Abstract
Background
Determining the cause for pulmonary hypertension (PH) is difficult in many patients. Pulmonary arterial hypertension (PAH) is differentiated from pulmonary venous hypertension (PVH) by a wedge pressure (PWP) >15 mmHg in PVH. Patients undergoing RHC for evaluation of PH may be dehydrated and have reduced intravascular volume, potentially leading to a falsely low measurement of PWP and an erroneous diagnosis of PAH. We hypothesized that a fluid challenge during RHC would identify occult pulmonary venous hypertension (OPVH).
Methods and Results
We reviewed the results of patients undergoing fluid challenge in our PH database from 2004-2011. Baseline hemodynamics were obtained and repeated following infusion of 0.5 liters of normal saline over 5-10 minutes. Patients were categorized as OPVH if PWP increased to >15 mm Hg after fluid challenge. Baseline hemodynamics in 207 patients met criteria for PAH. Following fluid challenge, 46 patients (22.2%) developed a PWP >15 mm Hg and were re-classified as OPVH. OPVH patients had a greater increase in PWP compared to PAH patients, p<0.001, and their demographics and comorbid illnesses were similar to PVH patients. There were no adverse events related to fluid challenge.
Conclusions
Fluid challenge at the time of RHC is easily performed, safe, and identifies a large group of patients diagnosed initially with PAH, but for whom OPVH contributes to PH. These results have implications for therapeutic trials in PAH and support the routine use of fluid challenge during RHC in patients with risk factors for PVH.
Keywords: hemodynamics, pulmonary hypertension, pulmonary heart disease, pulmonary arterial hypertension, fluid challenge
Pulmonary hypertension (PH) is defined by a mean pulmonary artery pressure (mPAP) ≥25 mm Hg1. Pulmonary arterial hypertension (PAH), group 1 in the Dana Point Classification, requires a pulmonary wedge pressure (PWP) ≤15 mm Hg2,3. Group 2, pulmonary venous hypertension (PVH), is defined by a PWP >15 mm Hg in addition to an elevated mPAP, and results from elevation in post-capillary pressure due to left ventricular systolic or diastolic dysfunction or left-heart valvular disease2.
Differentiating PAH from PVH is important to determine appropriate treatment. There are currently no medications approved specifically for the treatment PVH. However, treatment with prostaglandins or endothelin receptor antagonists is not only ineffective but can potentially worsen patients with PVH4-7. Further, PAH-approved medications are extremely costly and impose an unnecessary burden on the healthcare system when prescribed for inappropriate indications. Therefore, accurate measurement of left heart filling pressure at the time of right heart catheterization, either directly by measuring left ventricular end-diastolic pressure (LVEDP), or more commonly, indirectly by measuring PWP, is necessary to differentiate PAH from PVH.
Intravascular volume depletion may lead to underestimation of left heart filling pressure. Nearly all patients undergoing right heart catheterization (RHC) are in a fasting state for 12 or more hours. Additionally, many patients have undergone marked diuresis prior to invasive hemodynamic evaluation. In 1970, Bush and colleagues described rapid infusion of one liter of normal saline (NS) to uncover latent pericardial constriction8. In 2004, we began to administer a rapid fluid challenge in patients undergoing RHC as part of the evaluation of PH. Recent studies suggest that fluid challenge is a useful and safe maneuver to uncover occult PVH (OPVH) as a contributing cause for PH. Two case series, one in patients with heart failure with preserved ejection fraction (HFpEF) and one in patients with scleroderma and PH, reported an increase in PWP to >15 mm Hg following rapid infusion of ~0.5 L NS9,10. We hypothesized that fluid challenge would identify a significant group of PAH patients for whom OPVH contributes to PH.
Methods
This study was approved by the Institutional Review Board at Vanderbilt University Medical Center, IRB#130268. We reviewed all patients enrolled in our database from June, 2004-December, 2011 that underwent RHC with fluid challenge for known or suspected PH. All patients were evaluated using published guidelines to determine the presence, severity and etiology of PH11. PH was defined as a mean pulmonary artery pressure (mPAP) ≥ 25mmHg1. The diagnosis of PAH required a PWP ≤15 mmHg, while patients with a PWP >15mmHg were classified as having PVH3. Patients whose baseline hemodynamics met criteria for PAH but developed a PWP >15 mm Hg following fluid challenge were classified as having OPVH. Patients were diagnosed with chronic thromboembolic PH if imaging studies were consistent with this diagnosis3. Patients with chronic thromboembolic PH were included in the analysis of patients initially diagnosed with PAH based on previous studies showing pathological changes identical to those found in PAH and response to PAH-approved therapy12,13. Patients with PH and restrictive (total lung capacity<60% predicted) or obstructive (FEV1/FVC < 70% with and FEV1 <60%) defects or a diffusing capacity <60% and more than mild lung disease on chest computed tomography were diagnosed with PH associated with parenchymal lung disease3. In this study, PH associated with parenchymal lung disease or hypoxia (group 3 in the Dana Point Classification) or with unclear or multifactorial mechanisms (group 5) were classified as “other”. Patients with a reported left ventricular ejection fraction <50% or in whom a reliable PWP tracing was not available were excluded from this study.
