Abstract
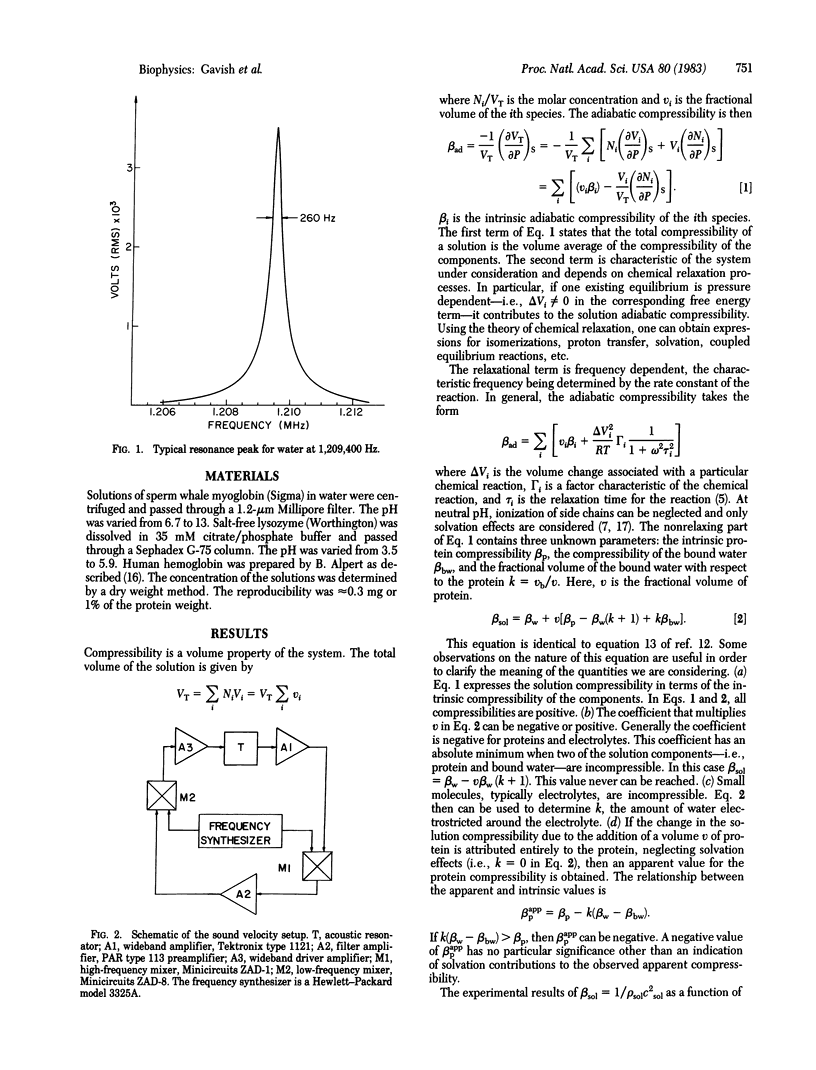
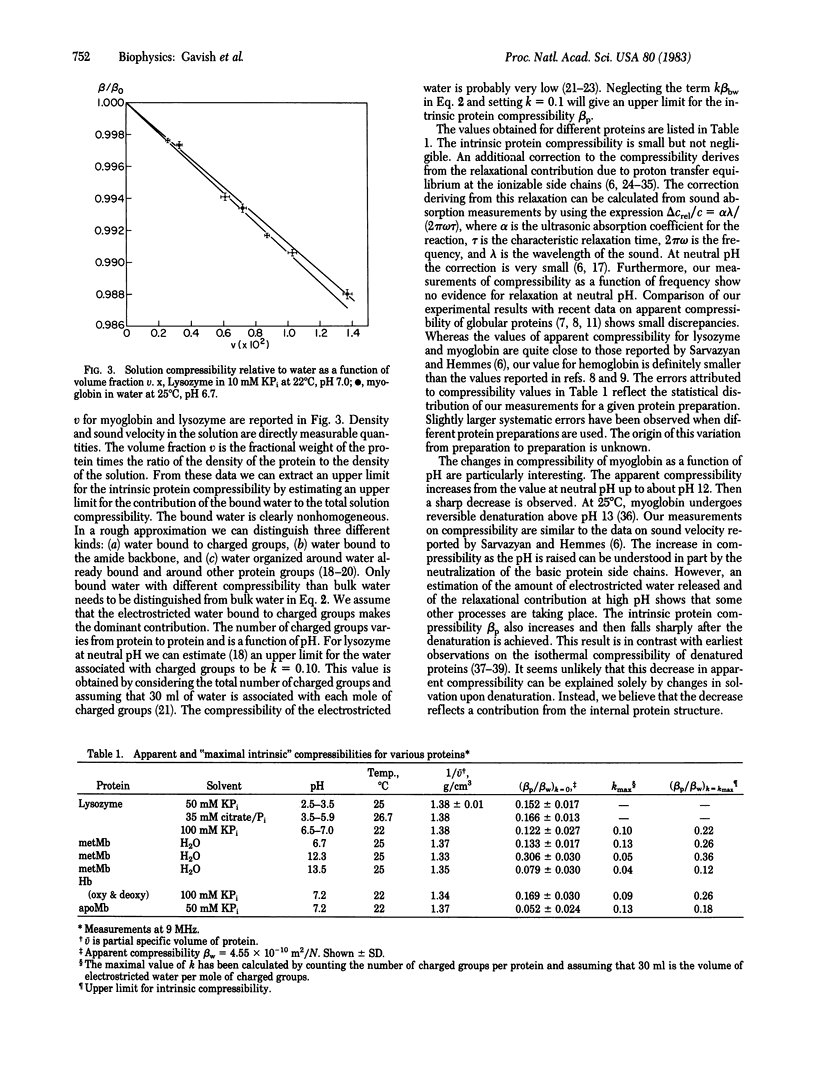
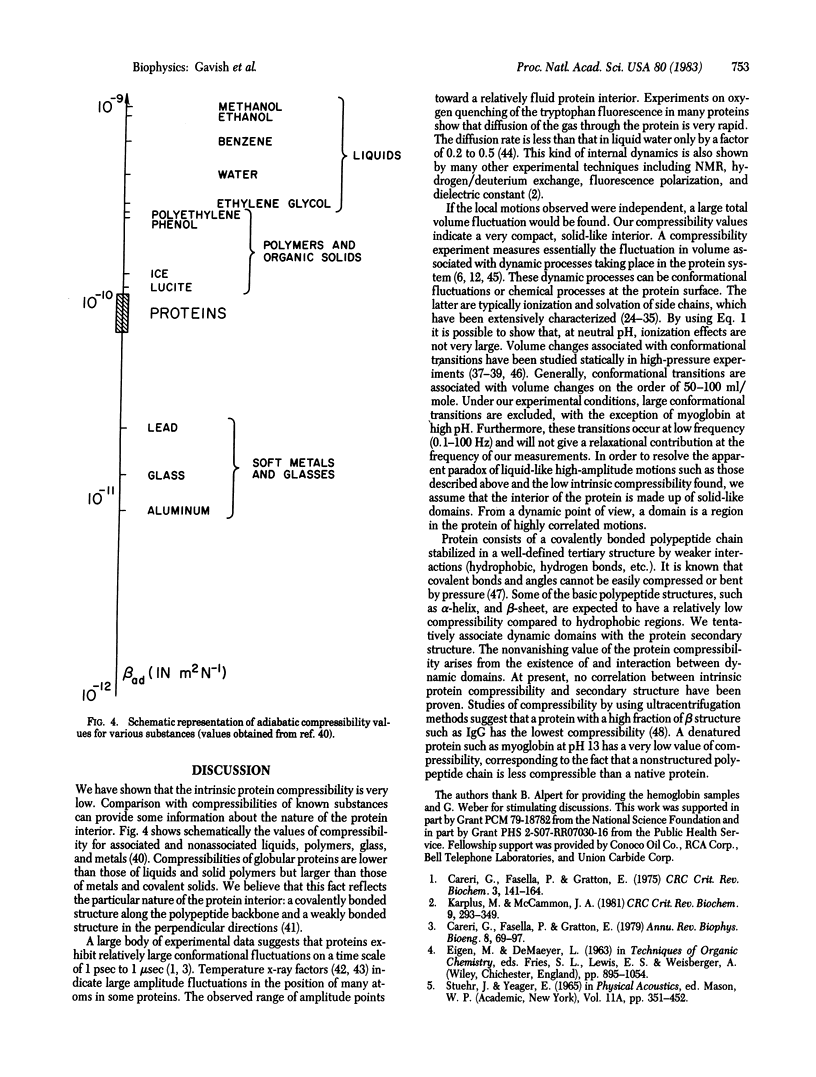
The adiabatic compressibility of several globular proteins has been measured by using an ultrasonic technique in the frequency range 0.5 to 10 MHz. The contributions to the measured compressibility from the protein matrix and from surface processes involving ionization of side chains and solvation effects are discussed. The internal protein compressibility is very low, indicating the existence of "dynamic domains" which are tentatively assigned to secondary structure elements.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acampora G., Hermans J., Jr Reversible denaturation of sperm whale myoglobin. I. Dependence on temperature, pH, and composition. J Am Chem Soc. 1967 Mar 29;89(7):1543–1547. doi: 10.1021/ja00983a001. [DOI] [PubMed] [Google Scholar]
- Applegate K., Slutsky L. J., Parker R. C. Kinetics of proton-transfer reactions of amino acids and simple polypeptides. J Am Chem Soc. 1968 Dec 4;90(25):6909–6913. doi: 10.1021/ja01027a003. [DOI] [PubMed] [Google Scholar]
- Artymiuk P. J., Blake C. C., Grace D. E., Oatley S. J., Phillips D. C., Sternberg M. J. Crystallographic studies of the dynamic properties of lysozyme. Nature. 1979 Aug 16;280(5723):563–568. doi: 10.1038/280563a0. [DOI] [PubMed] [Google Scholar]
