Abstract
Behavioral sensitization to cocaine is associated with increased AMPA receptor (AMPAR) surface expression in the nucleus accumbens (NAc). This upregulation is withdrawal-dependent, as it is not detected on withdrawal day (WD) 1, but is observed on WD7–21. Its underlying mechanisms have not been clearly established. Nitric oxide (NO) regulates AMPAR trafficking in the brain by S-nitrosylation of the AMPAR auxiliary subunit, stargazin, leading to increased AMPAR surface expression. Our goal was to determine if stargazin S-nitrosylation contributes to AMPAR upregulation during sensitization. First, we measured stargazin S-nitrosylation in NAc core and shell subregions on WD14 after 8 daily injections of saline or 15mg/kg cocaine. Stargazin S-nitrosylation was markedly increased in NAc shell but not core. To determine if this is associated with AMPAR upregulation, rats received 8 cocaine or saline injections followed by twice-daily treatments with vehicle or the nitric oxide synthase inhibitor L-NAME (50mg/kg) on WD1–6, the time when AMPAR upregulation is developing in cocaine-exposed rats. Cocaine/vehicle rats showed elevated stargazin and GluA1 surface expression on WD7 compared to saline/vehicle rats; the GluA1 increase was more robust in core, while stargazin increased more robustly in shell. These effects of cocaine were attenuated in shell but not core when cocaine injections were followed by L-NAME treatment on WD1–6. Together, these results indicate that elevated S-nitrosylation of stargazin contributes to AMPAR upregulation during sensitization selectively in the NAc shell. It is possible that AMPAR upregulation in core involves a different TARP, γ4, which also upregulates in the NAc of sensitized rats.
Keywords: AMPA receptor, cocaine, nitric oxide synthase, nitrosylation, nucleus accumbens, stargazin, TARP
1. Introduction
Repeated cocaine exposure leads to behavioral sensitization, a progressive increase in behavioral responses to cocaine that persists long after discontinuing cocaine treatment. Sensitization occurs to cocaine's locomotor activating effects as well as the incentive-motivational properties of cocaine that make it “wanted” (Robinson and Berridge, 2008). The nucleus accumbens (NAc), an interface between corticolimbic and motor regions, is critical for expression of motivated behaviors including behavioral sensitization (Sesack and Grace, 2010). Medium spiny neurons, the output neurons of the NAc, are excited primarily by AMPA-type glutamate receptors (AMPARs). During the first week of withdrawal from a sensitizing cocaine regimen, surface and synaptic levels of AMPARs increase in core and shell subregions of the NAc (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007; Boudreau et al., 2009; Ghasemzadeh et al., 2009; Schumann and Yaka, 2009; Ferrario et al., 2010; Schierberl et al., 2011). By increasing the reactivity of NAc neurons to corticolimbic glutamate inputs, this AMPAR upregulation is believed to augment cocaine-related behaviors (Wolf and Ferrario, 2010; Wolf, 2010).
Stargazin is a member of the family of transmembrane AMPAR regulatory proteins (TARPs) that serve as auxiliary AMPAR subunits (Jackson and Nicoll, 2011; Straub and Tomita, 2012). Stargazin is physiologically nitrosylated, which enhances its binding to GluA1 and thus increases AMPAR surface expression (Selvakumar et al., 2009). Supporting this, mutations that prevent stargazin nitrosylation, as well as NO synthase (NOS) inhibitors and genetic deletion of neuronal NOS (nNOS), decrease surface expression of AMPARs (Selvakumar et al., 2009). NMDA receptor (NMDAR) activation stimulates nNOS (Garthwaite, 2008), generating the NO that nitrosylates stargazin (Selvakumar et al., 2009). In the NAc of cocaine-sensitized rats, NMDAR transmission contributes to AMPAR upregulation (Schumann and Yaka, 2009; Huang et al., 2009). Furthermore, acute and chronic cocaine administration can increase NO levels via nNOS in the prefrontal cortex and dorsal striatum (Sammut and West, 2008; Lee et al., 2010; 2011). Based on these findings, we tested the hypothesis that nitrosylation of stargazin contributes to the upregulation of cell surface AMPARs that occurs in the NAc during withdrawal from a sensitizing regimen of cocaine. We also examined the surface expression of another TARP, γ-4, that is expressed in the NAc and regulated by cocaine (Ferrario et al., 2011a,b).
2. Materials and Methods
2.1. Animals and drug treatments
All experiments were approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats (275–300g on arrival; Harlan, Indianapolis, IN) were housed 2–3/cage and maintained on a 12 h light:dark cycle. They were acclimated to the colony for 7 days prior to beginning experiments. Four experiments were conducted. In Experiment 1, rats received repeated injections (i.p.) of cocaine (15 mg/kg, Sigma-Aldrich, St. Louis, MO) or saline (0.9%, 1mL/kg) for 8 consecutive days. On each day, rats were placed in photocell cages (San Diego Instruments, San Diego, CA), habituated for 40 min, and injected with cocaine or saline. Locomotor activity (beam breaks) was measured for 90 min. Locomotor sensitization was assessed by comparing beam breaks on Day 1 and Day 8 of cocaine injections. Then, rats remained in home cages until they were killed on withdrawal day (WD) 14 for analysis of stargazin nitrosylation and neuronal NOS expression. For some of these rats (n=6/group), whole NAc tissue was harvested. For other rats (n=16 saline, n=15 cocaine), core and shell subregions were harvested separately. In Experiment 2, rats were received 8 daily injections of saline or 15 mg/kg cocaine exactly as described for Experiment 1 (n=20 rats/group). Each group (saline and cocaine) was then divided into two subgroups (n=10 rats/group) that received twice-daily i.p. injections of either vehicle (saline) or 50mg/kg L-NG-Nitroarginine methyl ester (L-NAME) on WD1–6. This yielded 4 experimental groups: saline/vehicle, saline/L-NAME, cocaine/vehicle, and cocaine/L-NAME (n=10 rats/group). Rats were killed on WD7 to measure AMPAR and TARP surface expression in NAc core and shell by biotinylation as described in Section 2.3. In Experiment 3, drug-naïve rats were used to determine whether AMPAR surface expression is altered by acute inhibition or activation of NOS. To inhibit NOS, rats (n=10/group) were treated with vehicle or the NOS inhibitor L-NAME (50 mg/kg) 3 times within a 24 h period (10 a.m., 6 p.m., 2 a.m.) and killed 7.5 h after the last injection to measure AMPAR surface expression in NAc core and shell by protein crosslinking as described in Section 2.4. To activate NOS, rats were treated with the NOS donor sodium nitroprusside (SNP; 5 mg/kg, i.p.). After determining that the maximal elevation of NO occurred ~30 min after SNP injection (see Electrochemical detection of NO), additional rats (n=10 rats/group) were injected with SNP or vehicle and killed 30 min later for determination of AMPAR surface expression in NAc core and shell by protein crosslinking as described in Section 2.4.