RHCs were performed by one of three cardiologists with extensive experience with this procedure in patients with PH. Fluid challenge has become part of standard practice at our center over the past 5 years in patients with a right atrial pressure (RAP) of ≤15 mm Hg. Administration of a fluid challenge in patients with a RAP or PWP >15 mm Hg and multiple risk factors for PVH was at the discretion of the cardiologist performing the RHC. Baseline hemodynamics were obtained and an intravenous fluid bolus of 0.5 L normal NS was given over 5-10 minutes14, and hemodynamic measurements were then repeated. The results of fluid challenge in 16 patients in this study have previously been reported14. All patients with a mPAP >25 mm Hg at baseline and a PWP ≤15 mmHg received inhaled nitric oxide at 40 ppm for 10 minutes, prior to fluid challenge, to assess acute vasodilator response. While mPAP and PWP were obtained in all patients after fluid challenge, cardiac output (CO) and RAP were not measured in all patients.
CO was measured using either thermodilution method or Fick calculation, the latter using estimated oxygen uptake based on age and heart rate15. Mean PAP was calculated using the formula: (systolic PAP-diastolic PAP)/3 + diastolic PAP. Pulmonary vascular resistance was calculated using the formula: (mPAP-PWP)/CO. Transpulmonary gradient (TPG) was calculated as mPAP-PWP. Diastolic PAP to PWP gradient (DPG) was calculated as dPAP-PWP. LVEDP was measured in a minority of patients undergoing left heart catheterization for additional reasons. Systolic and diastolic blood pressure and peripheral saturation were obtained non-invasively in the majority of patients. All hemodynamic tracings were reviewed by one of two of the authors (IMR or ARH) with extensive experience in interpretation of hemodynamic tracings. Values are reported at end-expiration and represent the mean of ≥3 heart beats. Echocardiographic data were obtained within one year of RHC, either at our institution or by referring physicians, and the results used are those provided in the official report of the echocardiogram.
Statistics
Results are reported as mean±SD unless otherwise noted. Between-group differences were compared by Kruskal Wallis test or a χ2 test. We were interested in understanding differences between PAH and OPVH groups which were further compared with the Mann-Whitney U test and logistic regression models. A logistic regression model was developed to predict a change in diagnosis from PAH to OPVH after fluid challenge using the following clinical variables: age, hypertension, body mass index (BMI), and left ventricular hypertrophy. These variables were chosen based on previous finding of a strong association with PVH14. Statistical analyses were performed with using SPSS for Windows, version 20.0; SPSS; Chicago, IL. The α level was set at 0.05 for all analyses, 95% confidence intervals were calculated, and all comparisons were two tailed.
Results
Demographics and associated conditions
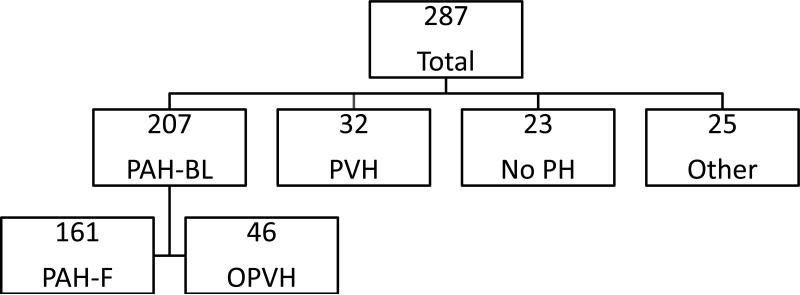
RHC with fluid challenge was performed in 292 patients during the study period; five patients were excluded from the study because an accurate, end-expiratory PWP tracing could not be determined, leaving 287 patients for analysis. After baseline hemodynamics were obtained, 207 patients were classified as having PAH (PAH-BL), 32 with PVH, 23 with no PH, and 25 with other diagnoses including two patients with an elevated PWP but no PH (Figure 1). Demographics, relevant medications and associated conditions are presented in Table 1. The majority of patients in all groups were female and functional class 3. Thirty-two patients reported a history of appetite suppressant use which included use of fenfluramine or dexfenfluramine in 23 patients.
