Abstract
Throughout development both the body and the brain change at remarkable rates. Specifically, the number of cells in the brain undergoes dramatic non-linear changes; first exponentially increasing in cell number and then decreasing in cell number. Importantly, different cell types, such as neurons and glia, undergo these changes at different stages of development. In the current investigation we used the isotropic fractionator method to examine the changes in cellular composition at multiple developmental milestones in the short-tailed opossum, Monodelphis domestica. Here we report several novel findings concerning marsupial brain development and organization. First, during the later stages of neurogenesis (P18) neurons comprise most of the cells in the neocortex, although the total number of neurons remains the same throughout the lifespan. In contrast, in the subcortical regions the number of neurons decreases dramatically after P18, and a converse relationship is observed for non-neuronal cells. In the cerebellum, the total number of cells gradually increases until P180 and then remains constant, and then the number of neurons is consistent across the developmental ages examined. For the three major structures examined, neuronal density and the percent of neurons within a structure is highest during neurogenesis and then decreases after this time point. Finally, the total number of neurons in the opossum brain is relatively low compared to other small-brained mammals such as mice. The relatively low number of neurons and correspondingly high number of non-neurons suggests that in the marsupial brain non-neurons may play a significant role in signal processing.
Keywords: development, marsupials, neocortex, evolution, comparative neuroanatomy, isotropic fractionation
Introduction
Throughout development, both the body and the brain undergo remarkable changes in both size and function. Because the nature of brain/body relationships and the cellular composition and state of connectivity of the brain itself is fundamentally different at various stages of development, it is important to consider each developmental stage in its own right, rather than as a continuum of the same structure that changes from simple to complex. This is particularly true since some cells play radically different roles at different stages of development (Polazzi and Contestabile, 2002). Traditionally, when we consider brain development we focus on neural development: how neurons are generated, how they migrate, and ultimately how they differentiate, connect, and refine their structure. While the importance of neural development is undeniable, the brain is not composed of neurons alone. Other cell types, including but not limited to endothelial cells (capillaries and blood vessels), mesothelial cells (pia mater), ependymal cells (lining of the ventricles), and glial cells are present as well, and of these, glia are the most prevalent (Morest and Silver, 2003; Temple, 2001). Recent studies have underscored the importance of microglia cells in both neurogenesis (Cunningham et al., in press) and programmed cell death (Kriegstein and Noctor, 2004; Polazzi and Contestabile, 2002; Upender and Naegele, 1999), and astrocytes in synaptic transmission and plasticity in adults (Nadarajah and Parnavelas, 2002; Rakic, 1990; Santello et al., 2012).
In the current study we examined the developmental relationships between neuronal and non-neuronal cells in the brains of short-tailed opossums using the isotropic fractionator technique. This relatively new methodology allows one to quickly and reliably estimate the number of neuronal and non-neuronal cells in different structures of the brain (Herculano-Houzel and Lent, 2005). This is done by transforming anisotropic structures of the brain, such as the six-layered neocortex, into an isotropic suspension of cell nuclei. These nuclei can be labeled using immunohistochemical techniques that identify them as neurons or other types of cells. The nuclei are then counted and the total number of cells, as well as the number of neuronal and non-neuronal cells, can be calculated. Because these techniques are independent of total brain volume, they are ideal for a number of important developmental comparisons across different age groups in which overall brain volume changes dramatically.
We used the short-tailed opossum because they are born in a very immature state, the equivalent to embryonic day (E) 13-14 in rats (Saunders et al., 1989)(See Table 1 for abbreviations). Further, infant opossums are not housed within a pouch, so they are easily accessible for manipulation and tissue harvest. Finally, compared to placental (eutherian) mammals such as rodents, the significant events of brain and sensory development occur over a protracted period, extending past the first postnatal month (Fig 1) (Saunders et al., 1989). Thus, the short-tailed opossum is an excellent model for studying both early brain development and adult cortical composition.
Table 1.
List of abbreviations
| E | Embryonic day |
| P | Postnatal day |
| DAPI | 4’,6-diamidino-2-phenylindole |
| NeuN | Neuronal nuclear protein |
| P35 | Phosphate buffered saline |
Figure 1.

Timeline of significant postnatal developmental milestones in the life of Monodelphis domestica. Thalamocortical connections are formed between P5 and P12 (red). Peak cortical neurogenesis occurs from P14-P24 (yellow). Eyes open between P31 and P34 (blue). Opossums are separated from their mothers at P56 (grey) and reach sexual maturity by P180 (green). Their peak reproductive period is from P270-P365, and after P365 they enter old age.
The goal of the present investigation was to examine the neuronal and non-neuronal composition of different structures of the developing brain including the neocortex, cerebellum and subcortical structures across important developmental time points. We found that while the total number of cells increases across development, the proportion of neurons generally decreases across development, and the cellular composition of different regions of the brain follows distinct and different developmental trajectories. These findings represent the first quantification of the cellular composition of a marsupial brain across several important developmental stages.
Methods
The isotropic fractionation procedure consists of multiple stages. First, tissue is dissected into major structures. Next, tissue was processed, which includes homogenization, DAPI staining and NeuN immunohistochemistry. The third stage involves quantifying the number of DAPI-labeled nuclei within a sample, and the fourth stage involves determining the proportion of NeuN-labeled nuclei within that same sample. Finally, in the last stage we use these values to calculate the total number of cells, total number of neurons, total number of non-neurons, cell density, neuronal density, and non-neuronal density. Each of these stages will be discussed in detail below.
Subjects
Thirty South American short-tailed opossums (Monodelphis domestica) raised in our breeding colony at the University of California, Davis were used in these experiments. Opossums were examined at six developmentally significant age groups: during cortical neurogenesis (P18, N=4), eye opening (P35 +/− 1 day, N=5), weaning (P56 +/− 1 day, N=5), sexual maturity (P180 +/− 1 day, N=5), adulthood (P270-365, N=4), and elderly adulthood (>P365, N=5). See Table 2 for sexes and weights. Two additional animals, one P18 and one P180, were used to show the relationship between neurons and non-neurons in non-homogenized tissue. All experiments were performed under National Institutes of Health guidelines for the care of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Table 2.
Age, sex, and weight of animals
| Case# | Group | Weight (g) | Sex |
|---|---|---|---|
| 12-060 | P18 | 3 | f |
| 12-061 | P18 | 3 | m |
| 12-062 | P18 | 2 | m |
| 12-064 | P18 | 2 | f |
| 11-148 | P35 | 7 | f |
| 11-149 | P35 | 9 | f |
| 11-235 | P35 | 7 | f |
| 11-236 | P35 | 5 | f |
| 11-237 | P35 | 7 | m |
| 11-157 | P56 | 13 | f |
| 11-158 | P56 | 13 | f |
| 11-160 | P56 | 15 | f |
| 11-240 | P56 | 13 | m |
| 11-241 | P56 | 13 | m |
| 11-150 | P180 | 77 | m |
| 11-159 | P180 | 96 | f |
| 11-162 | P180 | 59 | f |
| 11-267 | P180 | 99 | m |
| 11-268 | P180 | 131 | m |
| 11-154 | P270-365 | 75 | f |
| 11-156 | P270-365 | 137 | m |
| 11-161 | P270-365 | 64 | f |
| 11-242 | P270-365 | 86 | f |
| 11-151 | >P365 | 148 | m |
| 11-152 | >P365 | 121 | f |
| 11-153 | >P365 | 107 | f |
| 11-155 | >P365 | 134 | m |
| 11-243 | >P365 | 109 | m |
Tissue Dissection
Animals were euthanized with an overdose of sodium pentobarbital (Beuthanasia; 250 mg/kg) and transcardially perfused with phosphate buffered saline followed by 4% paraformaldehyde. The brain was extracted, photographed, and weighed. Under a microscope the brain was dissected into sections: the left cortical hemisphere, right cortical hemisphere, cerebellum, and the remaining subcortical regions (including the hypothalamus, thalamus, and brainstem) (Fig 2). The neocortex was then isolated by removing the hippocampus, basal ganglia, pyriform cortex, and olfactory bulb from the cortical hemisphere. These sections were photographed and weighed, and then placed in 5% paraformaldehyde for storage.
Figure 2.
The dissection of the brain for isotropic fractionation. The whole brain (A) is separated into the left and right cerebral hemispheres, subcortical structures (including the midbrain, thalamus, hypothalamus, and brainstem), and the cerebellum (B). The cerebral hemispheres are further dissected, removing the olfactory bulb, pyriform cortex, basal ganglia, and hippocampus, until only the neocortex remains (C). Scale = 1 mm.
