Abstract
Background
This study aims to apply the International mRCC Database Consortium (IMDC) Prognostic Model in metastatic non clear cell renal cell carcinoma (nccRCC). Additionally, the survival outcome of metastatic nccRCC patients was characterized.
Methods
Data on 2215 (1963 ccRCC/252 nccRCC) patients treated with first-line VEGF-and mTOR targeted therapies were collected from the IMDC. Time to treatment failure (TTF) and overall survival (OS) were compared in groups of favorable, intermediate, and poor prognosis groups according to the IMDC prognostic criteria
Results
The median OS of the entire cohort was 20.9 months. nccRCC patients were of younger age (p<0.0001), more often presented with low Hb (p=0.014) and elevated neutrophils (p=0.0001), but displayed otherwise similar clinicopathological features compared to ccRCC. OS (12.8 vs. 22.3 months; p<0.0001) and time to treatment failure (TTF) (4.2 vs. 7.8 months; p<0.0001) were worse in nccRCC compared to ccRCC. The hazard ratio for death and TTF when adjusted for the prognostic factors was 1.41 (95%CI 1.19, 1.67, p<0.0001) and 1.54 (95% CI 1.33, 1.79, p<0.0001), respectively. The IMDC prognostic model reliably discriminated three risk groups to predict OS and TTF in nccRCC; the median OS and TTF of favorable, intermediate, and poor prognosis groups were 31.4, 16.1, and 5.1 months (p<0.0001) and 9.6, 4.9, and 2.1 months (p<0.0001), respectively.
Conclusion
Although targeted agents have significantly improved the outcome of patients with nccRCC, for the majority survival is still inferior compared to ccRCC. The IMDC prognostic model reliably predicts OS and TTF in nccRCC and ccRCC patients.
Keywords: non-clear cell renal cell carcinoma, targeted therapies, overall survival, prognostication, Heng risk criteria, IMDC risk model
Introduction
Renal cell carcinoma (RCC) arises from the kidney parenchyma and is a complex aggregate of several malignant subtypes.1 According to the World Health Organization (WHO) classification system, the major subtypes are clear cell RCC (ccRCC), papillary RCC (pRCC), chromophobe RCC (chRCC), unclassified RCC (unRCC)RCC, and RCC of the collecting duct.2
In ccRCC, the von Hippel Lindau (VHL) gene is inactivated in 80-90%, and as a consequence the vascular endothelial growth factor (VEGF) and the mammalian target of rapamycin (mTOR) pathways are deregulated.1, 3 Agents that target members of these pathways have supplanted immunotherapies and have been internationally recognized as the standard of care therapy in metastatic RCC (mRCC).4 Since the VEGF- and mTOR pathways are particularly important for the biology of ccRCC, randomized phase III clinical trials were performed in patient cohorts that were exclusively or predominately comprised of ccRCC.5-9 To date, the 20% nccRCC patients included in the temsirolimus phase-III study are the largest cohort that was investigated in a randomized phase III trial of targeted agents.7 Therefore, the clinical outcomes of patients who have a nccRCC and were treated with VEGF- and mTOR targeted agents remains undefined.
The International mRCC Database Consortium (IMDC) or Heng model has proven to be a useful prognostic tool in major clinical trials of novel targeted therapies.10 This model includes six independent predictors of poor survival: Karnofsky performance status (KPS) <80%, time from diagnosis to treatment interval <1year, anemia, hypercalcemia, neutrophilia and thrombocytosis. According to the number of poor prognostic factors, patients were segregated into favorable (0 factors), intermediate (1-2 factors) and poor (≥3 factors) risk groups. The model was developed and externally validated without consideration of the histological RCC-subtypes.11, 12 It is assumed that the results were largely impacted by the ccRCC subtype because it was the predominant histological subtype in the development and validation cohort.11, 12 Therefore, it is unclear whether the IMDC prognostic model can be applied in nccRCC.