Figure 1. Patient diagnoses.
Two-hundred and seven patients were diagnosed with pulmonary arterial hypertension (PAH) after baseline hemodynamics were obtained. Following fluid challenge, the pulmonary wedge pressure in 46 patients increased to >15 mm Hg, and they were reclassified as occult pulmonary venous hypertension (OPVH). PAH-BL=pulmonary arterial hypertension baseline; PAH-F= pulmonary arterial hypertension final; PVH=pulmonary venous hypertension; No PH=no pulmonary hypertension.
Table 1.
Baseline Demographics, Co-morbid Illnesses and Echocardiographic Findings
| PAH-F N=161 | OPVH N=46 | PVH N=32 | No PH N=23 | P value | |
|---|---|---|---|---|---|
| Gender (% female) | 82.0 | 73.9 | 71.9 | 95.7 | 0.094 |
| Age (years) | 51.6±14.5 | 57.7±12.3* | 59.3±12.2 | 54.0±16.0 | 0.009 |
| Functional Class 1/2, 3/4 (%) | 36.7, 63.3 | 37.0, 63 | 21.9, 78.1 | 56.5, 43.5 | 0.010 |
| Body mass index (kg/m2) | 27.9±6.8 | 30.9±6.9* | 32.9±7.6 | 30.1±7.9 | 0.001 |
| Hypertension (%) | 32.9 | 63.0† | 62.5 | 39.1 | <0.001 |
| Diabetes (%) | 14.3 | 28.3* | 28.1 | 17.4 | 0.064 |
| Hyperlipidemia (%) | 14.9 | 21.7 | 40.6 | 30.4 | 0.009 |
| Coronary artery disease (%) | 11.8 | 8.7 | 15.6 | 13.0 | 0.824 |
| Obstructive sleep apnea (%) | 14.3 | 23.9 | 25.0 | 26.1 | 0.274 |
| Left ventricular hypertrophy (%) | 15.5 | 28.9* | 13.5 | 21.7 | 0.177 |
| Left atrial enlargement (%) | 23.6 | 39.1* | 56.2‡ | 26.1 | 0.005 |
| PAH-specific medication (%) | 53.4 | 41.3 | 18.8 | 13.0 | <0.001 |
| Calcium channel blockers (%) | 23.6 | 30.4 | 34.4 | 8.7 | 0.128 |
| Diuretics (%) | 60.9 | 69.6 | 71.9 | 47.8 | 0.214 |
| Risk factors for pulmonary hypertension | |||||
| Appetite suppressant use (%) | 6.8 | 19.6* | 21.9 | 26.1 | 0.004 |
| Connective tissue disease (%) | 34.8 | 15.2* | 3.1 | 17.4 | <0.001 |
| Congenital heart disease (%) | 6.2 | 4.3 | 6.3 | 4.3 | 0.954 |
| Portal Hypertension (%) | 9.3 | 10.9 | 6.3 | 0 | 0.416 |
| HIV infection (%) | 3.1 | 0 | 0 | 4.3 | 0.433 |
| CTEPH (%) | 6.8 | 6.8 | 6.5 | 6.3 | 0.646 |
P<0.05
P<0.001, PAH-F vs OPVH.
P<0.05, PVH vs OPVH
CTEPH=chronic thromboembolic pulmonary hypertension; HIV=human immunodeficiency virus; No PH= no pulmonary hypertension; OPVH=occult pulmonary venous hypertension; PAH=pulmonary arterial hypertension; PAH-F=pulmonary arterial hypertension final; PVH=pulmonary venous hypertension. Continuous variables are presented as mean±SD
After fluid challenge, 46 patients (22.2%) initially classified with PAH developed a PWP >15 mmHg and were re-classified as OPVH. The PWP remained ≤15 mmHg in the remaining 161 patients initially classified with PAH, and this group constitutes the PAH final (PAH-F) group. The characteristics and hemodynamics of OPVH patients are similar to those initially diagnosed with PVH and different from PAH-F patients (Tables 1 and 2). There was no difference between patients with OPVH and PAH-F in the proportion treated with PAH-approved medications, diuretics or calcium channel blockers (Table 1). Including five patients with no PH at baseline who developed PVH following fluid challenge, the number of patients ultimately classified as having PVH contributing to their PH (PVH plus OPVH) increased to 83 or 28.9% of the entire cohort.