Tissue Processing
Tissue was homogenized in a 15 ml glass KONTES* Tenbroek tissue grinder (Kimble Chase) with a dissociation solution composed of 10 ml triton X-100 and 11.76 g sodium citrate in 1000 ml distilled water. The homogenization process broke down cell membranes, producing a suspension of isolated cellular nuclei with no visible tissue clumps. An aliquot from the main suspension was centrifuged and re-suspended in a solution of PBS and DAPI (4’,6-diamidino-2-phenylindole), which binds to DNA and labels all cellular nuclei regardless of cell type (Fig 3 A, D, G). If the tissue had been stored in fixative for more than 4 weeks, the sample was suspended in a boric acid solution (12.37 g in 1000 ml distilled water) and placed in an oven at 70°C for one hour for epitope retrieval. A separate aliquot from the main suspension was stained for neuronal nuclei using immunocytochemical techniques with the anti-NeuN antibody (Millipore, Inc.; see Table 3 for antibody description). Alexa Fluor 647 or Alexa Fluor 700 goat anti-mouse IgG secondary antibody (Invitrogen, Inc.) was used to fluorescently tag NeuN labeled nuclei (Fig 3 B, E, H). In selected cases, we also used Alexa Fluor 594 goat anti-mouse IgG secondary antibody (Invitrogen, Inc.) to fluorescently tag NeuN labeled nuclei.
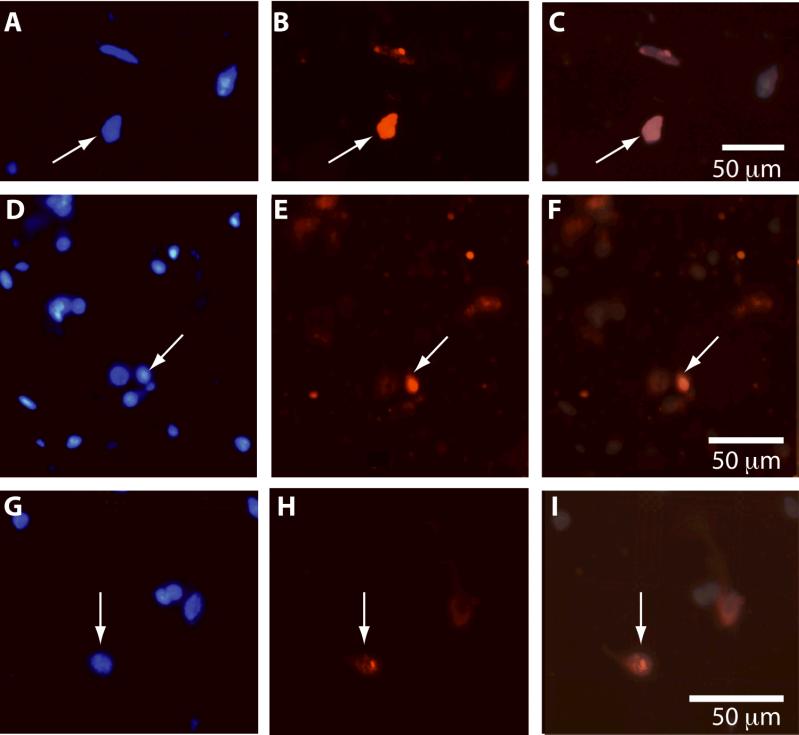
Figure 3.
Distribution of DAPI+ and NeuN+ nuclei following tissue dissociation. Cellular nuclei from the neocortex (top row), subcortical regions (middle row), and cerebellum (bottom row) were dissociated and stained for DAPI (labeled in blue; A, D, G) and NeuN (labeled in red; B, E, H). Nuclei that are stained for both DAPI and NeuN are considered to be neuronal (labeled in pink; C, F, I). White arrows identify one nucleus in each brain region that is both DAPI+ and NeuN+. Color and contrast have been adjusted using Adobe Photoshop. Scale for all images = 50 μm.
Table 3.
Weights of brain regions
| Age | Whole Brain | Neocortex | Subcortical Regions |
Cerebellum |
|---|---|---|---|---|
| P18 | 0.1191 ± .0026 | 0.0092 ± .0004 | 0.0525 ± .0014 | 0.0066 ± .0009 |
| P35 | 0.3450 ± .0176 | 0.0353 ± .0031 | 0.1401 ± .0055 | 0.0375 ± .0042 |
| P56 | 0.5112 ± .0062 | 0.0498 ± .0024 | 0.1586 ± .0060 | 0.0498 ± .0011 |
| P180 | 0.8949 ± .0568 | 0.0719 ± .0057 | 0.2767 ± .0142 | 0.1174 ± .0085 |
| P270-365 | 0.8533 ± .0404 | 0.0685 ± .0038 | 0.2705 ± .0142 | 0.1148 ± .0068 |
| >P365 | 0.9833 ± .0272 | 0.0726 ± .0029 | 0.3096 ± .0072 | 0.1235 ± .0070 |
| Age | Brain weight/ Body weight |
|---|---|
| P18 | 0.0522 ± .0087 |
| P35 | 0.0503 ± .0031 |
| P56 | 0.0382 ± .0011 |
| P180 | 0.0102 ± .0010 |
| P270-365 | 0.0100 ± .0011 |
| >P365 | 0.0080 ± .0004 |
| Age | % Neocortex | % Subcortical regions |
% Cerebellum |
|---|---|---|---|
| P18 | 7.72 ± 0.33 | 44.05 ± 0.36 | 5.52 ± 0.17 |
| PBS | 10.18 ± 0.44 | 40.73 ± 1.06 | 10.75 ± 0.80 |
| P56 | 9.73 ± 0.41 | 31.00 ± 0.86 | 12.40 ± 0.21 |
| P180 | 8.01 ± 0.22 | 31.03 ± 0.52 | 13.15 ± 0.55 |
| P270-365 | 8.03 ± 0.27 | 31.69 ± 0.43 | 13.44 ± 0.39 |
| >P365 | 7.26 ± 0.29 | 31.51 ± 0.36 | 12.57 ± 0.66 |
Antibody Characterization
Please see Table 3 for a list of the antibodies used. NeuN antibody (NEUronal Nuclei; clone A60) specifically recognizes the DNA-binding, neuron-specific protein NeuN, which is present in most central and peripheral nervous system neuronal cell types of all vertebrates tested. However, there are some exceptions that are described in the discussion. In a Western Blot analysis, this antibody recognized 2-3 bands in the 46-48 kDa range and possibly another band at approximately 66 kDa (adapted from product information). Since NeuN specifically stains neuronal nuclei, staining of non-neural tissue was used as a negative control.
Nuclei Quantification
The total number of nuclei was determined using a Neubauer cell-counting chamber (Optik Labor). Samples were vortexed and 10 μl aliquots were immediately loaded into a Neubauer cell-counting chamber and placed on a fluorescence microscope for visualization and counting of nuclei. Standard stereological protocols were used (Mouton, 2002). In order to obtain a reliable and representative sample of the number of DAPI-labeled nuclei within a given sample size we counted the nuclei in one 8-square by 8-square section of the Neubauer chamber (see Campi et al, 2011, for details). We repeated these counts on multiple samples until we had counted 10 unique 8x8 sections. We then calculated the mean number of nuclei in an 8×8 section and used that number to determine the total number of nuclei in the sample, using the equation described below.
Determining NeuN+ Percent
Using a separate aliquot from the main suspension, the ratio of neuronal to non-neuronal nuclei within a sample was determined using a flow cytometer at the UC Davis Flow Cytometry Shared Resource Center. The use of a flow cytometer to automate the detection and counting of neuronal and non-neuronal nuclei is both faster and more reliable than visual inspection under a fluorescent microscope (Collins et al., 2010b). To quantify the proportion of neuronal nuclei, we used a Becton Dickinson (BD) 5-Laser LSRII flow cytometer. The violet laser (50 mW 405 nm) excites DAPI-labeled nuclei and the red laser (50 mW 635 nm) excites both Alexa Fluor 647-labeled nuclei and Alexa Fluor 700-labeled nuclei. For all samples, between 1000-10,000 DAPI-positive nuclei were evaluated for AF 647 and AF 700 label. Samples run on the flow cytometer were forced through a 35-μm mesh cell filter, vortexed and immediately taken into the LSRII. Selection gates were determined by a flow cytometry expert who was blind to the developmental stage and brain areas of the samples. The selection gates used to estimate NeuN-IR nuclei were constructed to minimize the amount of both myelin debris and clumps of nuclei (i.e., doublets and triplets) that were counted within the samples. The samples were run for 120 seconds or until 10,000 nuclei were detected. The ratio of neuronal to non-neuronal nuclei was estimated from the gated population (see equations below).
To ensure the reliability of our results, in several cases we reanalyzed samples of dissociated and stained tissue. We did not see any significant differences between the first and second sets of analyses. Additionally, as mentioned above, in selected cases we labeled neuronal nuclei with Alexa Fluor 594 and counterstained them with DAPI. This allowed us to perform manual counts of NeuN+ and DAPI+ nuclei on a fluorescent microscope. The UV filter excited the DAPI-labeled nuclei and the TRITC filter excited the AF 594-labeled nuclei. We used a Neubauer cell counting chamber to count the number of DAPI-labeled and Alexa Fluor 594-labeled nuclei. We then calculated the proportion of NeuN+ to DAPI+ nuclei and compared that to the results obtained using the flow cytometer. We did not see any significant differences between these sets of analyses.