2.2. Biotin switch method
Nucleus accumbens tissue lysates from Experiment 1 were analyzed by the biotin-switch assay as described before with modifications (Selvakumar et al., 2009). Briefly, snap-frozen tissue was lysed using a glass homogenizer in HEN buffer (250 mM Hepes/1 mM EDTA/0.1 mM Neocuproine, pH 7.7) containing Triton X-100 (1%). Extracts were treated with methyl methanethiosulfonate (Sigma–Aldrich) and SDS (1%) at 50 °C for 20 min. Proteins were precipitated with acetone, and the precipitate was labeled with biotin-HPDP (0.2 mM) (Pierce, Rockford, IL) with or without ascorbate (50 mM) for 90 min at room temperature. Proteins were re-precipitated with acetone, and the precipitate was incubated with anti-stargazin antibody (1 μg/mg protein, AB9876, Millipore, Billerica, MA). The immune complex was eluted with the GST-tagged C-terminal fragment of stargazin (immunizing antigen), and the eluate was incubated with glutathione-Sepharose beads to remove the GST-fragments. The biotinylated proteins in the supernatant were purified by using neutravidin beads (Pierce), separated by SDS/PAGE, and analyzed by Western blotting.
2.3. Biotinylation
After completion of Experiment 2, bilateral core and shell subregions of the NAc were rapidly dissected from a 2 mm coronal section obtained using a brain matrix (ASI Instruments, Warren, MI) and minced with a scalpel. Biotinylation of surface-expressed proteins was performed as described previously (Ferrario et al., 2011a). Briefly, tissue was incubated (30 min with gentle agitation, 4°C) with 1 mM sulfo-NHS-S-S-Biotin (Thermo Scientific, Rockford, IL) dissolved in artificial cerebrospinal fluid. The reaction was quenched with 100 mM glycine (10 min, 4°C). The tissue was pelleted, re-suspended in ice-cold lysis buffer [25 mM HEPES pH 7.4, 500 mM NaCl, 2 mM EDTA, 20 mM NaF, 10 mM NaPPi, 1 mM PMSF, 0.1% NP-40 (v/v), 1 mM NaOV, 1 μM okadaic acid, 1μM microsystin-LF, 1X protease inhibitor cocktail (Calbiochem; 539131)], sonicated, and stored at −80°C. Biotinylated proteins were purified using NeutrAvidin beads (Thermo Scientific) and analyzed by SDS-PAGE and immunoblotting. The following primary antibodies were used: GluA1 (1:1000, 1308-1, Epitomics, Burlingame, CA); GluA2 (1:200, AB78-002, UC Davis/NIH NeuroMab Facility, Davis, CA); stargazin (1:1000, 1505-STAR, Phosphosolutions, Aurora, CO); γ-4 (1:1000, AB5795, Millipore).
2.4. Protein crosslinking
Bis(sulfosuccinimidyl)suberate (BS3.; Pierce Biotechnology, Rockford, IL) is a bifunctional crosslinking reagent that does not cross membranes. Therefore, it selectively modifies surface-expressed proteins, increasing their apparent molecular weight and enabling them to be separated from unmodified intracellular proteins by SDS-PAGE and quantified by immunoblotting (see Boudreau et al., 2012 for detailed protocols). BS3 protein crosslinking offers advantages over biotinylation for analysis of AMPAR surface expression, but some antibodies (e.g., the stargazin antibody) do not recognize their target protein after it has been crosslinked. Therefore biotinylation was used when analyzing TARPs (Experiment 2), whereas BS3 crosslinking was used for experiments in which only AMPAR subunits were analyzed (Experiment 3). Briefly, rats used for Experiment 3 were treated with L-NAME or SNP as described in Section 2.1. NAc core and shell tissue was dissected and minced as described in Section 2.3 and then incubated with 2 mM BS3 (30 min with gentle agitation, 4°C). After quenching the reaction with 100 mM glycine, tissue was processed as described for biotinylation and stored at −80°C. BS3 crosslinked samples were electrophoresed on 4%–15% Bis-Tris gradient gels (BioRad) under reducing conditions. Proteins were transferred (1.15 Amps, constant current, 1.25 h) onto PVDF membranes (Amersham Biosciences, Piscataway, NJ). The following primary antibodies were used: GluA1 (1:1000, PA1-37776, Thermo Scientific); GluA2 (1:200, AB78-002, UC-Davis/NIH NeuroMab Facility). Values (diffuse densities) for surface, intracellular and total (surface + intracellular) protein levels were normalized to a loading control (DARPP-32; 1:5000, 611520, BD Transduction Laboratories, San Jose, CA) analyzed in the same lane.
2.5. Electrochemical detection of NO
Extracellular levels of NO were measured in the NAc of urethane anesthetized rats using an NO-selective, amperometric microsensor (amiNO-100, Innovative Instruments, Inc., Tampa, FL). As previously described (Sammut and West, 2008; Park and West, 2009), prior to each experiment, microsensors were calibrated in a temperature controlled chamber using known solutions of the NO generating compound S-nitroso-N–acetyl-penicillamine. Calibration curves were calculated in order to determine the sensitivity of microsensors and confirm that the NO oxidation current exhibited a linear response to NO concentrations ranging from 0.6–48 nM. The lower detection limit of NO microsensors was ~0.1–1.0 nM NO. Following calibration, microsensors were implanted into the medial shell region of the NAc over a 30 min period using a micromanipulator (coordinates from Bregma: 1.6mm anterior, 0.7mm lateral, and 7.8mm ventral). All experiments were initiated ~1–2 h post-surgery. NO oxidation current (pA) was allowed to stabilize for at least 150 s prior to systemic administration of the NO generator sodium nitroprusside (SNP). Drug-induced changes in NO oxidation current were measured as previously described (Park and West, 2009). Briefly, the NO oxidation current recorded (50 Hz sampling frequency) during the last 30 s of the pre-injection period was averaged using Apollo 4000 software applications (WPI) and subtracted from the mean NO oxidation current recorded during drug administration. The amplitude and latency of the peak SNP-induced change in NO oxidation current was determined for each animal and averaged to generate mean group values. Data are expressed as concentration of NO (nM) as determined from in vitro calibration curves.