Table 2.
Baseline and Post-Fluid Hemodynamics in the PAH-F and OPVH Patients
| Pre-fluid | Post-fluid | |||
|---|---|---|---|---|
| PAH-F N=161 | OPVH N=46 | PAH-F N=161 | OPVH N=46 | |
| RAP1 | 7±5 | 9±5* | 10±5§ | 12±7*† |
| sPAP | 80±20 | 70±15* | 81±19 | 74±15*# |
| dPAP | 33±10 | 28±9* | 34±9 | 32±8# |
| mPAP | 50±13 | 42±10† | 49±12 | 46±8# |
| PWP | 9±3 | 12±2† | 11±4§ | 19±3†# |
| CO1 | 4.5±1.5 | 5.3±1.5* | 4.9±1.6§ | 5.6±1.6* |
| CI | 2.5±0.8 | 2.8±0.7* | 2.7±0.9§ | 2.9±0.7 |
| PVR | 10.1±5.4 | 6.0±2.6† | 8.8±4.3§ | 5.5±2.7†∥ |
| TPG | 40±13 | 30±10† | 39±12‡ | 27±9†∥ |
| SvO22 | 66±9 | 67±7 | 68±8‡ | 68±10 |
| HR | 78±14 | 76±16 | 77±14‡ | 74±13 |
| sBP | 125±20 | 133±21† | 125±18 | 132±20 |
| dBP | 80±13 | 80±12 | 79±13 | 79±15 |
| Sat | 96±4 | 95±3 | 96±3‡ | 95±4 |
Measured in only 112 PAH-F and 23 OPVH patients Post-fluid
Measured in only 125 PAH-F and 30 OPVH patients Post-fluid
p<0.05
p<0.001, PAH-F vs OPVH
p<0.05
p<0.001, PAH-F, Pre vs Post-fluid
p<0.05
p<0.001, OPVH, Pre vs Post-fluid.
CO=cardiac output; CI=cardiac index; dBP=diastolic blood pressures; dPAP=diastolic pulmonary artery pressure; DPG=diastolic pulmonary artery pressure to pulmonary wedge pressure gradient; HR=heart rate; mPAP=mean pulmonary artery pressure; OPVH=occult pulmonary venous hypertension; PAH-F=pulmonary arterial hypertension final; PVR=pulmonary vascular resistance; PWP=pulmonary wedge pressure; Sat=peripheral saturation; RAP=right atrial pressure; sBP=systolic blood pressure; sPAP=systolic pulmonary artery pressure; Sv02=mixed venous saturation; TPG=transpulmonary gradient. Continuous variables are presented as mean±SD
In the 161 patients whose PWP remain ≤15 mm Hg after fluid bolus, 60 patients (37.3) were diagnosed with idiopathic or heritable PAH, 55 (34.2) had connective tissue disease, 15 had (9.3%) portal hypertension, and 10 had congenital heart disease (6.2%) which included 7 patients with an atrial septal defect (3 repaired), 2 with a repaired ventricular septal defect and one with a repaired tetralogy of Fallot (Table 1). In the OPVH group, 18 patients (39.1%) had no identifiable risk factor for PAH, 7 (15.2%) had connective tissue disease, 5 (10.9%) had portal hypertension, 2 (4.3%) had congenital heart disease which included one patient with a repaired ventricular septal defect and one with a repaired patent ductus arteriosis, and one had a family history of PAH (Table 1). Fourteen patients had chronic thromboembolic PH, 3 of which were additionally diagnosed with OPVH following fluid challenge. Seventeen patients were believed to have primarily parenchymal lung disease and/or hypoxia as the predominant cause of PH. All had significant parenchymal abnormalities on imaging with an average DLCO of 45±14% of predicted. Seven of these patients exhibited an increase in PWP to >15 mm Hg following fluid challenge. Echocardiographic findings included mild left atrial enlargement in 5/7, mild LVH in 3 and moderate LVH in one. Five had hypertension.