Equations
Estimates of cellular composition for a given structure were derived from the following equations.
total nuclei = [(number of DAPI+ nuclei)/(volume of suspension counted in mm3)] x (total suspension volume in cm3) × 1000 percent neurons = (number of NeuN+ nuclei)/(number of DAPI+ nuclei) total neurons = percent neurons x total nuclei total non-neurons = total nuclei – total neurons cell density = number of cells/weight of structure neuron density = number of neurons/weight of structure non-neuron density = number of non-neurons/weight of structure
Analysis
Developmental changes in the weights of the whole brain, neocortex, subcortical structures, cerebellum, and brain weight to body weight ratio were assessed using analysis of variance (ANOVA; JMP; SAS, Cary, NC), and differences between specific age groups were determined using Student’s t-tests. Likewise, developmental differences in the percent neurons, total number of cells, total number of neurons, total number of non-neurons, cell density, neuronal density, and non-neuronal density were assessed using ANOVA, and differences between specific age groups were determined using Student’s t-tests. For all tests, alpha = 0.05.
Whole Section Staining
In two additional cases we show the relationship between DAPI vs. NeuN labeled cells in non-homogenized tissue (Figs 4, 5 and 6). Each brain was sliced on a freezing microtome into 50 μm horizontal sections. Briefly, the 50 μm thick free-floating sections were first rinsed (3×5 min) in phosphate buffered saline (PBS). To quench endogenous peroxidase, sections were incubated in an aqueous solution of 10% MeOH and 3% H2O2 for 30 minutes at room temperature. Following rinses in PBS-0.1% Triton X-100 (3×10 min), non-specific binding was suppressed by a preincubation in 10% normal goat serum (NGS; Invitrogen, Camarillo, CA) and PBS-0.1% Triton X-100 for 1 hour at room temperature. Sections were then transferred to the primary antibody solution (mouse anti-NeuN, 1:100; Millipore, Billerica, MA) containing 10% NGS and PBS-0.1% Triton X-100 overnight at 4°C. Tissue sections were then rinsed in PBS-0.1% Triton X-100 (4x10 min) and incubated in a secondary antibody solution (Cy3 Goat anti-mouse IgG, 1:200, Millipore, Billerica, MA) for 4 hours at room temperature. The tissue sections were thoroughly rinsed in PBS (3x10 min). The fluorescent sections were double-labeled with the nuclear stain DAPI (Figs 4, 5 and 6). Sections were rinsed in PBS (3×5 min), mounted on gelatin-subbed slides, and coverslipped. Adjacent tissue sections were stained for Nissl so that laminar patterns visualized with Nissl stains could be compared with DAPI and NeuN labeling (Fig 6).
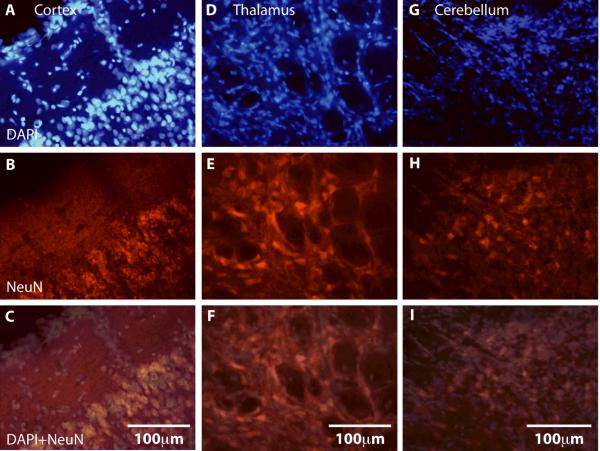
Figure 4.
The normal, anisotropic distribution of DAPI+ and NeuN+ cells in portions of the neocortex (A-C), dorsal thalamus (D-F), and cerebellum (G-I) in a P180 brain. The superimposition of DAPI+ (blue; A, D, G) and NeuN+ (red; B, E, H) cells reveals the distribution of neurons (pink; C, F, I) relative to all cells. In this low powered image the densely packed neurons in cortical layer 2/3 are clearly evident (C). Neurons in this portion of the dorsal thalamus are more evenly distributed. The high power image of the cerebellum (G –I) shows that the number of neurons is relatively sparse (H) compared to the total numbers of cells (G and I). Color and contrast have been adjusted using Adobe Photoshop. The scale for all images = 100 μm.
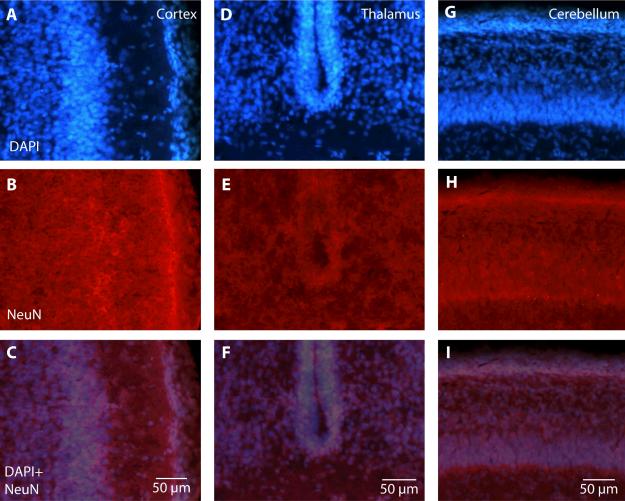
Figure 5.
The normal, anisotropic distribution of DAPI+ and NeuN+ cells in portions of the neocortex (A-C), thalamus (D-F), and cerebellum (G-I) in the brain of a P18 opossum. The superimposition of DAPI+ (blue; A, D, G) and NeuN+ (red; B, E, H) cells reveals the distribution of neurons (pink; C, F, I). In these images, the laminar structures of the cortex and cerebellum are clearly identifiable in both the DAPI+ and NeuN+ labeled cells. Within the thalamic section, there is a dense band of DAPI+ cells surrounding the ventricle. This band can also be seen in the NeuN+ cells, but it is very faint, indicating that this area contains a large proportion of non-neuronal cells or pre-mitotic cells. Color and contrast have been adjusted using Adobe Photoshop. The scale for all images = 50 μm.
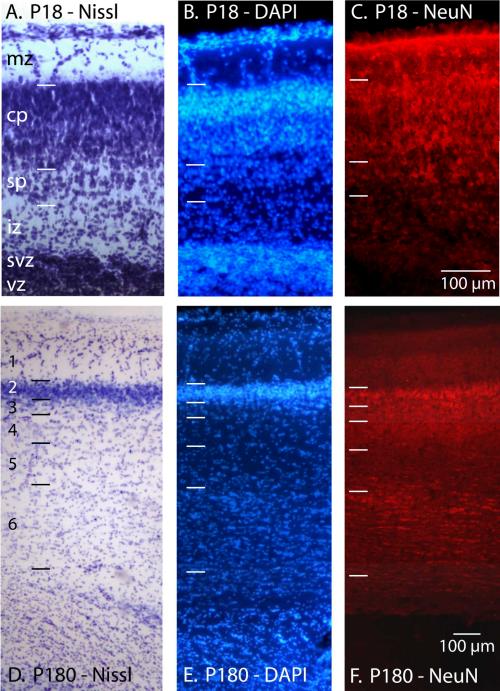
Figure 6.
The normal, anisotropic distribution of cells in cortical tissue stained for Nissl (A, D), DAPI (B, E), and NeuN (C, F) in P18 (A-C) and P180 (D-F) opossums. Both the P18 and P180 cortical sections are from a similar location in somatosensory cortex. At P18 the laminar organization of the developing neocortex is distinct and neurons within the cortical plate are clearly labeled with NeuN. While the ventricular zone is cell dense it contains no NeuN labeled cells. In the adult the characteristic layers of the neocortex are visible in both Nissl and DAPI stained tissue. Tissue stained for NeuN indicates a lack of neurons in layer 1, dense labeling of neurons in layers 2-3, and moderate labeling of neurons in layer 4 and 6. mz = marginal zone; cp = cortical plate; sp=subplate; iz = intermediate zone; svz = subventricular zone; vz = ventricular zone. Laminar division of early postnatal animals have been described by (Cheung et al., 2010; Saunders et al., 1989). Color and contrast have been adjusted using Adobe Photoshop. The scale for all images = 100 μm.