This study aims to characterize the applicability of the IMDC prognostic model and the survival outcome of patients with nccRCC who were treated with first line VEGF- and mTOR inhibitors. For this purpose, we assessed the time to treatment failure (TTF) and overall survival (OS) in ccRCC and nccRCC. We applied the IMDC prognostic model to the nccRCC patients and evaluated its discriminatory ability. This study was performed as a large retrospective analysis by the IMDC, a worldwide collaboration of academic centers.
Patients and Methods
Study populations
The International mRCC Database Consortiums includes 20 academic centers from Canada, USA, Japan, South Korea, Singapore, and Denmark. Data were collected from August 15, 2008 until October, 10, 2012. At the time of analysis, the database covered data of 2370 patients who have received first line targeted therapy between 2003 and 2012. Patients were excluded from analysis because of unknown histological subtypes (n=153 (7%)) and unknown treatment initiation date (n=2).
All centers obtained local Institutional Review Board approvals before including data into this large retrospective study. Baseline patient characteristics included demographic, clinicopathological, and laboratory data as described in the development study of the IMDC risk model.11 Survival data were retrospectively collected from medical chart reviews and publically available records. Uniform data templates were used to ensure consistent data collection at each institution. Patients may have been treated in part of clinical trials or as standard of care according to national cancer guidelines.
Statistical Analyses
The primary objective of this study was to prove the applicability of the IMDC prognostic model separately in nccRCC. The secondary objective was to characterize clinical outcomes in term of TTF and OS of nccRCC compared to ccRCC in patients treated with targeted therapies. OS was defined as the time period between targeted therapy initiation and the date of death, or it was censored on the day of the last follow up visit. TTF was defined as the time period between treatment initiation and progression, drug cessation, death, or it was censored at the last follow up visit. Progression was determined according to clinical criteria that made continuation of treatment impossible or radiographic criteria using the Response Evaluation Criteria in Solid Tumors (RECIST).
Patient and tumor characteristics were compared between ccRCC and nccRCC patients using the chi-square test. OS and TTF were estimated with the Kaplan-Meier method and differences between histological groups were examined with the log rank test or the Wald chi-square test from the Cox regression adjusted for the IMDC risk factors.
We applied the IMDC Model (presence/absence of the six pre-determined prognostic factors to determine the favorable, intermediate and poor risk groups)11, 12 to nccRCC patients using Cox regression. Concordance indices (C-Index) were computed in order to test the predictive accuracy of the IMDC prognostic model; a c-index of 0.5 indicates no predictive accuracy and an index of 1 shows perfect predictive accuracy.13
The statistical analyses were performed using SAS version 9 (SAS Institute, Cary, NC), and p<0.05 (two sided) was considered statistically significant.
Results
Characterization of the Clinical Outcome
The study cohort was comprised of 2215 patients. Overall, 1963 (88.6%) patients had ccRCCs and 252 (11.4%) nccRCCs. Tumors with clear cell component/mixed ccRCC and nccRCCs (n=21) were considered as ccRCC based on the study design of previously reported phase III randomized clinical trials in mRCC.10, 14 Tumors with nccRCC histology included papillary RCC (n= 151, 59.9%), chromophobe RCC (n= 37, 14.7%), collecting duct (n= 7, 2.8%), unclassified (n= 34, 13.5%), and RCC with Xp11 translocation (n=4, 1.6%). Nineteen patients were coded as nccRCC by the study center without further information on the exact nccRCC-subtype. We did not analyze pRCC separately in groups of type I and type II because our database does not contain these information. The comparison of patient characteristics revealed that nccRCC were of younger age (p<0.0001), had more baseline anemia (p=0.014), and neutrophilia (p=0.0001). Otherwise ccRCC and nccRCC had similar clinicopathological characteristics (Table 1).
Table 1.