Hemodynamics
Prior to fluid administration, the 207 PAH-BL patients had a lower RAP, CO and systolic blood pressure (p<0.05 for all 3), and a higher PVR (p<0.001), compared to patients with PVH (Table 3). CI was similar between the 2 groups indicating that higher weight in PVH patients accounted for much of the increased CO. There was no significant difference in systolic PAP (sPAP), diastolic PAP (dPAP) or mPAP between the two groups. RAP, mPAP, CO, CI, PWP, PVR and systolic BP were significantly different between the 161 PAH-F patients and the 46 OPVH patients prior to fluid challenge despite the fact that all patients had a PWP ≤15 mmHg (Table 2). Following fluid bolus, the PWP pressure of both groups increased significantly. However, the average increase in the OPVH group was greater than in those with PAH-F, 6±3 mmHg vs 2±4 mmHg, p<0.001. RAP, CO, CI, and PVR increased significantly in both groups, although RAP and CO were measured in only 30/46 patients with OPVH and 125/163 patients with PAH-F (Table 2). PAP increased significantly only in the OPVH patients. There was no effect of fluid administration on systemic BP in either group. The TPG was significantly greater in the PAH-F patients compared to the OPVH patients at baseline, 40±13 vs 29±10, and remained that way post-fluid, 39±12vs27±9, respectively, with a similar change in both groups, p<0.001 for both comparisons. Left ventricular end-diastolic pressure was measured in addition to PWP in 18 patients at baseline and 24 patients after fluid bolus. There was good correlation between the 2 values: average LVEDP was 14±6 mmHg and PWP 12±6 mmHg, r= 0.753, p<0.001. PWP was less than, greater than and equal to left ventricular end-diastolic pressure in 24, 14 and 4 sets of measurements, respectively.
Table 3.
Baseline Hemodynamics
| PAH-BL n=207 | PVH N=32 | No PH N=23 | P value | |
|---|---|---|---|---|
| RAP | 8±5 | 12±5* | 4±3 | <0.001 |
| sPAP | 78±19 | 73±21 | 27±8 | <0.001 |
| dPAP | 32±10 | 32±10 | 11±3 | <0.001 |
| mPAP | 47±13 | 46±13 | 16±5 | <0.001 |
| PWP | 10±4 | 19±3 | 8±3 | <0.001 |
| CO | 4.7±1.5 | 5.7±1.9* | 6.0±1.7 | <0.001 |
| CI | 2.5±0.8 | 2.8±1.0 | 3.5±1.1 | <0.001 |
| PVR | 9.2±5.2 | 5.8±5.2 | 1.4±0.9 | <0.001 |
| TPG | 38±13 | 26±13 | 8±4 | <0.001 |
| SvO2 | 66±8 | 68±10 | 74±8 | <0.001 |
| HR | 78±15 | 75±13 | 74±14 | 0.247 |
| sBP | 127±20 | 135±21* | 138±18 | 0.002 |
| dBP | 80±13 | 79±13 | 79±11 | 0.985 |
| Sat | 95±4 | 94±6 | 98±2 | 0.007 |
p<0.05
†p<0.001, PAH-Baseline vs PVH
CO=cardiac output, CI=cardiac index, dBP=diastolic blood pressures, dPAP=diastolic pulmonary artery pressure; DPG=diastolic pulmonary artery pressure to pulmonary wedge pressure gradient; HR=heart rate; mPAP=mean pulmonary artery pressure; No PH=no pulmonary hypertension; PAH-BL=pulmonary arterial hypertension baseline; PVH= pulmonary venous hypertension; PVR=pulmonary vascular resistance; PWP= pulmonary wedge pressure; Sat=peripheral saturation; RAP=right atrial pressure; sBP=systolic blood pressure; sPAP=systolic pulmonary artery pressure; Sv02=mixed venous saturation; TPG=transpulmonary gradient. Continuous variables are presented as mean±SD. P value reflects comparison by ANOVA across all groups.
Safety
Fluid challenge was tolerated without any side effects or clinical deterioration in all patients, including patients with a RAP as high as 29 mmHg. Although the RAP increased significantly in the entire cohort from 8±5 to 10±5 mm Hg (p<0.001), in the 24 patients with a pre-fluid RAP ≥15 mm Hg, 17 had a repeat RAP obtained, and there was no change in RAP after fluid administration, 19±4 vs 19±6, p=0.732.