Results
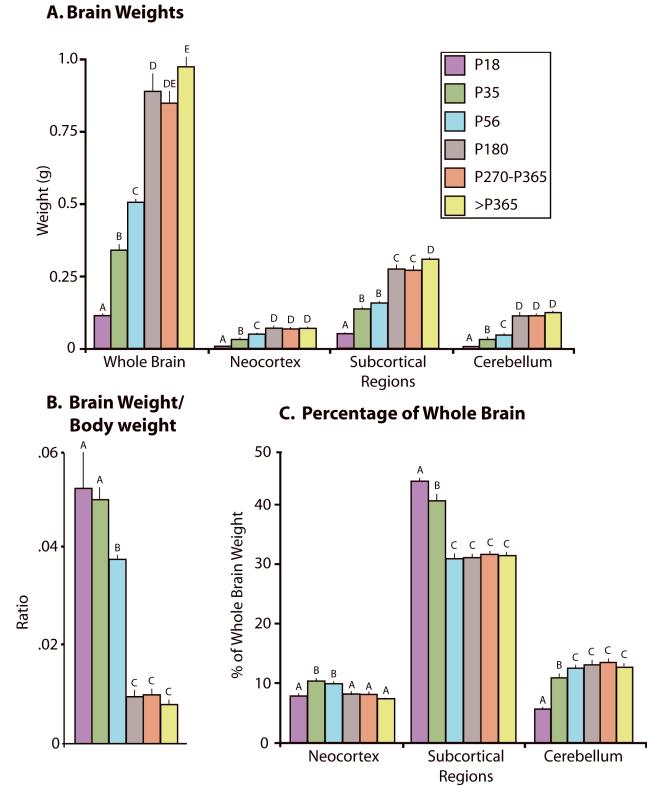
The weight of the whole brain (including both cortical hemispheres, the thalamus, hypothalamus, cerebellum, and brainstem; shown at each age in Fig 7) significantly increased across development (F5,27 = 111.88, p < .0001; see Table 4 for values). Likewise, the weights of the neocortex (F5,27 = 47.05, p < .0001), subcortical regions (F5,27 = 110.47, p < .0001), and cerebellum (F4,23 = 40.87, p < .0001) also significantly increased across development (Fig 8A). In contrast, the brain weight/body weight ratio significantly decreased across development (F5,27 = 69.09, p < .0001; Fig 8B), indicating that while both the body and brain increased across the lifespan, the body grew at a much faster rate than the brain.
Figure 7.
The relative size of Monodelphis domestica brains at P18, P35, P56, P180, P270-365, and >P365. The size and shape of the brain changes dramatically during the first six months of life. The caudolateral expansion of the cerebral cortex is particularly apparent. After sexual maturity (P180), there is little change in the size of the entire brain and the relative size of different structures. Images were converted to gray scale and contrast and brightness levels were adjusted using Adobe Photoshop. Scale = 2 mm.
Table 4.
Cellular composition of brain regions
| Neocortex: | |||||||
|---|---|---|---|---|---|---|---|
| Age | % Neurons | # cells (in millions) |
# neurons (in millions) |
# non-neurons (in millions) |
# cells/gram (in millions) |
# neurons/gram (in millions) |
# non-neurons/gram (in millions) |
|
| |||||||
| P18 | 60.55 ± 9.44 | 1.637 ± .058 | .985 ± .139 | .653 ± .163 | 180.032 ± 13.690 | 109.054 ± 18.773 | 70.977 ± 18.490 |
| P35 | 44.44 ± 9.21 | 1.580 ±.141 | .743 ± .093 | .836 ± .093 | 47.043 ± 6.952 | 22.792 ± 5.787 | 24.250 ± 3.103 |
| P56 | 43.52 ± 11.69 | 2.027 ± .220 | .970 ± .131 | 1.057 ± .131 | 40.454 ± 3.142 | 19.059 ± 6.024 | 21.394 ± 2.928 |
| P180 | 22.04 ± 3.63 | 3.224 ± .440 | .748 ± .283 | 2.476 ± .283 | 44.578 ± 4.136 | 10.111± 2.132 | 34.468 ± 2.678 |
| P270-365 | 33.55 ± 3.71 | 2.606 ± .308 | .847 ± .050 | 1.760 ± .306 | 37.901 ± 3.146 | 12.509 ± 1.226 | 25.391 ± 3.297 |
| >P365 | 25.62 ± 4.86 | 3.030 ± .209 | .803 ± .199 | 2.227 ± .120 | 42.067 ± 2.623 | 10.917 ± 2.545 | 30.779 ± 1.675 |
| Subcortical Regions: | |||||||
| Age | % Neurons |
# cells
(in millions) |
# neurons
(in millions) |
# non-neurons
(in millions) |
# cells/gram
(in millions) |
# neurons/gram
(in millions) |
# non-neurons/gram
(in millions) |
|
| |||||||
| P18 | 77.40 ± 5.00 | 3.184 ± .185 | 2.486 ± .267 | .698 ± .117 | 60.693 ± 3.293 | 47.436 ± 5.288 | 13.257 ± 2.198 |
| P35 | 6.77 ± 2.67 | 7.187 ± .659 | .503 ± .199 | 6.684 ± .610 | 51.355 ± 4.339 | 3.789 ± 1.677 | 47.565 ± 3.351 |
| P56 | 6.17 ± .69 | 8.240 ± .287 | .512 ± .066 | 7.728 ± .252 | 52.090 ± 1.759 | 3.186 ± .286 | 48.904 ± 1.868 |
| P180 | 6.30 ± .99 | 12.496 ± 1.009 | .803 ± .151 | 11.693 ± .912 | 44.941 ± 1.637 | 2.868 ± .498 | 42.073 ± 1.344 |
| P270-365 | 4.55 ± .32 | 12.073 ± 1.043 | .556 ± .079 | 11.517 ± .971 | 44.636 ± 3.108 | 2.053 ± .277 | 42.582 ± 2.853 |
| >P365 | 7.25 ± .90 | 13.147 ± 6.223 | .959 ± .172 | 12.044 ± .632 | 42.577 ± 2.326 | 3.079 ± .510 | 38.885 ± 2.541 |
| Cerebellum: | |||||||
| Age | % Neurons |
# cells
(in millions) |
# neurons
(in millions) |
# non-neurons
(in millions) |
# cells/gram
(in millions) |
# neurons/gram
(in millions) |
# non-neurons/gram
(in millions) |
|
| |||||||
| P18 | 86.93 ± 4.55 | 1.489 ± .191 | 1.282 ± .135 | .207 ± .086 | 229.061 ± 34.503 | 197.596 ± 26.204 | 31.465 ± 13.562 |
| P35 | 19.31 ± 8.14 | 11.084 ± .535 | 2.020 ± .740 | 9.064 ± 1.151 | 307.204 ± 26.825 | 65.787 ± 34.782 | 241.417 ± 19.457 |
| P56 | 30.82 ± 5.67 | 19.223 ± .522 | 5.954 ± 1.118 | 13.269 ± 1.039 | 303.494 ± 9.089 | 94.534 ± 18.142 | 208.961 ± 14.605 |
| P180 | 12.75 ± 4.20 | 30.496 ± 2.883 | 4.048 ± 1.353 | 26.448 ± 2.328 | 259.794 ± 15.348 | 34.082 ± 11.099 | 255.712 ± 14.011 |
| P270-365 | 9.97 ± 6.32 | 27.766 ± 2.378 | 2.537 ± 1.446 | 25.229 ± 3.305 | 241.285 ± 11.614 | 23.322 ± 14.085 | 217.963 ± 20.750 |
| >P365 | 8.45 ± 4.5 | 24.796 ± 1.969 | 2.018 ± .961 | 22.778 ± 2.308 | 200.907 ± 12.770 | 17.064 ± 8.764 | 183.843 ± 14.757 |
Figure 8.
Changes in brain weight (A), brain /body ratios (B) and relative size of major structures (C) across development. The entire brain and major structures increase in size until P180, and then remain relatively constant (A). Brain and body weight ratios decrease with age and then stabilized at P180 indicating that the body grows at a much faster rate than the brain until sexual maturity (B). The major structures that compose the brain undergo different trajectories of growth (C). Mean + s.e. Values with different letters are significantly different.
Different regions of the brain showed differential patterns of growth across development (Fig 8C). At each age the proportion of the brain comprised of the neocortex, subcortical regions, and cerebellum was determined by dividing the weight of the structure in question by the weight of the whole brain. The proportion of the brain consisting of the neocortex was 7.7% at P18, increased to 10.2% at P35 and significantly decreased to 8.0% at P180 and remained at that level throughout adulthood (F5,27 = 12.82, p < .0001; Fig 8C, Table 4). The proportion of the brain comprised by subcortical regions was highest at P18 at 44.0% and by P56 had significantly decreased to 31.0% where it remained through >P365 (F5,27 = 67.93, p < .0001). The cerebellum, on the other hand, significantly increased from 5.5% at P18 to 12.4% at P56 (F5,27 = 26.48, p < .05). These data indicate that while the brain grows steadily during the early part of the lifespan, it does not grow uniformly.