Patient Characteristics at initiation of targeted therapy
| ccRCC (N=1963) | nccRCC (N=252) |
||||
|---|---|---|---|---|---|
| N/Total N | % | N | % | P-value | |
| Age at therapy initiation >=60 | 1066/1963 | (54) | 102/252 | (40) | <0.0001 |
| KPS (<80) | 435/1881 | (23) | 53/233 | (23) | NS |
| Male Gender | 1452/1954 | (74) | 182/252 | (72) | NS |
| Number of metastases >1 | 1436/1959 | (73) | 182/251 | (73) | NS |
| Sarcomatoid pathology | 171/1816 | (9) | 22/228 | (10) | NS |
| Prior Nephrectomy | 1593/1961 | (81) | 197/252 | (78) | NS |
| Prior immunotherapy | 492/1963 | (25) | 54/252 | (21) | NS |
| Dx to TKI therapy <1yr | 1016/1960 | (52) | 143/251 | (57) | NS |
| Dx to metastasis<1yr | 1319/1943 | (68) | 183/250 | (73) | NS |
| Low Hb | 1069/1845 | (58) | 152/229 | (66) | 0.014 |
| Hypercalcaemia | 176/1785 | (10) | 16/223 | (7) | NS |
| High LDH (>1.5 ULN) | 234/1364 | (17) | 34/145 | (23) | NS(0.06) |
| Neutrophilia (>ULN) | 233/1775 | (13) | 51/226 | (23) | 0.0001 |
| Thrombophilia (>ULN) | 326/1839 | (17) | 48/229 | (21) | NS |
Abbreviations: ccRCC= clear cell renal cell carcinoma, nccRCC= non clear cell renal cell carcinoma, KPS= Karnofski performance status, Hb= hemoglobin, LDH= lactat dehydrogenase Dx= diagnosis, ULN= upper limit of normal, LLN= lower limit of normal
In the whole cohort, the median overall survival time after targeted therapy initiation was 20.9 months (95% CI: 19.7-22.6 months), with 798 (36%) of patients remaining alive at the time of analysis. The median follow-up in alive patients was 22.3 months (IQR: 10.8-38.4 months). The first-line targeted therapies had been stopped in 1898/2215 (86%) patients at the time of analysis. The median time on the first-line targeted therapies was 7.2 months (range: 0+-91+ months).
Comparing nccRCC with ccRCC in treatment outcomes
Response and time to Treatment Failure
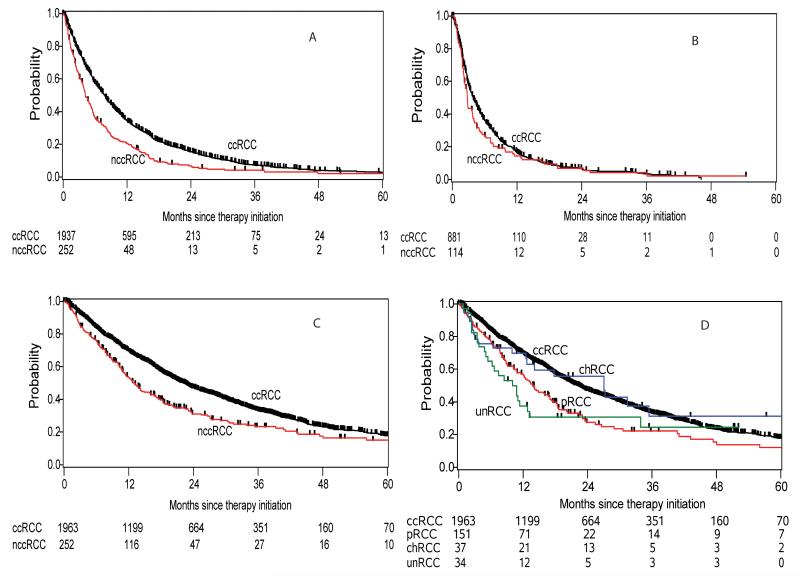
Patients with nccRCC were significantly more often treated with mTOR therapies in first (6.8% versus 1.5%, p<0.0001) and second line therapy (44.7% versus 30.9%, p=0.011). Treatment response data to first line therapy were available for 1801/2215 (81%) patients. In ccRCC, the best response rates to first line therapies were 1.1% CR, 26.8% PR, 50.2% SD, and 21.9% PD while it were 1.0% CR, 14.9% PR, 50.0% SD, and 34.1%PD in nccRCC (p<0.0001). For first line therapy, the median TTF was 7.8 months (95% CI: 7.2 – 8.1 months) and 4.2 (95% CI: 3.7 – 5.2 months) in ccRCC and nccRCC, respectively (HR: 1.57 (95% CI: 1.37 – 1.80) (p<0.0001) (Table 2; Figure 1 A). Second line therapy achieved best response rates of 0.4% CR, 11.3% PR, 49.6% SD, and 38.7% PD in ccRCC and 8.5% PR, 45.1% SD, and 46.3% PD in nccRCC (p=0.526). The detailed best response rates of each subtype are shown in Table 3. The median TTF on the second line therapy for ccRCC and nccRCC was 3.9 months (95% CI: 3.4 – 4.4 months) and 2.8 months (95% CI: 2.3 – 3.7 months) (p=0.142) (Table 2; Figure 1 B). Both groups had a similar median TTF for the third-line therapy; 4.0 (95% CI: 3.3 – 4.6) months in ccRCC and 3.4 (95% CI: 2.4 – 7.1) (p=0.544) months in nccRCC (Table 2). All treatment sequences are displayed in detail in Table 2.