Predictors of PVH and OPVH
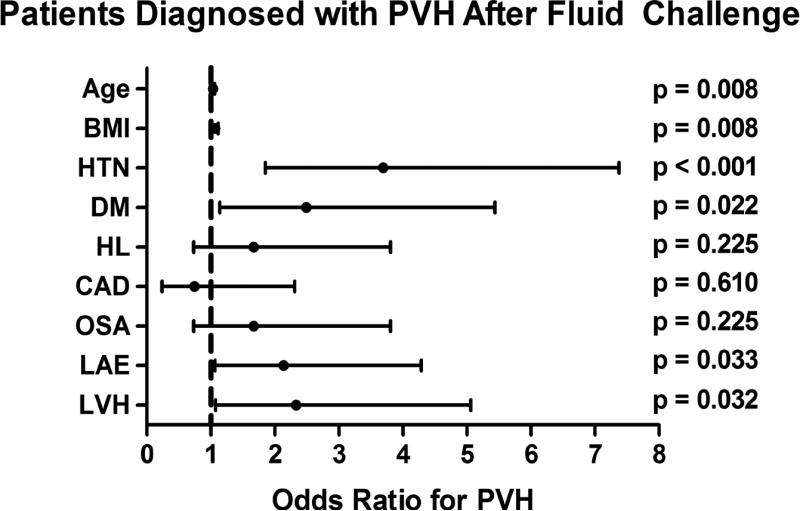
We analyzed demographic, hemodynamic and echocardiographic factors that could predict a diagnosis of OPVH. Univariate analysis of the entire cohort identified that factors associated with the metabolic syndrome (hypertension, hyperlipidemia, and obesity), older age, and left atrial enlargement on echocardiogram were associated with a diagnosis of PVH. Confining the analysis to the patients with OPVH, hypertension, body mass index (BMI), left atrial enlargement and older age remain as predictive factors. Additionally, the presence of left ventricular hypertrophy on echocardiogram is predictive of OPVH (Figure 2). However, the adjusted odds ratio demonstrates that age, hypertension and BMI are the only independent risk factors for OPVH (Table 4).
Figure 2. Univariate predictors for the development occult pulmonary venous hypertension (OPVH) following fluid challenge in patients initially diagnosed with pulmonary arterial hypertension.
Results are expressed as odds ratio with 95% confidence intervals. BMI=body mass index, CAD=coronary artery disease, DM=diabetes mellitus, HL=hyperlipidemia, HTN=hypertension, LAE=left atrial enlargement on echocardiogram, LVH=left ventricular hypertrophy on echocardiogram. OSA=obstructive sleep apnea.
Table 4.
Multivariate Predictors of change from PAH to OPVH
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.030 | 1.001-1.060 | 0.046 |
| HTN | 2.337 | 1.106-4.938 | 0.026 |
| BMI | 1.057 | 1.005-1.113 | 0.032 |
| LVH | 1.910 | 0.830-4.395 | 0.113 |
BMI=body mass index; HTN=hypertension; LVH=left ventricular Hypertrophy; OR=odds ratio; OPHV=occult pulmonary venous Hypertension; PAH=pulmonary arterial hypertension.
Discussion
Our results demonstrate that over 20% of patients initially meeting the hemodynamic criteria for PAH develop a PWP >15 mm Hg when given a rapid infusion of 0.5L NS. Although PWP pressure was ≤15 mm Hg in the 46 OPVH patients prior to fluid challenge, it was significantly higher than in the 161 PAH-F patients, suggesting that these are two distinct hemodynamic groups. In support of a different phenotype between the PAH-F and OPVH groups, although PWP increased in both following fluid challenge, the increase was significantly greater in the OPVH group. Conversely, OPVH patients have a hemodynamic profile similar to that seen in PVH patients. They also have clinical characteristics similar to those of PVH patients including older age, higher BMI and a greater prevalence of hypertension and diabetes when compared to PAH-F patients. Patients with these characteristics or LVH on echocardiogram, despite a normal PWP, should raise the possibility of PVH as a contributing factor to PH, and a fluid challenge can help to diagnose this.
Our findings confirm those recently reported in two smaller studies of rapid fluid loading during RHC. Fujimoto et al reported an increase in PWP from 14±4 mmHg to 20±4 mmHg in 11 HFpEF patients after an average saline challenge of just over half a liter9. Fox et al studied fluid challenge in scleroderma patients, and found that 6/29 patients with PH and a PWP <15 mmHg developed an elevated PWP following rapid infusion of 0.5 L of NS10. Our results extend their findings to a larger and broader group of patients with PH. It may be that a fluid challenge is not necessary to elicit this change. A previous study of HFpEF patients found that simply elevating the legs can help identify those patients who exhibit an increase in PWP with fluid challenge16. While this was not performed in our cohort, this should be considered in futures studies of patients with PH.