To demonstrate the relationship between DAPI and NeuN stains and the efficacy of NeuN in labeling neurons in both very young and adult brains the distribution of cells and neurons was visualized by staining whole sections of P18 and P180 tissue (Figs 4 – 6). All cells were labeled using DAPI (Fig 4A, D and G), and the tissue was counterstained for NeuN to identify neurons (Fig 4B, E, and H). The superimposition of the DAPI+ and NeuN+ images in Fig 4C, F and I allowed us to visualize individual nuclei and neurons and distinguish them from non-neuronal cells. The relationship between DAPI and NeuN also was demonstrated in the P18 opossum (Fig 5), and for the neocortex, DAPI and NeuN labeling were directly compared to adjacent Nissl stained sections (Fig 6). Both the P18 and P180 tissue showed a laminar distribution of neurons in the cortex and cerebellum. In the developing neocortex, laminar patterns, as revealed with both Nissl and DAPI stains indicate an abundance of cells in the ventricular zone and subventricular zones, although few of these cells were NeuN positive (Fig 6A – C). Some NeuN positive cells were labeled in the subplate and NeuN positive cells were found in abundance in the cortical plate. In the adult, Nissl and DAPI stained sections looked similar and show a similar laminar distribution with a prominent layer 2 −3 (Fig. 6D and E), and NeuN reveals an abundance of neurons in these same layers (Fig. 6 D and E). A moderate numbers of neurons were observed in deeper cortical layers (Fig. 6F). .
The isotropic fractionator method utilizes these same fluorescent stains, and follows the same principle of identifying neurons within a population of cells, but homogenizes the tissue, thus making different structures of the brain (e.g. neocortex, cerebellum) isotropic. In this way the cellular composition of large pieces of neural tissue can be quantified. It should be noted, however, that during the course of the homogenization areal and laminar information is necessarily lost. Following the homogenization of the tissue and nuclear dissociation, all nuclei were first labeled with DAPI then counterstained for NeuN in order to reveal neuronal nuclei (Fig 3). In figures 3, 4, 5, and 6 non-neuronal nuclei are visualized as blue, neuronal nuclei are visualized as red and nuclei that stain for both DAPI and NeuN are visualized as pink.
The cellular composition of the neocortex changed significantly across development (Fig 9). The total number of cells in the neocortex significantly increased across development (F5,27 = 7.19, p < .001; Fig 9A). While the total number of neurons did not significantly change (F5,27 = 0.27, NS), the total number of non-neurons significantly increased (F5,27 = 15.68, p < .0001). Further, the overall cell density decreased across development (F5,27 = 72.31, p < .0001; Fig 9B) as did neuronal density (F5,27 = 23.13, p < .0001) and non-neuronal density (F5,27 = 6.50, p < .001). The neuron percentage (i.e., the percent of total nuclei that were positive for NeuN) significantly decreased across development (F5,27 = 3.10, p < .05; Fig 9C; Table 5).
Figure 9.
Changes in cellular composition of the neocortex. A) Changes in the total number of cells (left), number of neurons (center), and number of non-neurons (right) in the neocortex at different developmental stages. While the total number of cells and non-neurons increases across the ages we sampled, the number of neurons does not change. B) Changes in total cell density (left), neuronal density (center), and non-neuronal density (right) across development. The density of both cell types was highest at P18, decreased by P35 and then remained unchanged through adulthood. C) The percentage of all cells within the neocortex that were neurons decreased across development. Mean + s.e. Values with different letters are significantly different.
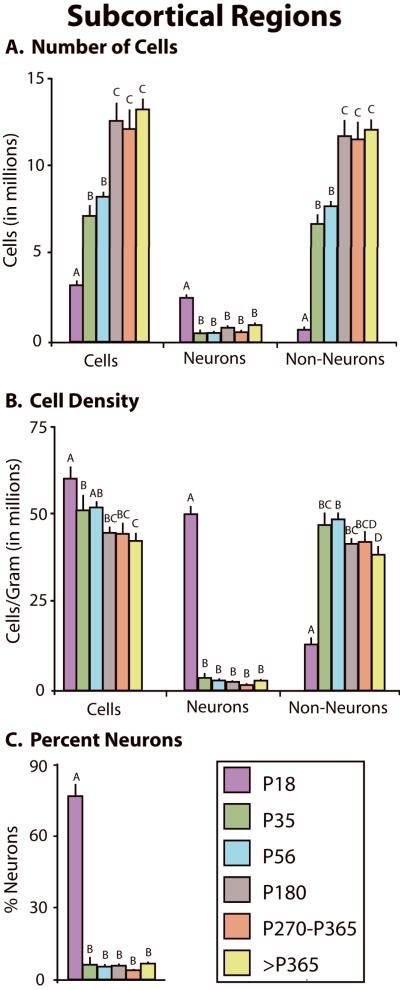
For subcortical regions the cellular composition also significantly changed across development (Fig 10). The total number of cells significantly increased with age (F5,27 = 20.06, p < .0001; Fig 10A; Table 4). Neuronal number was greatest at P18, during neurogenesis, and then significantly decreased with age (F5,27 = 3.48, p < .05), while the total number of non-neurons significantly increased over the same time period (F5,27 = 43.62, p < .0001). The overall cell density significantly decreased across development (F5,27 = 5.13, p < .005; Fig 10B), as did neuronal density (F5,27 = 74.01, p < .0001), while the density of non-neurons increased (F5,27 = 27.51, p < .0001). The percentage of neurons significantly decreased throughout the lifespan (F5,27 = 157.73, p < .0001; Fig 10C).
Figure 10.
Changes in cellular composition of subcortical regions. A) Changes in the total number of cells (left), number of neurons (center), and number of non-neurons (right) in the neocortex at different developmental stages. The total number of cells and non-neurons increased across development, but the number of neurons was highest at P18, decreased by P35 and remained unchanged across development. B) Changes in the total cell density (left), neuronal density (center), and non-neuronal density (right) across development. The total cell density and neuronal density was highest at P18. While the total cell density gradually decreased across development, neuronal density decreased dramatically by P35 and thereafter remained constant. In contrast, non-neuronal density was lowest at P18, increased by P35, then decreased slightly throughout adulthood. C) The percentage of neurons was highest at P18, significantly decreased by P35, and remained constant across development. Mean + s.e. Values with different letters are significantly different.
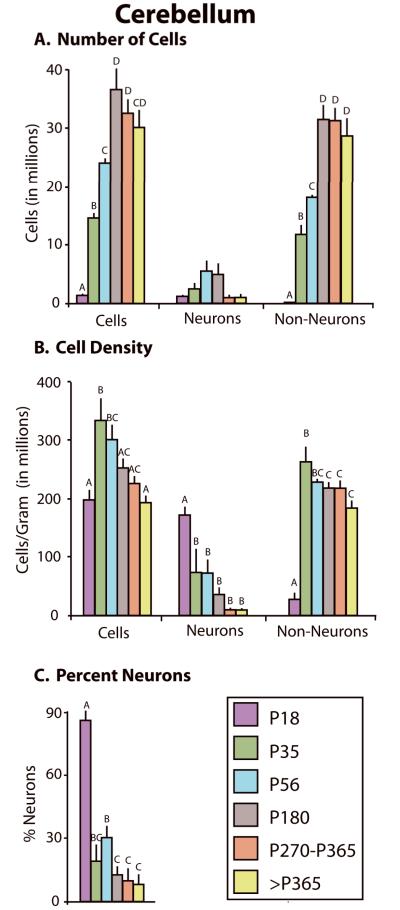
The cerebellum also showed significant changes in its cellular composition across development (Fig 11). The total number of cells sharply and significantly increased until P180, and then decreased through >P365 (F5,23 = 30.43, p < .0001; Fig 11A; Table 5). The total number of neurons did not significantly change across development (F5,23 = 2.45, p = .07), while the total number of non-neurons significantly increased until P180, at which point it remained steady (F5,23 = 44.36, p < .0001). Cell density increased from P18 through P35 and then gradually and significantly decreased with age (F5,23 = 5.43, p < .005; Fig 11B). In contrast, neuron density significantly decreased across development (F5,23 = 6.46, p < .005), and the non-neuron density increased from P18 to P35, then decreased from P35 to >P365 (F5,26 = 15.26, p < .0001). Finally, similar to both the neocortex and subcortical regions, the percentage of neurons within the cerebellum significantly decreased with age (F5,27 = 23.72, p < .0001; Fig 11C).
Figure 11.
Changes in cellular composition of the cerebellum. A) Changes in the total number of cells (left), number of neurons (center), and number of non-neurons (right) in the neocortex at different developmental stages. The total number of cells significantly increased from P18 through P180, then decreased through >P365. The number of neurons did not significantly change across development. In contrast, the number of non-neurons increased from P18 through P180, then remained constant. B) Changes in the total cell density (left), neuronal density (center), and non-neuronal density (right) across development. Both total cell density and non-neuronal density increased from P18 to P35, then decreased through >P365. In contrast, neuronal density was highest at P18 then decreased through adulthood. C) The percent neurons decreased across the lifespan. Mean + s.e. Values with different letters are significantly different.