2.6. Statistical analysis
Locomotor activity data for multiple groups were compared using two-way repeated measures ANOVA, followed by Bonferroni t-tests. For behavioral and biochemical experiments comparing two groups, data were analyzed using a Student's t-test (non-directional). Significance was set at P<0.05. We utilized SigmaPlot 11 (Systat Software Inc., San Jose, CA, USA) and Microsoft Excel.
3. Results
3.1. Experiment 1
Our first goal was to determine if a sensitizing regimen of cocaine leads to altered S-nitrosylation of stargazin. To elicit locomotor sensitization, we treated rats with 15 mg/kg cocaine daily for 8 days (Fig. 1A–C). We harvested NAc tissue on WD14 based on many results from our group and others demonstrating increased AMPAR surface expression in the NAc core and shell between WD7 and WD21 (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007; Boudreau et al., 2009; Ghasemzadeh et al., 2009; Schumann and Yaka, 2009; Ferrario et al., 2010; Schierberl et al., 2011), as confirmed in Experiment 2 (below). We measured stargazin nitrosylation in whole NAc, as well as core and shell subregions from a different cohort of identically treated animals. Sensitized rats exhibited a 1.5-fold increase in stargazin nitrosylation in whole NAc (Fig. 1D; t3=5.84, *P<0.05), no change in the core (Fig. 1E), and a 2.5-fold increase in the shell (Fig. 1F; t6=2.48, *P<0.05). Levels of neuronal NOS were also measured in these animals. Significantly higher levels were found in shell compared to core, regardless of whether rats were pretreated with saline or cocaine (Fig. 2; t7=5.42, *P<0.005 for the saline group; t7=4.79, **P<0.005 for the cocaine group). These results indicate that the shell subregion, but not the core, shows a robust increase in stargazin nitrosylation during cocaine withdrawal that corresponds to higher NOS levels in shell compared to core.
Figure 1. Cocaine-sensitized rats show increased S-nitrosylation of stargazin in NAc shell on withdrawal day 14.
(A) Time-line for Experiment 1. Rats received 8 daily injections of cocaine (Coc; 15 mg/kg, i.p.) or saline (Sal) in photocell cages. (B) Locomotor data (from animals used to generate whole NAc tissue) showing development of sensitization (main effect of injection day for cocaine group: F1,80=13.28, P<0.01; Bonferroni t-tests comparing individual time-points, *P<0.05; n=6/group). (C) Comparison of summed photocell counts demonstrating locomotor sensitization in a separate cohort of identically treated animals used to generate core and shell samples [main effect of injection day: F1,29=5.28, P<0.05; Bonferroni t-tests indicated a significant effect within the cocaine group (*P=0.01) but not the saline group; n=16 Sal, n=15 Coc]. (D–F) On withdrawal day 14, nitrosylation of stargazin was measured as described previously (Selvakumar et al., 2009) in whole NAc samples obtained from one cohort of rats (behavioral data shown in panel B) and in core and shell samples obtained from a different cohort of rats (behavioral data shown in panel C). Stargazin nitrosylation was significantly greater in whole NAc (D) (t3=5.84, *P<0.05) and NAc shell (F) (t6=2.48, *P<0.05) of cocaine-sensitized animals, with no difference in the NAc core (E).
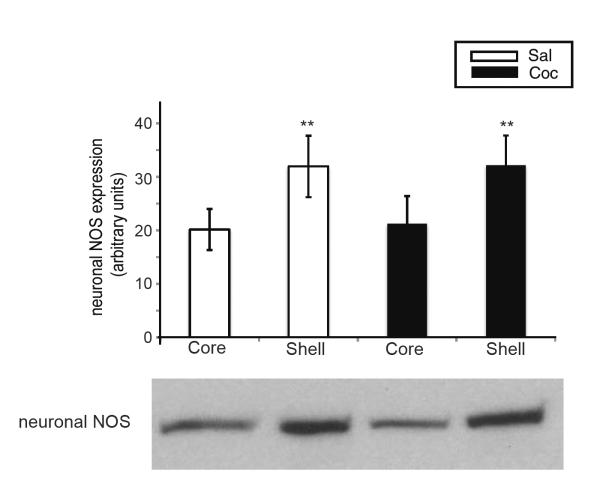
Figure 2. nNOS expression is higher in NAc shell than core.
nNOS protein was measured from cocaine-sensitized and saline control rats killed on WD14 (rats from Experiment 1, Fig. 1). nNOS expression was significantly greater in shell compared to core regardless of pretreatment (t7=5.42, *P<0.005 for the saline group; t7=4.79, **P<0.005 for the cocaine group).
3.2. Experiment 2
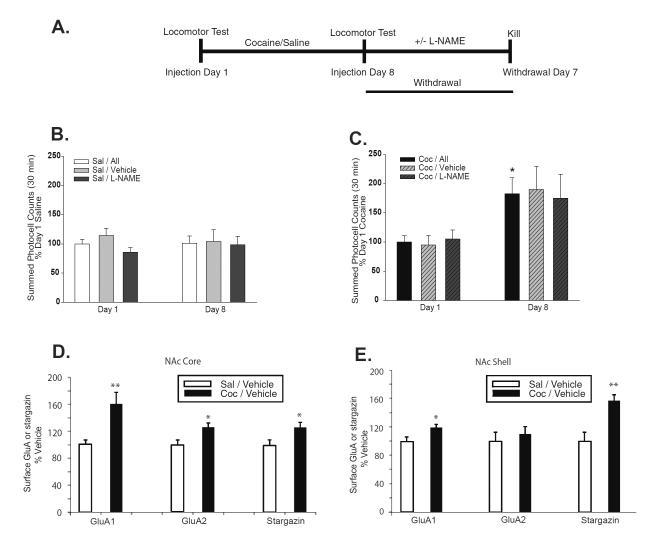
Next, we asked whether NO and nitrosylation of stargazin contribute to the upregulation of surface AMPARs that occurs in the NAc of cocaine-sensitized rats. Our prior studies have shown that AMPAR upregulation in the NAc is not yet present on WD1 but can be detected by WD7, indicating that it develops during the first week of withdrawal (Boudreau and Wolf, 2005; Boudreau et al., 2009). Therefore, we tested whether AMPAR upregulation would be prevented by NOS inhibition during this critical first week of withdrawal. Rats received 8 daily injections of saline or cocaine. Then, based on locomotor activity scores on day 8, saline and cocaine groups were each subdivided into two behaviorally equivalent groups, destined for treatment on WD1-6 with either the NOS inhibitor L-NAME or vehicle (Fig. 3A–C). L-NAME or vehicle injections (50 mg/kg) were given twice daily; this L-NAME regimen reduces NOS activity by >90% after 4 or more injections (Dwyer et al., 1991). Thus, four experimental groups were generated: saline/vehicle, saline/L-NAME, cocaine/Vehicle, cocaine/L-NAME (n=10 rats/group). For each group, NAc core and shell were harvested on WD7, approximately 3 h after the final L-NAME or vehicle injection, and biotinylated to measure cell surface levels of GluA1, GluA2, and stargazin.