Table 2.
Therapy-sequences and TTF in ccRCC and nccRCC
| First Line Therapy | Second Line Therapy | Third Line Therapy | ||||
|---|---|---|---|---|---|---|
| ccRCC | nccRCC | ccRCC | nccRCC | ccRCC | nccRCC | |
| Total N | 1963 | 252 | 894 | 114 | 336 | 35 |
| Type of therapy | N(%) | N(%) | N(%) | N(%) | N(%) | N(%) |
|
| ||||||
|
Anti-VEGF
Therapy |
1934(98.5) | 235(93.2) | 570(63.8) | 57(50.0) | 169(50.3) | 20 (57.1) |
| Sutent | 1417(72) | 181(72) | 224(25) | 20(18) | 59(18) | 7(20) |
| Sorafenib | 388(20) | 45(18) | 273(31) | 27(24) | 58(17) | 7(20) |
| Axitinib | 3(0.2) | - | 11(1) | 3(3) | 7(2) | |
| Bevacizumab | 87(4) | 6(2) | 34(4) | 3(3) | 22(7) | 2(6) |
| Pazopanib | 34(2) | 1(0.4) | 25(3) | 3(3) | 22(7) | 4(11) |
| Tivozanib | 5(0.3) | 2(1) | 3(0.3) | 1(1) | 1(0) | |
|
| ||||||
|
mTOR
inhibitors |
29(1.5) | 17(6.8) | 276(30.9) | 51 (44.7) | 133(39.6) | 12(34.3) |
| Temsirolimus | 24(1) | 15(6) | 104(12) | 30(26) | 64(19) | 7(20) |
| Everolimus | 5(0.3) | 2(1) | 172(19) | 21(18) | 69(21) | 5(14) |
|
| ||||||
| Other Therapy† | 48(5.4) | 6(5.3) | 34(10.1) | 3(8.6) | ||
|
| ||||||
| TTF | ||||||
| No. of failure/Total |
1666/1937 | 232/252 | 755/881 | 98/114 | 271/317 | 26/33 |
| Median, months | 7.8 (7.2 - 8.1) |
4.2 (3.7 - 5.2) |
3.9 (3.4 - 4.4) |
2.8 (2.3,3.7) |
4.0 (3.3 - 4.6) |
3.4 (2.4 - 7.1) |
|
| ||||||
| Univariate | ||||||
| HR | 1.57 (1.37,1.80) |
1.17 (0.95,1.45) |
0.88 (0.59,1.32) |
|||
| P-value | <0.0001 | 0.142 | 0.544 | |||
|
| ||||||
|
Multivariable HR* |
1.54 (1.33,1.79) |
|||||
|
Adjusted p-
value* |
<0.0001 | |||||
Adjusted for IMDC risk group criteria
including ABT-869, AG117+Taxotere, ARQ-197, BMS 93655, BMS-275183, CRLX101, DOXORUBICIN, GSK1363089, Gemcitabine/Cisplatin, IMCL1121, Gemcitabine/Xeloda, MCL1121B, Imclone, MLN trial, Perifosine, Revlamid (CC5013), SIROLIMUS, Temsirolimus-Bevacizumab, XL-880, XL-999, Xeloda
Figure 1.