Discussion
In the current study we found that the size of the body and brain increased with age, and the ratio between the brain and the body was relatively high early in development, but both dropped at 6 months of age and remained constant. In terms of cellular composition, the total number of cells in the neocortex, subcortical structures and the cerebellum increased with age until 6 months. However, there were some important differences in the growth patterns and cellular composition across the major structures, particularly with regard to neuronal number. For example, in the neocortex, the cellular density was highest at P18 and dropped off at P35 and remained constant across progressively older age groups. The number of neurons remained relatively constant while the percent of neurons declined with age (Figs 9 and 12) suggesting that the growth of the neocortex is due to the addition of non-neuronal cells and that naturally occurring neuronal cell death occurs before P18, or is not prevalent in the developing marsupial neocortex. The increase in non-neuronal cells is not surprising since our earliest sampled age (P18) was during peak cortical neurogenesis, which is then followed by gliogenesis within the neocortex (Cheung et al., 2010; Miller and Gauthier, 2007; Puzzolo and Mallamaci, 2010). In subcortical regions neuron number was high at P18, decreased at P35 and stayed constant at progressively older ages, as did percent of neurons; a converse relationship was observed for non-neuronal cells. Neuronal density and percent neurons also dropped significantly at P35 compared to P18 and a converse relationship was observed for non-neuronal cells. Finally, in the cerebellum, cell number increased with age and was accounted for mainly by an increase of non- neuronal cells. As in the cortex, neuronal number remained constant across all ages. The density and percent of neurons decreased dramatically after P18 and a converse relationship in cell density was observed for non-neuronal cells.
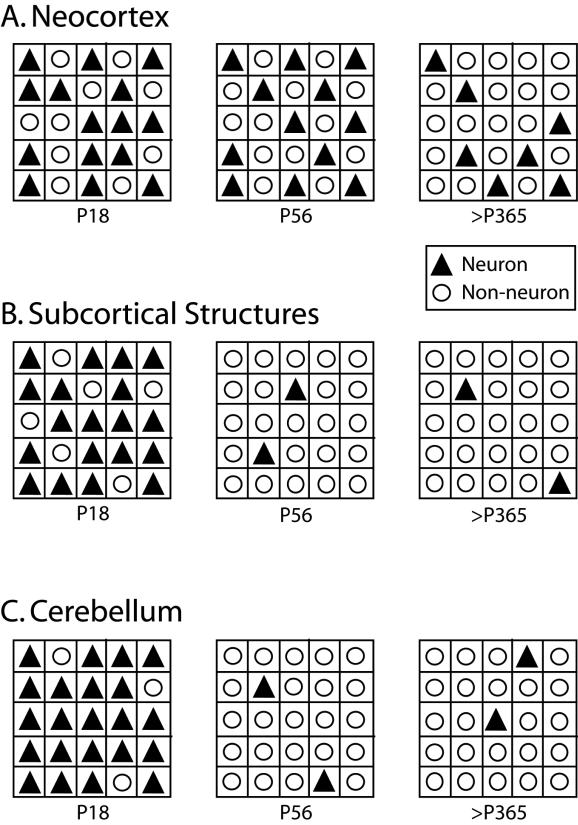
Figure 12.
Schematic of changes in cellular composition across development. The major assumption here is that cells (neuronal and non-neuronal) occupy the entire structure and that cell size does not change dramatically. This assumption is purposely simplified to better appreciate changes in number and density of cells across development. Each outer square represents a set volume of tissue for each age group. Neurons are represented by black triangles and non-neurons are represented by white circles. The ratio of neurons to non-neurons follows a distinct developmental trajectory in each brain region. A) In the neocortex, the total number of cells increases with the overall size of the structure. However, the number of neurons remains constant from P18 through adulthood. Thus, as the size of the structure increases the neuronal density decreases. B) In the subcortical structures, the total number of cells is lowest at P18 while the total number of neurons is highest at that age, resulting in a high neuronal density. Throughout development, the number of neurons decreases as the total number of cells increases, resulting in a significantly lowered neuronal density. C) In the cerebellum, the total number of cells are lowest at P18, but because a large proportion of those cells are neuronal we see a very high neuronal density. The number of neurons remains constant throughout development into adulthood while the number of cells (non-neurons) increase resulting in a lower neuronal density. All schematics are based on the total cell density and neuronal density of brain regions at P18, P56, and <P365.
It should be noted that NeuN is a marker for post mitotic neurons (Mullen et al., 1992; Sarnat et al., 1998). That is, NeuN is not expressed in neuronal precursor cells, but is expressed as the neurons exit the cell cycle (Martinez-Cerdeno et al., 2012; Noctor et al., 2008; Oomman et al., 2004; Yan et al., 2001). Thus, the large proportion of NeuN-positive cells during early development was not a result of labeling blastocysts or other immature cell types.
There are several important caveats that must be considered when interpreting data that use NeuN as a neuronal marker in different tissue and in different species. First, NeuN fails to label several types of neurons in the adult brain such as mitral cells in the olfactory bulb, retinal photoreceptors, and Purkinje cells in the cerebellum (Mullen et al., 1992). Second, NeuN fails to label some groups of postmitotic neurons, such as layer VIa cells in the neocortex (Lyck et al., 2007) and non-granule cell interneurons in the mouse cerebellum (Weyer and Schilling, 2003) until later developmental ages. The latter investigation also indicates that the expression of NeuN during development may be dependent on the physiological status of the developing neurons (Weyer and Schilling, 2003). Thus, studies that utilize NeuN to examine patterns of cellular composition across multiple developmental time points must be interpreted with caution. Finally, while there is good evidence that NeuN labels neurons in adult mammalian nervous tissue, and the use of the isotropic fractionator methodology in over 30 species including capybaras, star-nosed moles, bonnet macaques, and baboons is based on this assumption [e.g. (Burish et al., 2010; Collins et al., 2010a; Herculano-Houzel et al., 2006; Sarko et al., 2009)], it is likely that there are species specific differences in its labeling pattern. For example NeuN does not label substantia nigra neurons in the gerbil, but does label these neurons in rats (Kumar and Buckmaster, 2007). Thus, comparative studies on specific details of its labeling patterns for species other than mice and rats are important for accurate interpretation of data using this methodology.
An example of how these issues impact our own investigation comes from our estimates of neuronal and non-neuronal populations in the cerebellum where the numbers are low compared to other species. As noted above, NeuN does not label Purkinje cells in the adult cerebellum, and may not label all interneurons in the cerebellum or layer 6 cortical neurons until later developmental ages. If this is the case for the Monodelphis, then at earlier development ages, neurons in these structures may be under represented. However, the low numbers of cells in the developing cortex and cerebellum in the developing Monodelphis are also observed in adult suggesting that there may be true species differences in marsupials and small-brained eutherian mammals. These differences are discussed below.
Neural development in marsupials and rodents
Although this is the first study in marsupials to examine and quantify the cellular composition across major neural structures through different developmental time points, there are other studies of neural development in marsupials, particularly in the neocortex. As in other mammals, neurogenesis in the marsupial neocortex occurs in a rostrolateral to mediocaudal progression (Molnar et al., 1998; Sanderson and Weller, 1990). In marsupials this process is prolonged and occurs almost completely postnatally, and in some marsupials, such as the native cat, brush-tailed possum and wallaby, it occurs over a two to three month postnatal period (Aitkin et al., 1991; Marotte and Sheng, 2000; Sanderson and Weller, 1990). Neurogenesis and gliogenesis have been examined specifically in Monodelphis as well, and their duration may be somewhat shorter than in Australian marsupials (Puzzolo and Mallamaci, 2010). Using BrdU pulse-chase birth dating analysis these investigators suggest that neurogenesis was complete by P16. Neurons born after P18 remain mostly beneath the cortical plate; ages past P18 were not examined. By P30 cells born at P16 have migrated to the superficial layers of the neocortex indicating that the laminar development of the neocortex is complete. It should be noted that the samples in this previous study were taken from mid-frontal cortex, where the wave of neurogenesis begins and ends earlier than in other portions of the neocortex. Other studies that examine development of the neocortex in Monodelphis indicate that cortical neurogenesis occurs over a longer postnatal period, that the characteristic developmental layers, including the ventricular zone and subventricular zone are still clearly apparent at P45 (Saunders et al., 1989), and that late stage neurogenesis occurs in the middle of the fourth postnatal week (Molnar et al., 1998). Our own data indicate that peak neurogenesis of the neocortex and subcortical structures is complete by P35 since neuronal number is relatively constant across ages sampled for the neocortex (P18 to adulthood), and decreases at P35 for subcortical structures. Our studies also indicate that gliogenesis is prolonged in all structures of the brain that were examined and extends into the 6th postnatal month of life (P180).
There are only two other studies in which cellular composition of the brain has been examined across developmental age groups: one in mice (Lyck et al., 2007) and one in rats (Bandeira et al., 2009). The former study only examined the neocortex. However, neither of these studies captured early embryonic stages during which the vast majority of cortical and subcortical neurons are reported to be generated in mice and rats (Dehay and Kennedy, 2007; Robinson and Dreher, 1990). In rats cortical neurogenesis begins on E12 and ends on E21 and in mice cortical neurogenesis begins on E11 and ends on E19. These dates correspond to E12 and P24 in Monodelphis, respectively. Thus, the only data from the current study that we can compare with these previous studies would be at P35 and progressively later stages.
