib with bevacizumab + paclitaxel/carboplatin in non-small cell lung cancer. J Thorac Oncol. 2010 Mar;5(3):354–60. doi: 10.1097/JTO.0b013e3181c7307e. [DOI] [PubMed] [Google Scholar]
- 47.Patel PH, Senico PL, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009 Jan;7(1):24–7. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Research. 2004;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]