Table 3.
Best response rates and overall survival (OS) between ccRCC and nccRCC
| General Comparison | nccRCC Subtypes Compared to ccRCC | |||||
|---|---|---|---|---|---|---|
| ccRCC | nccRCC | pRCC | chRCC | unRCC | Other/Unknown | |
|
BR 1-st line
CR/PR/SD/PD (%) |
17 / 428 / 803 / 350 (1.1 / 26.8 / 50.2 / 21.9) |
2 / 30 / 101 / 69 (1.0 / 14.9 / 50.0 / 34.2) |
0 / 17 / 63 /41 (0 / 14.0 / 52.1 / 33.9) |
1 / 6 / 16 / 9 (3.1 / 18.8 / 50.0 / 28.1) |
1 / 6 / 9 / 9 (4.0 / 24.0 / 36.0 / 36.0) |
0 / 1 / 13 / 10 (0/4.2 / 54.2 / 41.7) |
|
BR 2-nd line
CR/PR/SD/PD (%) |
3 / 76 / 334 / 261 (0.4 / 11.3 / 49.6 / 38.7) |
0 / 7 / 37 / 38 (0 / 8.5 / 45.1 / 46.3) |
0 / 2 / 22 / 20 (0 / 4.5 / 50.0 / 45.5) |
0 / 2 / 5 / 7 (0 / 14.3 / 35.7 / 50.0) |
0 / 1 / 4 / 5 (0 / 10 / 40 / 50) |
0 / 2 / 6 / 6 (0 / 14.3 / 42.9 / 42.9) |
|
OS analyses
N |
1963 | 252 | 151 | 37 | 34 | 30 |
| No. deaths | 1240 | 177 | 105 | 21 | 24 | 27 |
|
Median
(95% CI) |
22.3 (20.7,23.5) |
12.8 (11.0,16.1) |
14.0 (10.9,17.1) |
27.1 (12.6,75.3) |
10.1 (5.1,13.2) |
11.3 (9.6,19.4) |
|
Unadjusted
HR (95% CI) |
reference | 1.44 (1.23,1.69) |
1.48 (1.21,1.81) |
0.98 (0.64,1.51) |
1.71 (1.14,2.56) |
1.67 (1.14,2.45) |
|
Unadjusted
p-value |
<0.0001 | 0.0001 | 0.923 | 0.010 | 0.008 | |
|
Adjusted HR*
(95% CI) |
reference | 1.41 (1.19, 1.67) |
1.57 (1.27,1.94) |
0.89 (0.55,1.45) |
1.51 (0.98,2.31) |
1.30 (0.84,2.00) |
|
Adjusted
p-value * |
<0.0001 | <0.0001 | 0.646 | 0.060 | 0.243 | |
Abbreviations: ccRCC= clear cell renal cell carcinoma, nccRCC= non clear cell renal cell carcinoma, HR= hazard ratio; BR=best response
adjusted for the IMDC risk group criteria (time from diagnosis to treatment, Karnofsky performance status <80, hemoglobin <upper limit of normal, neutrophilia, thrombocytosis, hypercalcemia)
Overall Survival Outcomes
The entire nccRCC cohort had a significantly shorter OS than ccRCC; median OS was 22.3 months (95% CI: 20.7 – 23.5) and 12.8 months (95% CI: 11.0 – 16.1; p<0.0001) in ccRCC and nccRCC (Figure 1 C), respectively. After adjustment for the IMDC risk group criteria, patients with nccRCC tumors had a HR for death of 1.41 (95% CI: 1.19 – 1.67; p<0.0001) (Table 3). In subgroup analyses, median OS was 27.1 (95% CI: 12.6 – 75.3), 14.0 (95% CI: 10.9 – 17.1), and 10.1 (95% CI: 5.1 – 13.2) months in chRCC, pRCC, and unRCC, respectively (Table 3, Figure 1 D). When pRCC was compared to ccRCC, the adjusted HR for death was 1.57 (95% CI: 1.27 – 1.94; p<0.0001). Likewise, unRCC subtype had a higher risk for death (HR 1.71, 95%CI 1.14 – 2.56; adjusted HR 1.51, 95% CI: 0.98 – 2.31). By contrast, the chRCC subtype had comparable survival outcome with ccRCC in both univariate and multivariable analysis (adjusted HR 0.89, 95% CI: 0.55 – 1.45; p=0.646) (Table 3).