- BURKE J. J., HAMMES G. G., LEWIS T. B. ULTRASONIC ATTENUATION MEASUREMENTS IN POLY-L-GLUAMIC ACID SOLUTIONS. J Chem Phys. 1965 May 15;42:3520–3525. doi: 10.1063/1.1695754. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Oliveira R. J., Westort C. Thermodynamics of protein denaturation. Effect of pressu on the denaturation of ribonuclease A. Biochemistry. 1970 Feb 17;9(4):1038–1047. doi: 10.1021/bi00806a045. [DOI] [PubMed] [Google Scholar]
- Bull H. B., Breese K. Protein hydration. II. Specific heat of egg albumin. Arch Biochem Biophys. 1968 Nov;128(2):497–502. doi: 10.1016/0003-9861(68)90056-8. [DOI] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Enzyme dynamics: the statistical physics approach. Annu Rev Biophys Bioeng. 1979;8:69–97. doi: 10.1146/annurev.bb.08.060179.000441. [DOI] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Statistical time events in enzymes: a physical assessment. CRC Crit Rev Biochem. 1975 Aug;3(2):141–164. doi: 10.3109/10409237509102555. [DOI] [PubMed] [Google Scholar]
- Careri G., Giansanti A., Gratton E. Lysozyme film hydration events: an ir and gravimetric study. Biopolymers. 1979 May;18(5):1187–1203. doi: 10.1002/bip.1979.360180512. [DOI] [PubMed] [Google Scholar]
- Cooper A. Thermodynamic fluctuations in protein molecules. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2740–2741. doi: 10.1073/pnas.73.8.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn F., Kessler L. W. Further remarks on the ultrasonic properties of bovine serum albumin solutions. J Phys Chem. 1970 Jun 25;74(13):2736–2736. doi: 10.1021/j100707a032. [DOI] [PubMed] [Google Scholar]