Figure 3. AMPAR and stargazin surface expression increase in both core and shell subregions of the NAc during cocaine withdrawal, but stargazin increases more robustly in the shell.
(A) Time-line for Experiment 2. Rats received 8 days of saline or 15mg/kg cocaine injections in photobeam cages as described in Fig. 1 (n=20 rats/group). Data for all saline rats (Sal/All) showed no change in activity between Day 1 and Day 8 (B), but locomotor sensitization developed in the cocaine-treated rats (Coc/All) on Injection Day 8 compared to Injection Day 1 (t15=3.19, *P<0.01) (C). After collecting Day 8 activity data, saline rats (n=20) were divided into two equivalent subgroups, destined for treatment with vehicle (Sal/Veh, n=10) or L-NAME (Sal/L-NAME, n=10) during the first week of withdrawal (B). Cocaine rats were similarly divided into Coc/Veh (n=10) or Coc/L-NAME (n=10) subgroups (C). Vehicle or 50mg/kg L-NAME was injected twice daily on withdrawal days (WD) 1–6. NAc core and shell were harvested from rats shown in panels B and C on WD7 (~3 h after the last L-NAME or vehicle injection), biotinylated, and analyzed by immunoblotting. (D) Surface GluA1, GluA2 and stargazin levels increased in the NAc core of cocaine-sensitized rats (cocaine/vehicle) compared to controls (saline/vehicle) (GluA1 t18=3.29, **P<0.005; GluA2 t18=2.46, *P<0.05; stargazin t18=2.36, *P<0.05). (E) Surface GluA1 and surface stargazin levels increased in the NAc shell of cocaine-sensitized rats (cocaine/vehicle) compared to controls (saline/vehicle) (GluA1 t18=2.66, *P<0.05; stargazin t18=3.84, **P<0.005). Surface GluA2 did not increase significantly. Analysis of saline/L-NAME and cocaine/L-NAME treatment groups from this experiment is shown in Fig. 4.
To achieve accurate results despite the many manipulations involved in purifying biotinylated proteins (particularly when the starting material is a small dissection from an individual rat), we find that groups to be compared should be pulled down together and analyzed on the same gel. Therefore, we first pulled down and compared NAc core from saline/vehicle and cocaine/vehicle groups (Fig. 3D) and NAc shell from saline/vehicle and cocaine/vehicle groups (Fig. 3E). Our initial goal was to confirm AMPAR upregulation in cocaine/vehicle rats. As expected based on prior results (see Introduction), both shell and core regions from the cocaine/vehicle group showed a significant elevation of surface GluA1 (core: t18=3.29, **P<0.005; shell: t18=2.66, *P<0.05), although the elevation was greater in core (~60% increase) than shell (~20% increase). Surface GluA2 was significantly elevated in the core (26% increase; Fig. 3D; t18=2.46, *P<0.05) but there was only a trend in the shell (~10% increase; Fig. 3E). As discussed in Section 4.3, we speculate that less robust increases in surface GluA2 after sensitization, as well as less robust effects of L-NAME on GluA2 (Fig. 4), reflect the existence of unresponsive GluA2A3 receptors that dilute the effect of altering GluA1A2 receptor levels. Using the same samples, we showed that stargazin surface expression was elevated in both core and shell subregions of the cocaine/vehicle group compared to the saline/vehicle group (core: t18=3.84, *P<0.05; shell: t18=2.36, **P<0.005). However, the elevation of stargazin was more robust in the shell (~55% increase; Fig. 3E) than in the core (~25% increase; Fig. 3D). Overall, results in Fig. 3 indicate that surface expression of stargazin-associated AMPARs is increased most prominently in the shell subregion of the NAc from cocaine-sensitized rats.
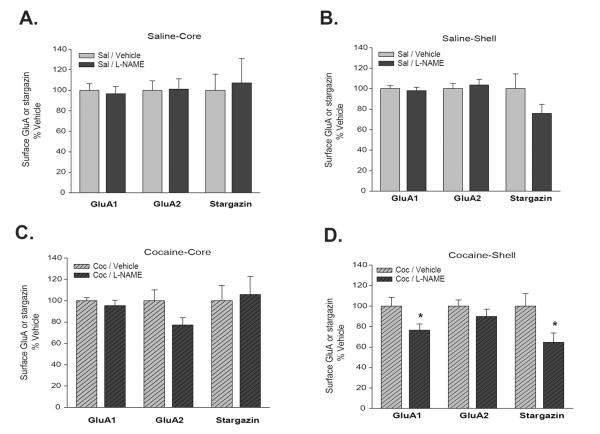
Figure 4. Inhibition of NOS activity during cocaine withdrawal opposes upregulation of AMPAR and stargazin surface expression in the NAc shell.
Data are from the same experimental groups described in Fig. 3, except that here we directly compare saline/vehicle to saline/L-NAME rats (A,B) and cocaine/vehicle to cocaine/L-NAME rats (C,D). In saline pretreated rats, L-NAME treatment during withdrawal had no effect in core (A) or shell (B). L-NAME treatment during withdrawal had no significant effects in the core (C) of sensitized rats. However, in the NAc shell of cocaine-sensitized rats (D), L-NAME treatment during withdrawal reduced surface GluA1 (t18=2.25, *P<0.05) and surface stargazin (t17=2.29, *P<0.05) as compared to cocaine-sensitized rats treated with vehicle during withdrawal (Student's t-tests comparing cocaine/vehicle versus cocaine/L-NAME groups).
Next, using new aliquots of tissue from Experiment 2, NAc core samples from saline/vehicle and saline/L-NAME groups were pulled down and immunoblotted in parallel (Fig. 4A). Similarly, NAc shell samples from the same two groups were pulled down and immunoblotted in parallel (Fig. 4B). All saline/L-NAME data were normalized to the saline/vehicle group. In these saline rats, we found no significant effect of L-NAME treatment on WD1-6 on GluA1 or stargazin surface expression in core or shell (Fig. 4A,B), although there was a trend towards a reduction in surface stargazin in the shell (Fig. 4B). Thus, NOS does not play a significant role in regulating AMPAR surface expression in the NAc of saline treated rats.