IMDC risk model for nccRCC
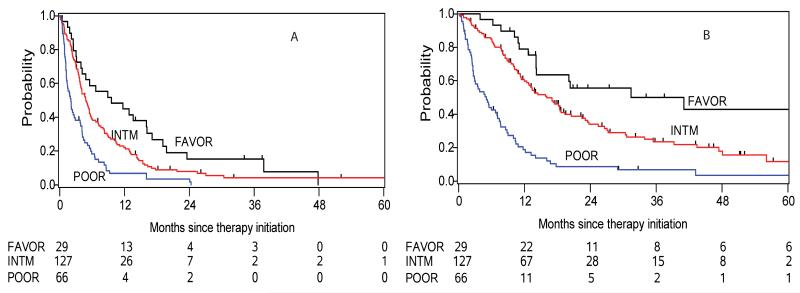
We next investigated the applicability of the IMDC risk model as a prognostic tool for TTF and OS in nccRCC. According to the six pre-defined IMDC risk criteria, 29 (13%), 127 (57%), and 66 (30%) nccRCC and 337 (19%), 972 (55%), and 463 (26%) ccRCC patients were assigned to favorable, intermediate, and poor prognosis groups (p=0.08). Among the nccRCC patients, the median TTF to the first line treatment in the three risk groups were 9.6 (95% CI: 3.9-16.2), 4.9 (95% CI:3.9-5.7), and 2.1 (95% CI:1.3-2.9) months (Figure 2 A); the HRs were 1.57 (95% CI: 1.02 – 2.42) and 3.10 (95% CI: 1.94 – 4.95) in the intermediate and poor prognosis group compared with favorable risk patients, respectively (p<0.0001). The estimated median OS of the three IMDC risk groups was 31.4 (95% CI: 14.2-78.3), 16.1 (95% CI: 12.5-18.7), and 5.1 (95% CI: 2.7-7.1) months (Figure 2 B); the intermediate risk group had an increased HR for death of 1.97 (95% CI: 1.13 – 3.42) and those with a poor prognosis had a HR of 5.69 (95% CI: 3.20 – 10.1) (p<0.0001). The c-indices for OS with the three groups was 0.66 (95% CI: 0.63 – 0.70) for the IMDC model and 0.64 (95% CI: 0.60 – 0.68) for the Memorial Sloan Kettering Cancer Center (MSKCC) risk criteria, respectively.
Figure 2.
We then correlated each of the individual six IMDC risk criteria with OS outcome in nccRCC. There was a significant association of all prognostic factors with OS in univariable analyses (HRs range 1.5-3.2, p<0.01 Table 4 B). Given limited patient numbers and low prevalence of some laboratory risk factors, we did not have sufficient power to test all the six risk factors in the multivariable model. However, the model yielded a c-index of 0.70 (95% CI: 0.66-0.74) when using the individual six risk factors instead of collapsing them into three risk groups, suggesting a good discriminatory ability.
Tabe 4.