In addition to studying fluid challenge in HFpEF patients, Fujimoto and colleagues studied 60 subjects without cardiopulmonary disease, and reported an increase in PWP from 10±2 mmHg to 20±3 mm after rapid infusion of~ 2 L of NS9. While their results indicate that PWP can increase to >15 mm Hg in normal subjects, it is difficult to extrapolate this finding to patients with PH. Further, none of the normal subjects appear to have had an increase in PWP >15 mm Hg after just 0.5 L of NS, and the slope of the increase in PWP with fluid challenge was significantly greater in those with HFpEF.
The lower increase in PWP with fluid challenge in our PAH patients is consistent with disease in the resistance pulmonary arteries which would limit the effects of fluid loading on the post-capillary circulation. RAP was similar in PAH-F and OPVH patients at baseline, indicating that it is not a sensitive marker of left heart filling pressure. This is supported by the finding of a similar increase in RAP in both groups with fluid challenge despite the significantly greater increase in PWP in the OPVH patients. As with the findings reported in HFpEF patients9, the filling pressure in a less compliant LV increases more rapidly, and to a greater extent, when subjected to increased flow from a rapid fluid challenge. Thus, greater increases in PWP may be used to differentiate the two groups. It is possible that diuresis or mild dehydration reduced the PWP to normal levels in some OPVH patients, and diastolic dysfunction was revealed only after volume infusion. Prospective hemodynamic studies of fluid loading and outcomes are needed to determine if there is a cut-point for increase in PWP which differentiates PAH from PVH.
There is a substantial group of patients with PVH or OPVH who have considerable elevations of PAP along with large TPGs and DPGs. In our study, which excluded patients with more than mild LV systolic dysfunction, 84.3% of patients categorized as having PVH, either pre- or post-fluid challenge, had a TPG≥ 15 mm Hg, ranging from 15-51 mm Hg. These patients have features of both PAH and PVH, and have previously been described as having PH “out of proportion” to the degree of PWP elevation2, but may be more aptly described as having “combined disease”. There is no universal definition of combined disease17, although previous studies have reported a TPG< 12 mm Hg or <15 mm Hg as indicative of predominantly passive PH resulting from elevated post-capillary pressure18,19. It is clear from this study and other recent publications that combined disease is common in patients with PH14,18,20.
In support of a combined etiology of PH in the majority of our OPVH patients, nearly 40% of them were treated with PAH-approved therapies. Several of them were treated with phosphodiesterase type 5 inhibitors based on studies showing some benefit, and no deterioration, in patients with elevated post-capillary pressure due to either systolic or diastolic LV dysfunction21,22. This may be the safest approach in the treatment of patients with combined disease and only mildly elevated PVR or elevated TPR. A small group of patients was treated with other PAH-approved therapy as their PH was believed to be markedly out of proportion to the elevation in PWP. While many of these patients had several risk factors for PVH, they also had risk factors for PAH, further highlighting the weakness of the current classification of PH and the need to study PAH therapies specifically in patients with combined disease.
The results of the current study have implications not only for the classification of PH but for clinical trials in PAH. Based on our findings, over one-fifth of patients enrolled in PAH clinical trials may have a component of PVH. Whether an increase in PWP ≥15 mm Hg after fluid challenge should be a criterion for exclusion from PAH clinical trials requires validation of our results, but should be considered in patients with multiple risk factors for PVH, prior to enrollment in studies evaluating new PAH treatments.
There are several limitations to this study. This is a single center study but a large number of patients with multiple etiologies for PAH argues for general applicability of the results. LVEDP was measured in only a minority of patients and one study has suggested that there is not infrequently a discrepancy between LVEDP and PWP in the same patient23. However, we found a good correlation between the 2 measurements in our cohort and excluded patients with PWP tracings that were not interpretable. PWP is routinely used at our, and most centers, and endorsed in guidelines for evaluation of PH as a measure of left heart filling pressure, making our results applicable to standard practice (3). It should also be noted that an accurate PWP, or even LVEDP, cannot be obtained in a small number of patients despite multiple attempts due to body habitus and/or large pleural pressure swings. Echocardiogram results used in this study were not reviewed by a single cardiologist, and we included studies not done at our institution. However, this supports a more general applicability of our results.