- Eden D., Matthew J. B., Rosa J. J., Richards F. M. Increase in apparent compressibility of cytochrome c upon oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):815–819. doi: 10.1073/pnas.79.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds P. D., Bauld T. J., 3rd, Dyro J. F., Hussey M. Ultrasonic absorption of aqueous hemoglobin solutions. Biochim Biophys Acta. 1970 Jan 20;200(1):174–177. doi: 10.1016/0005-2795(70)90058-9. [DOI] [PubMed] [Google Scholar]
- Eggers F., Funck T. Ultrasonic measurements with milliliter liquid samples in the 0.5-100 MHz range. Rev Sci Instrum. 1973 Aug;44(8):969–977. doi: 10.1063/1.1686339. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Hawley S. A. Reversible pressure--temperature denaturation of chymotrypsinogen. Biochemistry. 1971 Jun 22;10(13):2436–2442. doi: 10.1021/bi00789a002. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Kratky O., Leopold H., Stabinger H. The determination of the partial specific volume of proteins by the mechanical oscillator technique. Methods Enzymol. 1973;27:98–110. doi: 10.1016/s0076-6879(73)27007-6. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. M., Hook J. W., 3rd, Drickamer H. G., Weber G. Plurality of pressure-denatured forms in chymotrypsinogen and lysozyme. Biochemistry. 1976 Dec 14;15(25):5571–5580. doi: 10.1021/bi00670a023. [DOI] [PubMed] [Google Scholar]
- Millero F. J., Ward G. K., Chetirkin P. Partial specific volume, expansibility, compressibility, and heat capacity of aqueous lysozyme solutions. J Biol Chem. 1976 Jul 10;251(13):4001–4004. [PubMed] [Google Scholar]
- O'Brien W. D., Jr, Dunn F. Ultrasonic absorption mechanisms in aqueous solutions of bovine hemoglobin. J Phys Chem. 1972 Feb 17;76(4):528–533. doi: 10.1021/j100648a014. [DOI] [PubMed] [Google Scholar]