IMDC risk group criteria for OS in nccRCC
| Univariate analysis | Multivariable model* | |||
|---|---|---|---|---|
| Parameter | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Hb low (<LLN) | 1.72(1.23,2.40) | 0.002 | 1.48(1.03,2.14) | 0.034 |
| Neutrophilia (>ULN) | 3.23(2.24,4.65) | <.0001 | 2.43(1.58,3.74) | <.0001 |
| KPS <80 | 3.15(2.23,4.45) | <.0001 | 2.02(1.35,3.02) | 0.0006 |
| Thrombophilia (>ULN) | 2.56(1.80,3.63) | <.0001 | 1.13(0.72,1.79) | 0.592 |
| Hypercalcaemia (>ULN) | 2.13(1.24,3.65) | 0.006 | 1.42(0.78,2.60) | 0.256 |
| Dx to treatment initiation <1year | 1.53(1.12,2.07) | 0.007 | 1.22(0.87,1.71) | 0.251 |
Abbreviations: Hb= hemoglobin, KPS= Karnofski performance status, Dx= diagnosis, ULN= upper limit of normal, LLN= lower limit of normal
c-index of 0.70 (95% CI: 0.66-0.74),
Discussion
In the current study, we report two findings: 1) the IMDC risk model reliably prognosticates clinical outcome in nccRCC, and 2) in the targeted therapy era, the majority of nccRCC patients still have an inferior clinical outcome compared to patients with ccRCC.
The introduction of agents targeting the VEGF- and mTOR pathways into clinical practice has revolutionized the treatment of mRCC. Median overall survival time has doubled when compared to historical control treatment with immunotherapy.12 The improvement of the survival rates is particularly true for ccRCC which represents the majority of mRCC. Less is known about the survival outcome of nccRCC which account only for 10-15% of RCCs.1, 2 Patients with metastatic nccRCC have worse response to immunotherapies than ccRCC.15 Motzer et al. have described a median OS of 9.4 months in a cohort treated with several kinds of cytokines.16 An early retrospective study of the targeted therapy era described a median OS of 19.4 months in a cohort of pRCC and chRCC patients and this was significantly longer than the OS outcome of 13.4 months in the sunitinib expanded access program.17, 18 The OS was very heterogeneous with 25.6 and 16.8 months in two recent sunitinib phase II trials, and 14.0 months in a phase II everolimus study.19-21 In the two studies demonstrating the longest OS (19.4 and 25.6 months) the rate of death events(~40%) was very low, and thus both studies may have overestimated the median OS.17, 19 In our study, 70% of nccRCC patients had died at the time of analysis and the majority of nccRCC patients (93.2%) were treated with an anti-VEGF therapy, sunitinib being the most often used drug. This may explain the shorter median OS compared to the smaller studies and the high concordance with the sunitinib open access trial.18 Moreover, the sunitinib open access program and the current study are the largest cohorts that investigated survival outcome in nccRCC. Other studies that revealed better survival outcome of nccRCCs may be biased by their small sample size.
In our study, nccRCC patients were more often treated with mTOR targeted therapies than ccRCC patients. Dutcher et al. have shown that nccRCC and ccRCC who were treated with temsirolimus have comparable OS and progression free survival.22 Our study found that nccRCC patients had a significantly inferior survival outcome compared to ccRCC. Some studies restricted the nccRCC population to certain subtypes17, 19 while others included a wider range of nccRCC subtypes.20, 21 Similar to the sunitinib open access trial our study cohort included patients without restriction of nccRCC subtype.18 However, the sunitinib open access program described the survival outcome not separately by nccRCC subtype.18 We found that patients with chRCC had the best OS and those with pRCC and unRCC the worst OS. These findings confirm results of smaller studies.11, 23-25 In chRCC preclinical studies have demonstrated that mTOR is activated by inactivation of its negative regulatory protein folliculin.26 Higher mTOR activity is related to a higher hypoxia inducible factor-α (HIF-α) production.27 Both pathways are major targets of the drugs used in our patients. Recently, a recent phase II trial with foretinib, a dual inhibition of VEGFR2 and MET, has demonstrated an overall response rate of 13.5% (all PR) and a PFS of 9.3 months with an intriguing 50% response in pRCC patients with a germline MET mutation.28 In the future, MET inhibitors may improve the survival outcome of pRCCs patients, possibly in a selected population. However, new drugs that better consider the unique biological properties of each nccRCC subtype are needed.