In conclusion, administration of a rapid infusion of 0.5 L NS during RHC was associated with an increase in PWP in 22% of patients in our cohort. Fluid challenge is an easy and safe maneuver that can be performed in any catheterization lab without specialized equipment. Our results support the use of a fluid challenge in patients undergoing diagnostic RHC with risk factors for PVH.
Acknowledgments
Sources of Funding
This work was in part supported by NIH grants UL1 RR024975 (MEP), T32 HL087738 (MEP), K08 HL093363 (ARH), and P01 HL108800 (IMR, ARH, JHN).
Footnotes
Disclosures
None.
References
- 1.Hatano S, Strasser T, editors. Primary Pulmonary Hypertension: Report on a WHO Meeting. World Health Organization; Geneva: 1975. pp. 7–45. Figure Legends. [Google Scholar]
- 2.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G, ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 4.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FE, Jr, Wheeler W, Soler-Soler J, Swedberg K. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 5.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Lüscher TF, EARTH investigators Long-term effects of darusentan on left ventricular remodeling and clinical outcomes in endothelin receptor antagonist trial in heart failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaluski E, Cotter G, Leitman M, Milo-Cotter O, Krakover R, Kobrin I, Moriconi T, Rainisio M, Caspi A, Reizin L, Zimlichman R, Vered Z. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension--a multi-center randomized study. Cardiology. 2008;109:273–280. doi: 10.1159/000107791. [DOI] [PubMed] [Google Scholar]
- 7.Teerlink JR, McMurray JJ, Bourge RC, Cleland JG, Cotter G, Jondeau G, Krum H, Metra M, O'Connor CM, Parker JD, Torre-Amione G, Van Veldhuisen DJ, Frey A, Rainisio M, Kobrin I, VERITAS Investigators Tezosentan in patients with acute heart failure: design of the value of endothelin receptor inhibition with tezosentan in acute heart failure Study (VERITAS). Am Heart J. 2005;150:46–53. doi: 10.1016/j.ahj.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Bush CA, Stang JM, Wooley CF, Kilman JW. Occult constrictive pericardial disease. Diagnosis by rapid volume expansion and correction by pericardiectomy. Circulation. 1977;56:924–930. doi: 10.1161/01.cir.56.6.924. [DOI] [PubMed] [Google Scholar]
- 9.Fugimoto N, Borlaug B, Lewis GD, Hastings JL, Shafer KM, Bhella PS, Carrick-Ranson G, Levine BD. Hemodynamic respons to rapid saline infusion: The impact of age, sex and heart failure. Circulation. 2013;127:55–62. doi: 10.1161/CIRCULATIONAHA.112.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox BD, Shimony A, Langleben D, Hirsch A, Rudski L, Schlesinger R, Eisenberg MJ, Joyal D, Hudson M, Boutet K, Serban A, Masetto A, Baron M. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension. Eur Respir J. 2013;42:1083–1091. doi: 10.1183/09031936.00091212. [DOI] [PubMed] [Google Scholar]
- 11.McGoon D, Gutterman V, Steen Barst R, McCrory DC, Fortin TA, Loyd JE. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 12.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103:685–692. doi: 10.1378/chest.103.3.685. [DOI] [PubMed] [Google Scholar]
- 13.Jaïs X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zaba J, Perchenet L, Morganti A, Simonneau G, Rubin LJ. Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension Study Group. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 14.Robbins IM, Newman JH, Johnson RF, Hemnes AR, Fremont RD, Piana RN, Zhao DX, Byrne DW. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136:31–36. doi: 10.1378/chest.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pugh ME, Hemnes AR, Newman JH, Robbins IM. Treatment of Non-Group 1 Pulmonary Hypertension: Results of a Survey of Pulmonary Hypertension Centers in the United States. J Heart Lung Transpl. 2012;31:S211. (abstract) [Google Scholar]
- 18.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic Pulmonary Vascular Pressure Gradient: A Predictor of Prognosis in “Out-of-Proportion” Pulmonary Hypertension. Chest. 2013;143:758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 19.Murali S, Kormos RL, Uretsky BF, Schechter D, Reddy PS, Denys BG, Armitage JM, Hardesty RL, Griffith BP. Preoperative pulmonary hemodynamics and early mortality after orthotopic cardiac transplantation: the Pittsburgh experience. Am Heart J. 1993;126:896–904. doi: 10.1016/0002-8703(93)90704-d. [DOI] [PubMed] [Google Scholar]
- 20.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 21.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 23.Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136:37–43. doi: 10.1378/chest.08-2784. [DOI] [PubMed] [Google Scholar]