To determine if NOS plays a more significant role in cocaine-sensitized rats, we used additional aliquots to pull down and compare NAc core from cocaine/vehicle and cocaine/L-NAME groups (Fig. 4C), as well as NAc shell from cocaine/vehicle and cocaine/L-NAME groups (Fig. 4D). All cocaine/L-NAME data were normalized to the cocaine/vehicle group. The hypothesis to be tested was that NOS-induced nitrosylation of stargazin mediates the increases in AMPAR and stargazin surface expression observed during cocaine withdrawal. If this hypothesis is correct, we predicted that NOS inhibition during withdrawal should prevent or attenuate these increases, i.e., there should be lower AMPAR and stargazin surface expression in the cocaine/L-NAME group than the cocaine/vehicle group. In accordance with this prediction, surface expression of GluA1 and stargazin in the NAc shell were significantly reduced in cocaine/L-NAME rats compared to cocaine/vehicle rats (Fig. 4D; GluA1: t18=2.25, *P<0.05; Stargazin: t17=2.29, *P<0.05), while a trend towards a reduction was observed for GluA2 (Fig. 4D). These results indicate that NOS activity during withdrawal is required for the increase in AMPAR and stargazin surface expression that occurs in the NAc shell. In contrast to the shell, L-NAME treatment did not significantly reduce GluA1 or stargazin surface expression in the core, i.e., the cocaine/L-NAME and cocaine/vehicle groups did not differ significantly (Fig. 4C). Thus, even though AMPAR upregulation during withdrawal occurs in both core and shell subregions (Fig. 3D,E and prior studies reviewed in Wolf and Ferrario, 2010), L-NAME treatment selectively interfered with this effect in the shell subregion.
It is possible that the differential response to L-NAME reflects substantially higher levels of nNOS in shell compared to core (Fig. 2; see Discussion). However, if a NOS/stargazin interaction is not important in promoting AMPAR upregulation in the core, this raises the question of whether a different regulatory mechanism and/or TARP is important in the core. While a thorough examination of this question is beyond the scope of this study, we returned to NAc tissue pulled down to compare GluA1, GluA2 and stargazin in saline/vehicle and cocaine/vehicle groups (Fig. 3D,E). Using this tissue, we measured surface expression of another TARP, γ4, that we have previously shown is expressed in the NAc (Ferrario et al., 2011a,b). Interestingly, significant increases in surface γ4 were found in cocaine-sensitized rats relative to saline/vehicle controls in both core and shell subregions (Core, saline/vehicle 100 ± 9.74, cocaine 159.42 ± 6.32, t18=4.24, P<0.001; Shell: saline/vehicle 100 ± 14.72, cocaine/vehicle 150.74 ± 7.30, t18=3.09, P<0.01; Student's t-tests; data expressed as percent of saline/vehicle groups). These results suggest that γ4 may play an important role in AMPAR upregulation in the NAc of sensitized rats.
3.3. Experiment 3
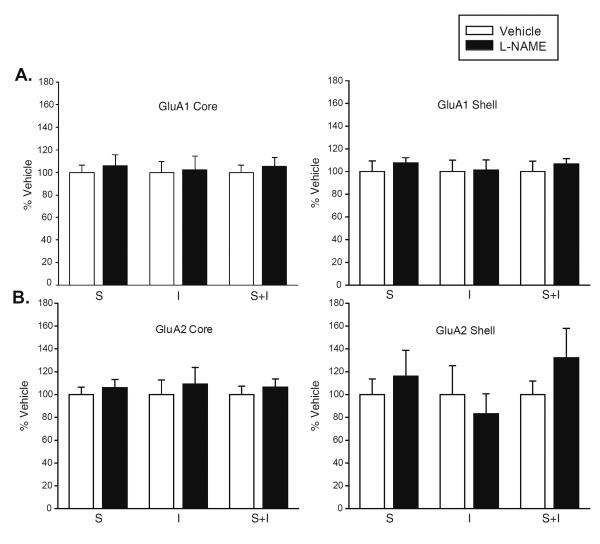
Here, we investigated whether acute reduction or elevation of NO signaling would alter AMPAR surface expression in the NAc of drug naïve rats. To reduce NO levels, rats were injected with the NOS inhibitor L-NAME (50 mg/kg, n=10) three times within a 24 h period (10 am, 6 pm, 2 am), a regimen expected to reduce NOS activity by 50–95% (Dwyer et al., 1991). Controls received vehicle (saline) on the same schedule. Rats were killed 7.5 h after the last injection. Using a BS3 protein crosslinking assay, we found no changes in surface, intracellular or total levels of GluA1 or GluA2 in NAc core or shell (Fig. 5). Conversely, we tested the effect of acutely elevating NO levels using the NO donor SNP (5mg/kg, i.p.). Using electrochemical methods, we determined that the maximal elevation of NO levels in the NAc shell occurred ~30 min after SNP injection (Fig. 6A). Therefore, additional rats were killed 30 min after injection of SNP or vehicle, and AMPAR subunit distribution was examined using BS3 protein crosslinking. No changes in surface, intracellular or total levels of GluA1 or GluA2 were found in NAc core or shell (Fig. 6B and C, respectively).
Figure 5. NOS activity is not required to maintain basal AMPAR surface expression in the NAc of drug-naïve rats.
Rats were injected with vehicle or the NOS inhibitor L-NAME (50mg/kg) three times within a 24 h period (n=10 rats/group), and killed 7.5 h after the last injection. Core and shell subregions of the NAc were rapidly dissected and surface-expressed proteins were selectively modified using BS3 (Boudreau et al., 2012). Surface (S), intracellular (I) and total (S+I) levels of GluA1 (A) or GluA2 (B) were measured using SDS-PAGE and immunoblotting. Student's t-tests failed to reveal significant differences between vehicle and L-NAME groups.
Figure 6. Acute administration of the NO donor, SNP, does not increase AMPAR surface expression in the NAc of drug-naïve rats.
NO oxidation current (i.e., NO efflux) was recorded prior to and immediately following systemic administration of vehicle or sodium nitroprusside (SNP; 5mg/kg, i.p.) using previously described methods (Park and West, 2009). (A) Systemic administration of SNP induced an increase in NAc extracellular NO levels (mean concentration = 36.40±10.22 nM, n=3) which typically peaked ~35 min post-injection (mean peak time = 34.98±4.2 min, n=3). Vehicle injection did not alter NO efflux and smaller effects were seen with 2.5mg/kg SNP (data not shown). (B–C) Based on the electrochemical data, additional drugnaïve rats were injected with vehicle (n=10) or 5mg/kg SNP (n=10), and killed 30 min later. Core and shell subregions of the NAc were rapidly dissected and surface-expressed proteins were selectively modified using BS3 (Boudreau et al., 2012). Surface (S), intracellular (I) and total (S+I) levels of GluA1 (B) or GluA2 (C) were measured using SDS-PAGE and immunoblotting. Student's t-tests failed to reveal significant differences between vehicle and SNP groups (t-tests).