Probably the most notable difference between the current study and these previous investigations is our observation that the number of cortical neurons stabilizes or declines following the completion of neurogenesis as defined by earlier studies (see above), while these previous studies in mice and rats (Bandeira et al., 2009; Lyck et al., 2007) show a two fold or more increase in the number of neurons that occurs at P5 in the rat and P16 in the mouse, well after most studies indicate that neurogenesis has ended. These studies also demonstrate that this initial increase in neurons is followed by a reduction in neurons at later postnatal ages. In rats this reduction is as large as 70%.
While other studies have reported postnatal neurogenesis of GABAergic neurons in mice, the number of these neurons that actually migrate to the neocortex was estimated to be relatively modest (Inta et al., 2008). Lyck and colleagues (Lyck et al., 2007) suggest that this increase in the number of neurons in postnatal mice could come from neurons migrating into the neocortex from other regions such as the telencephalic wall (Molyneaux et al., 2005; Noctor et al., 2004) and medial ganglionic eminence (Anderson et al., 1997; Kriegstein and Noctor, 2004; Wichterle et al., 2001). Bandiera and colleagues (Bandeira et al., 2009) ascribe increases in neuronal population to massive postnatal neurogenesis, a notion counter to all that we know about neurogenesis in rodents; in fact their data suggest that most of neurogenesis occurs postnatally. However, given the limitation of the methods and the variable efficacy of NeuN for labeling particular populations of neurons and neurons present at early developmental ages (see above), it is not possible to determine if the cells they encounter at these early postnatal stages are newly-born cells, migrating cells, or cells which had not previously expressed NeuN at these earlier ages. The methods used in the rat suggest but do not specify that the pyriform cortex was included as part of the neocortex, which could also account for some of the differences described (Bandeira et al., 2009).
The cerebellum differs from the other brain regions described here in that at P18 it comprises only a small proportion of the weight of the whole brain (5.52%), and that proportion more than doubles by the time the opossum reaches adulthood (13.15% at P180; Fig 6, 7, and Table 4). Structurally, at P18 the opossum cerebellum is very immature, consisting of only an external granular layer, but by P35 all of the cerebellar layers are apparent, including the external granular, molecular, and internal granular layers as well as white matter (Sanchez-Villagra and Sultan, 2002). This late growth pattern is similar to that seen in rats, where the vast majority of cerebellar growth, including the development of its characteristic fissures and folia, occurs postnatally (Bandeira et al., 2009; Carletti and Rossi, 2008; Goldowitz and Hamre, 1998).
As noted above, the number of cerebellar neurons in Monodelphis throughout all stages of development, including adults, is low compared to eutherian mammals. This may be due in part to a lack of labeling of Purkinje cells, lack of labeling of non-granule cell interneurons (at least at earlier stages), lack of efficacy of labeling cerebellar cells other than the Purkinje cells, or true species differences. If our small number of cerebellum neurons in adults is due to a lack of Purkinje cell labeling, and if the ratio of Purkinje cells to granule cells is similar to that estimated for mice (Goldowitz and Hamre, 1998; Wetts and Herrup, 1983), than our results would have underestimated the number of neurons in the cerebellum by less than 1%.
Cellular composition of the neocortex in other small-brained animals
Another important difference between the current study and previous studies utilizing similar techniques is that there are an extremely small number of neurons that compose the adult marsupial brain compared to estimates from other small-brained mammals such as mice (Lyck et al., 2007), shrews, and moles (Sarko et al., 2009). For example, in adult mice the neocortex contains 14.4 million cells; about 50% are neurons and 50% are glial cells. Shrews and moles demonstrate a similarity in the composition of cells within the neocortex; the number of cells in the small neocortex (0.7 grams) of the smoky shrew was 14 million. Importantly, like mice, neurons comprised about 50% of these cells. As the size of the neocortex increased in shrews and moles, the proportion of neurons to non-neurons changed with larger brained insectivores having a greater percentage of non-neurons (e.g. 78% in the hairy tailed mole (See (Sarko et al., 2009), Table 2), which is similar to the percentage of non-neurons we observed in adult opossums.
The total number of cells in an adult Monodelphis neocortex (0.7 grams) was 3.2 million cells; 750 thousand or 22% were neurons and 2.5 million (78%) were non-neurons. Thus, both the total number of cells as well as the proportions of neurons versus non-neurons was dramatically different than in rodents and insectivores with a similar sized neocortex. This suggests that there may be fundamental differences in signal processing and transmission in marsupial brains, and that glia may play a more important role in information processing in marsupials compared to eutherian mammals (see below). There are no other studies that have examined the cellular composition of marsupial brains so it is not known whether this observation on overall number as well as proportion of neurons to non-neurons is a general characteristic of marsupials or specific to Monodelphis.
However, if this were a general feature of marsupials it suggests that early mammals had brains that were composed of substantially fewer neurons and that glial cells may have played a more central role in processing. Changes in the proportion of neurons (increases in number and density) may have arisen in eutherian mammals along with more neuron centered processing networks. Given the potential role of glial cells in synaptic transmission (see below), it also suggests that the neocortex of early mammals may have had a greater capacity for plastic changes in the adult.
Benefits and limitations the isotropic fractionator method
When considering data generated using the isotropic fractionator methodology it is important to consider both the benefits of this technique as well its limitations. Although isotropic fractionator methodology does not replace traditional stereological methods for quantifying various aspects of neuroanatomical organization and development, it offers the extraordinary advantage of estimating the number and composition of cells in the entire brain or entire neural structure in a relatively rapid, consistent manner. Not since the analogous comparative brain morphometry studies of Stephan and colleagues (Baron et al., 1990; Frahm et al., 1982; Stephan et al., 1981) has critical and extensive cross species comparisons been possible. These early morphometry studies of gross brain organization and size generated numerous and important theories regarding brain scaling in mammals (Finlay and Darlington, 1995; Stevens, 2001). Similarly, the isotropic fractionator techniques have been used to compare cellular composition and generate data driven theories of cellular evolution and brain scaling in a variety of mammals including several primates (Collins et al., 2010a), different rodents (Herculano-Houzel et al., 2006), shrews and moles (Sarko et al., 2009), and now marsupials. This accumulation of cross species data serves as an important data repository for any number of neurocomputational, developmental and evolutionary studies.
Of course the most obvious limitation of the technique when used in large structures as a whole is the deconstruction of tissue to rapidly and accurately estimate the number of cells, neurons and non-neurons that compose a structure. Thus, laminar divisions as well as cortical and nuclear divisions are lost. Second, there is some destruction of nuclei due to the homogenization process, as well as some loss of nuclei during the immunohistochemical processing of the tissue, however this loss is estimated to be minimal (Collins et al., 2010b; Herculano-Houzel and Lent, 2005). Furthermore, as mentioned above, there are selected neuron types that NeuN does not label (Mullen et al., 1992), and the efficacy of NeuN has not been well characterized in the brains of the many different animals in which the isotropic fractionator technique has been used. These limitations must be considered when interpreting data.
Finally, as we have already mentioned, the isotropic fractionation method uses dissociated cellular nuclei to generate data concerning the number and density of cells within a structure. Because all the cell membranes, axons, and dendrites have been removed during the cellular dissociation process, this technique cannot provide any concrete information about the size, shape, extracellular spaces, or connections of whole cells and neurons.
What about glial cells?
The current discussion is based on the assumption that the non-neuronal cells are predominantly comprised of glial cells. The other types of cells that constitute the non-neuronal group, endothelial cells, mesothelial cells, and ependymal cells, are relatively restricted in their distribution. Endothelial cells form the thin lining of blood vessels and compose the blood-brain barrier. The mesothelial cells comprise the pia mater, which in lissencephalic brains is relatively small compared to the volume of tissue it encloses. The ependymal cells line the ventricles, whose membrane volume is substantially smaller than cortical gray matter. Thus, of the non-neuronal cell types, glial cells represent the vast majority of this cellular population (Morest and Silver, 2003; Temple, 2001).
As noted in the introduction, given the changing role of different glial cells at various stages of development and in the adult brain, it is not surprising that their numbers and density varies across the developmental time points that we measured as well as in different structures. Importantly, in adult Monodelphis, the number of glial cells far exceeds that of neurons. This observation is particularly important given the present understanding of glial cells in the adult CNS. Glial cells are no longer considered to be primarily supportive cells that passively maintain homeostatic conditions necessary for neurotransmission, but rather they actively participate in synaptic transmission. In recent years, the tripartite synapse, which contains pre- and post-synaptic neuronal elements as well as astrocytes that encapsulate the synapse, has been described. Astrocytes have both ionotropic and metabotropic receptors that detect neurotransmitters, which increases internal stores of calcium within the astrocyte. This in turn causes gliotransmitters to be released at a slower rate than neurotransmitters and with a more prolonged affect. This bidirectional process is thought to modulate neurotransmission and plasticity (Pirttimaki and Parri, 2012; Santello et al., 2012; Verkhratsky et al., 2012). Given the importance of these cells in both homeostasis and active synaptic function it is critical to appreciate the neuronal/glial cell relationships at a systems level. The relatively large proportion of glial cells in the adult Monodelphis suggests that their brains may rely heavily on glial cells (such as astrocytes) for assisting in the synaptic transmission of substantially fewer neurons. This supposition could be explored using the isotropic fractionator method following the generation of nuclear markers for different types of glial cells, such as microglia and astrocytes. Ultimately, these studies will lead to a greater understanding of how neuronal and glial populations interact, and how those interactions may influence neural processing.