To the best of our knowledge, there is actually no other modern prognostic model that has been assessed exclusively in advanced nccRCC. Herein, we have demonstrated that the IMDC risk criteria reliably segregated nccRCC into three risk groups similar to our previous studies.11, 12 Moreover, the accuracy in prognosticating the OS was slightly higher than with the MSKCC risk model which was one of the most often used prognostic models in the past.29
The current study has several limitations that merit discussion. First, the evaluation of TTF and OS was done at each center without an independent radiological assessment. Our results are based on a retrospective investigation of a highly heterogeneous study population. We had to exclude 7% of the patients because of an unknown histology. There was no central pathology review and therefore, some tumors may have been misclassified but this may better reflect daily clinical practice where dedicated kidney cancer pathologists may not be available, and therefore results may be more generalizable.
Conclusions
The IMDC risk model is a reliable prognostication tool that can be employed to prognosticate OS in nccRCC for patient counseling and clinical trials design. The survival outcome for the majority of nccRCC patients remains lower than their clear cell counterparts. However, chRCC patients have a survival outcome that is comparable to ccRCC.
Acknowledgments
Funding for this study: None
Footnotes
Financial Disclosure of all Authors: NK, WX, SS, SKP, TY, NA, MHT have no conflict of interests, JLL Honoraria: Novartis, Bayer, Pfizer, Research Funding: Bayer; GAB consultant and advisory role at Pfizer; honoraria and research funding from Pfizer; JJK consultant and advisory role AVEO and has received research funding from Pfizer. MM advisory role at Novartis and Pfizer and has received research funding from both. LW has an advisory role at Pfizer and Novartis and has received research funding from Pfizer, Novartis, and GlaxoSmithKline. UNV has received honoraria and research funding from Pfizer, Novartis, and GlaxoSmithKline. S-YR has an advisory role at Novartis, Pfizer, and GlaxoSmithKline, and has received research funding from Novartis and Bayer Korea. FD has received research funding from Novartis. CK has an advisory role at Pfizer, Novartis, and GlaxoSmithKline and has received honoraria and research funding from Pfizer, Novartis, and GlaxoSmithKline. SAN consultant and advisory role at Novartis, Bayer, GlaxoSmithKline, Pfizer, BIR has an advisory role at Pfizer, GlaxoSmithKline, Aveo, Bayer, Onyx, and has received research funding from GlaxoSmithKline and Pfizer. TKC has received research funding from Pfizer and has an advisory role at Aveo, Pfizer, Novartis, GlaxoSmithKline, Genentech, Bayer, and Onyx. DYCH advisory role at Aveo, Pfizer, Novartis, and Bayer;
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 3.Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14:4726–4734. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patard JJ, Pignot G, Escudier B, et al. ICUD-EAU International Consultation on Kidney Cancer 2010: treatment of metastatic disease. Eur Urol. 2011;60:684–690. doi: 10.1016/j.eururo.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Escudier BJ, Ravaud A, Negrier S, et al. Update on AVOREN trial in metastatic renal cell carcinoma (mRCC): Efficacy and safety in subgroups of patients (pts) and pharmacokinetic (PK) analysis. ASCO Meeting Abstracts. 2008;26:5025. [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 11.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 12.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 14.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upton MP, Parker RA, Youmans A, McDermott DF, Atkins MB. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28:488–495. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 17.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 18.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee JL, Ahn JH, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. 2012;23:2108–2114. doi: 10.1093/annonc/mdr586. [DOI] [PubMed] [Google Scholar]
- 20.Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62:1013–1019. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann Oncol. 2012 doi: 10.1093/annonc/mds582. [DOI] [PubMed] [Google Scholar]
- 22.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 23.Choueiri TK, Garcia JA, Elson P, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110:543–550. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 24.Manola J, Royston P, Elson P, et al. Prognostic model for survival in patients with metastatic renal cell carcinoma: results from the international kidney cancer working group. Clin Cancer Res. 2011;17:5443–5450. doi: 10.1158/1078-0432.CCR-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–1558. doi: 10.1002/cncr.23776. [DOI] [PubMed] [Google Scholar]
- 26.Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Kaelin WG., Jr New insights into the biology of renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:667–686. doi: 10.1016/j.hoc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and Biomarker Study of the Dual MET/VEGFR2 Inhibitor Foretinib in Patients With Papillary Renal Cell Carcinoma. J Clin Oncol. 2013;31:181–186. doi: 10.1200/JCO.2012.43.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]