4. Discussion
We tested the hypothesis that S-nitrosylation of the AMPAR auxiliary subunit stargazin contributes to the increased AMPAR surface expression that occurs in the NAc after withdrawal from a sensitizing regimen of cocaine (see Introduction). In Experiment 1, we showed that cocaine sensitization is associated with an increase in NOS-mediated S-nitrosylation of stargazin selectively in the NAc shell. In Experiment 2, we found that cell surface levels of stargazin and AMPAR subunits increase in both core and shell subregions of the NAc in cocaine-sensitized rats. While the increase in AMPAR surface expression was more robust in core, the increase in surface stargazin was more robust in shell, suggesting that stargazin may play a relatively more important role in AMPAR trafficking or function in the shell. Furthermore, inhibition of NOS during the first week of withdrawal, the time-period during which AMPAR upregulation develops (Boudreau and Wolf, 2005; Boudreau et al., 2009), decreases surface expression of both stargazin and GluA1 selectively in the NAc shell of cocaine-treated rats. These results indicate a role for stargazin nitrosylation in AMPAR upregulation, selectively in the NAc shell, during withdrawal from a sensitizing regimen of cocaine. In contrast to this effect of long-term NOS inhibition in cocaine-sensitized rats, we found in Experiment 3 that AMPAR surface expression in the NAc was not affected by acutely reducing or elevating NO signaling in drug-naïve rats. This suggests a requirement for more prolonged NO activation to accomplish the cocaine withdrawal-dependent increase in AMPAR surface expression, and/or a potential requirement for physiologically stimulated NO and its associated spatio-temporal specificity (Hess et al., 2005).
4.1. Mechanisms linking NO, S-nitrosylation, stargazin and AMPAR trafficking
In the NAc shell, we observed increased stargazin and AMPAR subunit surface expression after cocaine sensitization, and showed that this was accompanied by increased S-nitrosylation of stargazin and blocked by NOS inhibition. This is consistent with earlier results demonstrating a linkage between stargazin S-nitrosylation and increased surface expression of both stargazin and GluA1 (Selvakumar et al., 2009). Therefore, we propose that increased NMDAR transmission during cocaine withdrawal (Schumann and Yaka, 2009; Huang et al., 2009) activates NOS, which in turn leads to S-nitrosylation of stargazin and increased AMPAR surface expression. However, we acknowledge that there are other mechanisms by which NO may regulate AMPAR trafficking. First, nitrosylation of NSF enhances its binding to GluA2, thereby promoting surface expression of GluA2-containing AMPARs (Huang et al., 2005). However, this seems unlikely to explain our results, which appear to reflect a selective effect on GluA1-containing AMPARs (see Section 4.3). Second, activation of cGMP, a downstream effector of NO, increases AMPAR surface expression in cultured neurons (Serulle et al., 2007). However, our data implicate S-nitrosylation, which is independent of cGMP signaling, as the critical regulatory mechanism. Finally, while the present results point to S-nitrosylation of stargazin as mediating AMPAR upregulation in sensitized rats, S-nitrosylation of GluA1 itself also influences AMPAR plasticity. Thus, we showed recently in cultured neurons that GluA1 phosphorylation at S831, the site phosphorylated by protein kinase C and Ca2+/calmodulin-dependent protein kinase II (CaMKII), is enhanced by the NMDAR-dependent generation of NO and subsequent S-nitrosylation of GluA1 (Selvakumar et al., 2012). However, at least in cultured neurons treated with 40μM NMDA, this facilitates AMPAR endocytosis (Selvakumar et al., 2012) and is therefore unlikely to be directly related to the increased AMPAR surface expression observed in the NAc of cocaine-sensitized rats. Together, these findings underscore the complexity of mechanisms through which NO and S-nitrosylation can regulate AMPAR function and localization. Thus, we cannot rule out a contribution of other NOS-dependent events, beyond S-nitrosylation of stargazin, to AMPAR upregulation during withdrawal (Selvakumar et al., 2012).
Our previous data demonstrating a mechanistic link between stargazin S-nitrosylation and increased GluA1 surface expression (Selvakumar et al., 2009) were obtained in expression systems and in cerebellar granule cells; the latter express stargazin as their primary TARP (Jackson and Nicoll, 2009). It is expected that the relationship between stargazin and AMPAR trafficking has the potential to be more complex in cells that express more than one TARP. As discussed in the next section, this may help explain our results in the core subregion of the NAc. Furthermore, while our study has focused on TARPs, it should be noted that previous work has implicated ERK (Boudreau et al., 2007; Schumann and Yaka, 2009) as well as other protein kinases including CaMKII (Boudreau et al., 2009; Schierberl et al., 2011) in cocaine-induced AMPAR upregulation in the NAc.
4.2. Selective effect of S-nitrosylation in the NAc shell
It is surprising that regulation by NO of cell surface stargazin and GluA1 was restricted to the NAc shell of cocaine-sensitized rats, given that sensitization to cocaine is associated with increased AMPAR surface expression in both core and shell subregions (Wolf and Ferrario, 2010; Wolf, 2010) and increased stargazin surface expression in both subregions (present results). Selectivity of NO regulation for the shell might reflect the substantially higher levels of nNOS that we detect in the shell of saline- or cocaine-treated rats contrasted to the core (Fig. 2), fitting with very recent observations of greater NADPH diaphorase staining, reflecting nNOS activity, in the NAc shell than core (Hoque and West, 2011). The nNOS-expressing interneurons in the NAc shell differ from those in the core in several respects. For example, nNOS cells localized in the shell give rise to larger and more plentiful NOS-immunolabeled terminals (Hidaka and Totterdell, 2005), suggesting that when activated, shell interneurons produce more NO. Furthermore, although nNOS interneurons in both the NAc shell and core receive afferent glutamatergic and dopaminergic inputs from the hippocampal ventral subiculum and ventral tegmental area, respectively, shell neurons exhibit a larger proportion of asymmetric (largely glutamatergic) compared to symmetric (largely dopaminergic) synapses (Hidaka and Totterdell, 2001, French et al., 2005). Thus, nNOS interneurons in the shell are likely to be preferentially activated by glutamatergic input from the ventral subiculum compared to core interneurons. Shell interneurons are also considerably more responsive to dopamine and glutamate receptor manipulations compared to counterparts in the core (Hoque and West, 2011). Apart from differences in NO signaling, it is interesting that core and shell also differ markedly in the responsiveness of extracellular glutamate levels, measured using enzyme-based biosensors in freely moving rats, to cocaine as well as other behavioral manipulations; in all cases, shell was significantly more sensitive than core (Wakabayashi and Kiyatkin, 2012).