- O'Brien W. D., Jr, Dunn F. Ultrasonic examination of the hemoglobin dissociation process in aqueous solutions of guanidine hydrochloride. J Acoust Soc Am. 1971 Oct;50(4):1213–1215. doi: 10.1121/1.1912758. [DOI] [PubMed] [Google Scholar]
- Schneider F., Müller-Landau F., Mayer A. Acoustical properties of aqueous solutions of oxygenated and deoxygenated hemoglobin. Biopolymers. 1969;8(4):537–544. doi: 10.1002/bip.1969.360080411. [DOI] [PubMed] [Google Scholar]
- Sebban P., Coppey M., Alpert B., Lindqvist L., Jameson D. M. Fluorescence properties of porphyrin-globin from human hemoglobin. Photochem Photobiol. 1980 Dec;32(6):727–731. doi: 10.1111/j.1751-1097.1980.tb04049.x. [DOI] [PubMed] [Google Scholar]
- White R. D., Slutsky L. J., Pattison S. Kinetics of the proton-transfer reactions of serine and threonine. J Phys Chem. 1971 Jan 7;75(1):161–163. doi: 10.1021/j100671a027. [DOI] [PubMed] [Google Scholar]
- Zana R., Lang J. Effect of pH on the ultrasonic absorption of aqueous solutions of proteins. J Phys Chem. 1970 Jun 25;74(13):2734–2736. doi: 10.1021/j100707a031. [DOI] [PubMed] [Google Scholar]
- Zana R., Tondre C. [Ultrasonic studies in relation to the helix-coil transition in aqueous solutions of poly-L-lysine]. Biopolymers. 1971;10(12):2635–2638. doi: 10.1002/bip.360101226. [DOI] [PubMed] [Google Scholar]
- Zipp A., Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973 Oct 9;12(21):4217–4228. doi: 10.1021/bi00745a028. [DOI] [PubMed] [Google Scholar]