Acknowledgments
Thanks to Carol Oxford for her assistance at the UC Davis Flow Cytometry Shared Resource. Thanks also to Cindy Clayton, DVM, and the rest of the animal care staff at the UC Davis Psychology Department Vivarium.
Support: NIH R21NS071225 to LAK and NIH T32EY015387 to JCD
Footnotes
Conflict of Interest Statement:
The authors, AMHS, JCD, and LAK, have no conflicts of interest.
Role of Authors:
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AMHS, JCD, and LAK. Acquisition of data: AMHS. Analysis and interpretation of data: AMHS. Drafting of the manuscript: AMHS and LAK. Critical revision of the manuscript for important intellectual content: AMHS, JCD, and LAK. Statistical analysis: AMHS. Obtained funding: LAK. Administrative, technical, and material support: JCD. Study supervision: LAK.
References
- Aitkin L, Nelson J, Farrington M, Swann S. Neurogenesis in the brain auditory pathway of a marsupial, the northern native cat (Dasyurus hallucatus) J Comp Neurol. 1991;309(2):250–260. doi: 10.1002/cne.903090206. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278(5337):474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A. 2009;106(33):14108–14113. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron G, Stephan H, Frahm HD. Comparison of brain structure volumes in Insectivora and primates. IX. Trigeminal complex. Journal fur Hirnforschung. 1990;31(2):193–200. [PubMed] [Google Scholar]
- Burish MJ, Peebles JK, Baldwin MK, Tavares L, Kaas JH, Herculano-Houzel S. Cellular scaling rules for primate spinal cords. Brain Behav Evol. 2010;76(1):45–59. doi: 10.1159/000319019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti B, Rossi F. Neurogenesis in the cerebellum. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2008;14(1):91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- Cheung AF, Kondo S, Abdel-Mannan O, Chodroff RA, Sirey TM, Bluy LE, Webber N, DeProto J, Karlen SJ, Krubitzer L, Stolp HB, Saunders NR, Molnar Z. The subventricular zone is the developmental milestone of a 6-layered neocortex: comparisons in metatherian and eutherian mammals. Cereb Cortex. 2010;20(5):1071–1081. doi: 10.1093/cercor/bhp168. [DOI] [PubMed] [Google Scholar]
- Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci U S A. 2010a;107(36):15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CE, Young NA, Flaherty DK, Airey DC, Kaas JH. A rapid and reliable method of counting neurons and other cells in brain tissue: a comparison of flow cytometry and manual counting methods. Front Neuroanat. 2010b;4:5. doi: 10.3389/neuro.05.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate neurogenesis in the developing cerebral cortex. The Journal of Neuroscience. doi: 10.1523/JNEUROSCI.3441-12.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8(6):438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268(5217):1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Frahm HD, Stephan H, Stephan M. Comparison of brain structure volumes in Insectivora and Primates. I. Neocortex. Journal fur Hirnforschung. 1982;23(4):375–389. [PubMed] [Google Scholar]
- Goldowitz D, Hamre K. The cells and molecules that make a cerebellum. Trends Neurosci. 1998;21(9):375–382. doi: 10.1016/s0166-2236(98)01313-7. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25(10):2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci U S A. 2006;103(32):12138–12143. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A. 2008;105(52):20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27(7):392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Neuron-specific nuclear antigen NeuN is not detectable in gerbil subtantia nigra pars reticulata. Brain Res. 2007;1142:54–60. doi: 10.1016/j.brainres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck L, Kroigard T, Finsen B. Unbiased cell quantification reveals a continued increase in the number of neocortical neurones during early post-natal development in mice. The European journal of neuroscience. 2007;26(7):1749–1764. doi: 10.1111/j.1460-9568.2007.05763.x. [DOI] [PubMed] [Google Scholar]
- Marotte LR, Sheng X. Neurogenesis and identification of developing layers in the visual cortex of the wallaby (Macropus eugenii) J Comp Neurol. 2000;416(2):131–142. [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, Noctor SC. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PloS one. 2012;7(1):e30178. doi: 10.1371/journal.pone.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54(3):357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Knott GW, Blakemore C, Saunders NR. Development of thalamocortical projections in the South American gray short-tailed opossum (Monodelphis domestica) J Comp Neurol. 1998;398(4):491–514. [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47(6):817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Morest DK, Silver J. Precursors of neurons, neuroglia, and ependymal cells in the CNS: what are they? Where are they from? How do they get where they are going? Glia. 2003;43(1):6–18. doi: 10.1002/glia.10238. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology : an introduction for bioscientists. Johns Hopkins University Press; Baltimore: 2002. p. 214. x. [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3(6):423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7(2):136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508(1):28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomman S, Finckbone V, Dertien J, Attridge J, Henne W, Medina M, Mansouri B, Singh H, Strahlendorf H, Strahlendorf J. Active caspase-3 expression during postnatal development of rat cerebellum is not systematically or consistently associated with apoptosis. J Comp Neurol. 2004;476(2):154–173. doi: 10.1002/cne.20223. [DOI] [PubMed] [Google Scholar]
- Pirttimaki TM, Parri HR. Glutamatergic input-output properties of thalamic astrocytes. Neuroscience. 2012;205:18–28. doi: 10.1016/j.neuroscience.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polazzi E, Contestabile A. Reciprocal interactions between microglia and neurons: from survival to neuropathology. Reviews in the neurosciences. 2002;13(3):221–242. doi: 10.1515/revneuro.2002.13.3.221. [DOI] [PubMed] [Google Scholar]
- Puzzolo E, Mallamaci A. Cortico-cerebral histogenesis in the opossum Monodelphis domestica: generation of a hexalaminar neocortex in the absence of a basal proliferative compartment. Neural development. 2010;5:8. doi: 10.1186/1749-8104-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Principles of neural cell migration. Experientia. 1990;46(9):882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Dreher B. The visual pathways of eutherian mammals and marsupials develop according to a common timetable. Brain Behav Evol. 1990;36(4):177–195. doi: 10.1159/000115306. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villagra MR, Sultan F. The cerebellum at birth in therian mammals, with special reference to rodents. Brain Behav Evol. 2002;59(3):101–113. doi: 10.1159/000064158. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ, Weller WL. Gradients of neurogenesis in possum neocortex. Brain research Developmental brain research. 1990;55(2):269–274. doi: 10.1016/0165-3806(90)90208-g. [DOI] [PubMed] [Google Scholar]
- Santello M, Cali C, Bezzi P. Gliotransmission and the tripartite synapse. In: Kreutz MR, Sala C, editors. Synaptic plasticity: Dynamics, development, and disease. Springer; New York: 2012. pp. 307–331. [Google Scholar]
- Sarko DK, Catania KC, Leitch DB, Kaas JH, Herculano-Houzel S. Cellular scaling rules of insectivore brains. Front Neuroanat. 2009;3:8. doi: 10.3389/neuro.05.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain & development. 1998;20(2):88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Adam E, Reader M, Mollgard K. Monodelphis domestica (grey short-tailed opossum): An accessible model for studies of early neocortical development. Anatomy and Embryology. 1989;180:227–236. doi: 10.1007/BF00315881. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia primatologica; international journal of primatology. 1981;35(1):1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Stevens CF. An evolutionary scaling law for the primate visual system and its basis in cortical function. Nature. 2001;411(6834):193–195. doi: 10.1038/35075572. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414(6859):112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Upender MB, Naegele JR. Activation of microglia during developmentally regulated cell death in the cerebral cortex. Developmental neuroscience. 1999;21(6):491–505. doi: 10.1159/000017416. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V. Neurotransmitters and Integration in Neuronal-Astroglial Networks. Neurochemical research. 2012 doi: 10.1007/s11064-012-0765-6. [DOI] [PubMed] [Google Scholar]
- Wetts R, Herrup K. Direct correlation between Purkinje and granule cell number in the cerebella of lurcher chimeras and wild-type mice. Brain Res. 1983;312(1):41–47. doi: 10.1016/0165-3806(83)90119-0. [DOI] [PubMed] [Google Scholar]
- Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. Journal of neuroscience research. 2003;73(3):400–409. doi: 10.1002/jnr.10655. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128(19):3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Yan XX, Najbauer J, Woo CC, Dashtipour K, Ribak CE, Leon M. Expression of active caspase-3 in mitotic and postmitotic cells of the rat forebrain. J Comp Neurol. 2001;433(1):4–22. doi: 10.1002/cne.1121. [DOI] [PubMed] [Google Scholar]