While higher levels of NOS in the shell are likely to contribute importantly to the core-shell differences observed in this study, regional differences in TARP function may also contribute. Specifically, we wondered if such differences could help explain our observation that AMPAR upregulation occurs in core of sensitized rats but is not opposed by L-NAME treatment. We were particularly interested in TARP γ-4. We previously showed that γ-4 and stargazin are expressed at similar levels in the NAc, and that levels of both proteins increase in the NAc after withdrawal from cocaine self-administration (Ferrario et al., 2011a,b). In the present study, we extended these findings by demonstrating significant increases in γ-4 surface expression in both shell and core of cocaine-sensitized rats. An important contribution of γ-4 in the core could explain why we observed greater AMPAR surface expression in the core than shell after sensitization (~65% increase versus ~20% increase), despite the fact that the increase in surface stargazin in the core was quite modest (~25% in core compared to ~60% in shell). However, interpretation of the γ-4 data is complicated for two reasons. First, γ-4 is expressed primarily in extrasynaptic membranes of the NAc whereas stargazin is expressed primarily in the PSD (Ferrario et al., 2011a,b). Second, it is unknown whether γ-4 is regulated by nitrosylation. Overall, these results suggest that further work is needed to understand the role of different TARPs in AMPAR upregulation in the NAc of sensitized rats.
4.3. Effects of cocaine and L-NAME on GluA1 versus GluA2
Some differences in results obtained for GluA1 versus GluA2 deserve comment. In Experiment 2, cocaine-sensitized rats (cocaine/vehicle group) showed a statistically significant increase in surface GluA1 in both core and shell compared to saline/vehicle controls. However, while a statistically significant increase in surface GluA2 was also observed in core, only a trend (~10% increase) was observed in the shell. Furthermore, NOS inhibition during the first 6 days of withdrawal from cocaine (cocaine/L-NAME group) significantly decreased GluA1 surface expression on WD7 compared to cocaine-sensitized animals receiving vehicle treatment during withdrawal (cocaine/vehicle), but produced only a very modest decrease in surface GluA2. These findings might seem surprising because it is well established that the AMPARs that upregulate after sensitization are GluA1A2 receptors (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007; Ghasemzadeh et al., 2009; Ferrario et al., 2010; Schierberl et al., 2011; McCutcheon et al., 2011). We speculate that the absence of significant changes in GluA2 in some experiments reflects the presence of a substantial pool of GluA2A3 receptors in the NAc (Reimers et al., 2011) which dilute the effect of changes in GluA1A2 receptor surface expression. Given that stargazin S-nitrosylation enhances AMPAR surface expression by increasing binding between stargazin and GluA1 (Selvakumar et al., 2009), it is to be expected that inhibiting S-nitrosylation would selectively interfere with maintenance of surface pools of GluA1-containing AMPARs such as the GluA1A2 receptors implicated in sensitization.
4.4. NO contributes to multiple aspects of cocaine's actions
The present study identifies a role for NO in a neuroadaptation (AMPAR upregulation in the NAc) that occurs during withdrawal, after sensitization has already developed. In contrast, previous studies have demonstrated a requirement for nNOS signaling in the development of sensitization to cocaine (Haracz et al., 1997; Itzhak, 1997; Itzhak et al., 1998a,b; Byrnes et al., 2000; Nasif et al., 2011). This latter effect of NO appears to involve the ventral tegmental area (VTA), as bilateral infusions of a nNOS inhibitor directly into the VTA prior to each cocaine injection completely blocked the development of sensitization in rats (Byrnes et al., 2000). In addition, several studies have examined the effect of NOS inhibition during cocaine self-administration and found that it decreases maintenance of cocaine seeking (Pulvirenti et al., 1996; Orsini et al., 2002; Collins and Kantak, 2002; Pudiak and Bozarth, 2002) and reinstatement (Orsini et al., 2002). Systemic pretreatment with nNOS inhibitors also blocks cocaine-induced conditioned place preference (Kim and Park, 1995; Itzhak et al., 1998; Itzhak, 2008). However, to our knowledge, none of these studies assessed the effect of NOS inhibition during withdrawal in the absence of a cocaine challenge. Our study is also the first to implicate NOS signaling in a sensitization-related adaptation in excitatory synaptic transmission in the NAc.
5. Conclusions
Our findings suggest that activation of NOS in the NAc shell of cocaine-sensitized rats leads to increased S-nitrosylation of stargazin, which in turn contributes to AMPAR upregulation. NOS activation may occur as a result of increased NMDAR transmission in the NAc during early cocaine withdrawal, a phenomenon that has been previously linked to cocaine-induced AMPAR upregulation (Schumann and Yaka, 2009; Huang et al., 2009). Given that sensitization and the associated AMPAR upregulation may be steps leading to increased drug craving (Wolf and Ferrario, 2010), our results suggest NOS as a potential target for anti-craving pharmacotherapies.
Highlights
Cocaine sensitized rats show increased AMPAR surface expression in the NAc
Sensitization is accompanied by NOS-mediated stargazin S-nitrosylation in NAc shell
Blocking NOS decreases the AMPAR upregulation normally occurring during withdrawal
Stargazin S-nitrosylation contributes to AMPAR upregulation in NAc shell
Acknowledgements
Supported by DA015835 and K05 DA029099 (MEW), NARSAD (MEW and ARW), and DA000266 (SHS).
Abbreviations
- AMPAR
AMPA receptor
- BS3
bis(sulfosuccinimidyl)suberate
- (CaMKII)
Ca2+/calmodulin-dependent protein kinase II
- L-NAME
L-NG-Nitroarginine Methyl Ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- NAc
nucleus accumbens
- SNP
sodium nitroprusside
- TARP
transmembrane AMPA receptor regulatory protein
- WD
withdrawal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no conflict of interest.
6. References
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J. Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Milovanovic M, Conrad KL, Nelson C, Ferrario CR, Wolf ME. A protein cross-linking assay for measuring cell surface expression of glutamate receptors subunits in the rodent brain after in vivo treatments. Curr. Protocols. Neurosci. April, Units. 2012;5(30):1–19. doi: 10.1002/0471142301.ns0530s59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Pantke MM, Onton JA, Hammer RP. Inhibition of nitric oxide synthase in the ventral tegmental area attenuates cocaine sensitization in rats. Prog. Neuro-Psychopharmacol. Biol. Psych. 2000;24:261–273. doi: 10.1016/s0278-5846(99)00094-9. [DOI] [PubMed] [Google Scholar]
- Collins SL, Kantak KM. Neuronal nitric oxide synthase inhibition decreases cocaine self-administration behavior in rats. Psychopharmacology. 2002;159(4):361–369. doi: 10.1007/s00213-001-0935-8. [DOI] [PubMed] [Google Scholar]
- Dwyer MA, Bredt DS, Snyder SH. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem. Biophys. Res. Commun. 1991;176:1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca+2-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011a;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neurosci. Lett. 2011b;490:180–184. doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Ritson GP, Hidaka S, Totterdell S. Nucleus accumbens nitric oxide immunoreactive interneurons receive nitric oxide and ventral subicular afferents in rats. Neuroscience. 2005;135:121–131. doi: 10.1016/j.neuroscience.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neurosci. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Haracz JL, MacDonall JS, Sircar R. Effects of nitric oxide synthase inhibitors on cocaine sensitization. Brain Res. 1997;746:183–189. doi: 10.1016/s0006-8993(96)01193-6. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell. Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Totterdell S. Ultrastructural features of the nitric oxide synthase-containing interneurons in the nucleus accumbens and their relationship with tyrosine hydroxylase-containing terminals. J. Comp. Neurol. 2001;431:139–154. doi: 10.1002/1096-9861(20010305)431:2<139::aid-cne1061>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hoque KE, West AR. Dopaminergic modultion of nitric oxide synthase activity in subregions of the rat nucleus accumbens. Synapse. 2011;66:220–231. doi: 10.1002/syn.21503. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, Schlüter OM, Zukin RS, Dong Y. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Man H-Y, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Modulation of cocaine- and methamphetamine-induced behavioral sensitization by inhibition of brain nitric oxide synthase. J. Pharmacol. Exp. Ther. 1997;282:521–527. [PubMed] [Google Scholar]
- Itzhak Y, Ali SF, Martin JL, Black MD, Huang PL. Resistance of neuronal nitric oxide synthase-deficient mice to cocaine-induced locomotor sensitization. Psychopharmacology. 1998a;140:378–386. doi: 10.1007/s002130050779. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Black MD, Huang PL. The role of nitric oxide synthase in cocaine-induced conditioned place preference. NeuroReport. 1998b;9(11):2485–8. doi: 10.1097/00001756-199808030-00011. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann. NY. Acad. Sci. 2008;1139:350–357. doi: 10.1196/annals.1432.051. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. Neuroscience: AMPA receptors get `pickled'. Nature. 2009;458:585–586. doi: 10.1038/458585a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Park WK. Nitric oxide mediation of cocaine-induced dopaminergic behaviors: ambulation-accelerating activity, reverse tolerance and conditioned place preference in mice. J. Pharmacol. Exp. Ther. 1995;275:551–557. [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Koh WCA, Shim YB, Shim I, Choe ES. Repeated cocaine administration increases nitric oxide efflux in the rat dorsal striatum. Psychopharmacology. 2010;208:245–256. doi: 10.1007/s00213-009-1724-z. [DOI] [PubMed] [Google Scholar]
- Lee DK, Ahn SM, Shim YB, Koh WCA, Shim I, Choe ES. Interactions of dopamine D1 and NMDA receptors are required for acute cocaine-evoked nitric oxide efflux in the dorsal striatum. Exp. Neurobiol. 2011;20(2):116–122. doi: 10.5607/en.2011.20.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after long withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain. Struct. Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Hu X-T, Ramirez OA, Perez MF. Inhibition of nitric oxide synthase prevents alterations in medial prefrontal cortex excitability induced by repeated cocaine administration. Psychopharmacol. 2011;218:323–330. doi: 10.1007/s00213-010-2105-3. [DOI] [PubMed] [Google Scholar]
- Orsini C, Izzo E, Koob GF, Pulvirenti L. Blockade of nitric oxide synthesis reduces responding for cocaine self-administration during extinction and reinstatement. Brain. Res. 2002;925:133–140. doi: 10.1016/s0006-8993(01)03267-x. [DOI] [PubMed] [Google Scholar]
- Park DJ, West AR. Regulation of striatal nitric oxide synthesis by local dopamine and glutamate interactions. J. Neurochem. 2009;111:1457–1465. doi: 10.1111/j.1471-4159.2009.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudiak CM, Bozarth MA. The effect of nitric oxide synthesis inhibition on intravenous cocaine self-administration. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(1):189–196. doi: 10.1016/s0278-5846(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Koob GF. Inhibition of nitric oxide synthesis reduces intravenous cocaine self-administration in the rat. Neuropharmacol. 1996;35:1811–1814. doi: 10.1016/s0028-3908(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammut S, West AR. Acute cocaine administration increases NO efflux in the rat prefrontal cortex via a neuronal NOS-dependent mechanism. Synapse. 2008;62(9):710–713. doi: 10.1002/syn.20537. [DOI] [PubMed] [Google Scholar]
- Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, Garraway SM, Hofmann F, Moosmang S, Striessnig J, Inturrisi CE, Rajadhyaksha AM. Cav1.2 L-type Ca+2 channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Ca(v)1.3 channels. J. Neurosci. 2011;31:13562–13575. doi: 10.1523/JNEUROSCI.2315-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J. Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar B, Huganir RL, Snyder SH. S-nitrosylation of stargazin regulates surface expression of AMPA-glutamate neurotransmitter receptors. Proc. Natl. Acad. Sci. U S A. 2009;106:16440–16445. doi: 10.1073/pnas.0908949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar B, Jenkins MA, Hussain NK, Huganir RL, Traynelis SF, Snyder SH. S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation, single-channel conductance, and endocytosis. Proc. Natl. Acad. Sci. 2012;110(3):1077–82. doi: 10.1073/pnas.1221295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxilliary subunits. Curr. Opin. Neurobiol. 2012;22:488–495. doi: 10.1016/j.conb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Kiyatkin EA. Rapid changes in extracellular glutamate induced by natural arousing stimuli and intravenous cocaine in the nucleus accumbens shell and core. J. Neurophysiol. 2012;108:285–299. doi: 10.1152/jn.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda triangle of cocaine-induced neuroadaptations. Trends. Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci. Biobehav. Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]