Abstract
Human, monkey, and bovine retinal pigment epithelial (RPE) cells exhibit an M-type K+ current, which in many other cell types is mediated by channels composed of KCNQ α-subunits and KCNE auxiliary subunits. Recently, we demonstrated the expression of KCNQ1, KCNQ4, and KCNQ5 in the monkey RPE. Here, we investigated the expression of KCNQ and KCNE subunits in native bovine RPE. RT-PCR analysis revealed the expression of KCNQ1, KCNQ4, and KCNQ5 transcripts in the RPE, but, in Western blot analysis of RPE plasma membranes, only KCNQ5 was detected. Among the five members of the KCNE gene family, transcripts for KCNE1, KCNE2, KCNE3, and KCNE4 were detected in bovine RPE, but only KCNE1 and KCNE2 proteins were detected. Immunohistochemistry of frozen bovine retinal sections revealed KCNE1 expression near the apical and basal membranes of the RPE, in cone outer segments, in the outer nuclear layer, and throughout the inner retina. The localization of KCNE1 in the RPE basal membrane, where KCNQ5 was previously found to be present, suggests that this β-subunit may contribute to M-type K+ channels in this membrane.
Keywords: potassium channels, M-type K+ current, RPE, cone photoreceptors
1. Introduction
Previous studies established that RPE cells from human (Hughes et al., 1995; Wen et al., 1993), monkey (Wen et al., 1993; Zhang et al., 2011), and bovine (Takahira and Hughes, 1997) retinas exhibit a sustained, outwardly rectifying M-type K+ current. Recently, we provided evidence that this current is generated by channels composed of members of the KCNQ/Kv7 gene family, namely KCNQ4 and KCNQ5, and that KCNQ5 protein is present in the RPE basolateral membrane (Pattnaik and Hughes, 2012; Zhang et al., 2011). Because the M-type K+ conductance in the RPE is partially activated in the physiological voltage range (−50 to −60 mV), it is likely that these channels participate in the active transport of K+ in the retina-to-choroid direction. In this way, M-type K+ channels probably play important roles in K+ homeostasis in the subretinal space and, secondarily, in fluid absorption across the RPE.
KCNQ subunits have a six-transmembrane domain motif with a single P-loop that contains the K+ pore selectivity sequence GYG. Like other K+ channels, KCNQ channels are composed of four separate subunits and, in vitro, all five members of the KCNQ family are capable of assembling into homomeric or heteromeric channels. KCNE genes (KCNE1-E5) encode single transmembrane spanning peptides (minK and minK-related peptides (MiRPs)) that associate with and alter the surface expression, voltage-dependence, kinetics, and pharmacology of KCNQ channels (Kanda and Abbott, 2012; McCrossan and Abbott, 2004). When co-expressed in Xenopus oocytes, KCNQ4 current amplitude was increased by KCNE1, KCNE2, and KCNE4 and decreased by KCNE3 (Strutz-Seebohm et al., 2006), but interpretation of these results is complicated by the possible presence of endogenous KCNQ1 (Sanguinetti et al., 1996) and KCNE subunits (Gordon et al., 2006). Co-expression of KCNQ5 with KCNE1 in Xenopus oocytes slowed activation and reduced current magnitude (Schroeder et al., 2000), but when co-expressed in HEK293 cells, KCNE1 increased KCNQ5 currents (Roura-Ferrer et al., 2009). On the other hand, KCNE3 markedly decreased KCNQ5 current density in both expression systems (Roura-Ferrer et al., 2009; Schroeder et al., 2000).
In addition to KCNQ, KCNE subunits also associate with other voltage-gated cation channels including Kv1.3 (Sole et al., 2009), Kv2.1 (McCrossan et al., 2009) (McCrossan et al., 2003), Kv3.1 (McCrossan et al., 2003), Kv4.2 (Zhang et al., 2001), Kv4.3 (Deschenes and Tomaselli, 2002), Kv11/HERG (Um and McDonald, 2007), and HCN2 (Yu et al., 2001). For example, KCNE3 alters the gating of Kv3.4 (Abbott and Goldstein, 2001) and reduces Kv2.1 and Kv3.1 currents in brain and smooth muscle (McCrossan et al., 2003). This raises the possibility that KCNE subunits may interact with and modify the properties of multiple types of voltage-gated K+ channels in the RPE and elsewhere in the retina.
To date, there are no published studies on the expression of KCNE subunits in the RPE or neural retina. In the present study, we applied RT-PCR, Western blot, and immunohistochemical analyses to determine the expression and localization of KCNQ and KCNE subunits in bovine RPE and neural retina. Some preliminary results of this study were presented previously in abstract form (Zhang, 2009).
2. Materials and methods
2.1 Preparation of membrane proteins from bovine RPE sheets, neural retina, skeletal muscle, and heart
Bovine eyes, heart, and skeletal muscle were obtained from a local abattoir and transported to the laboratory on ice. All protein procedures were performed at 4°C. Bovine heart and skeletal muscle plasma membrane proteins were isolated according to methods previously published (Galante et al., 1995; Trumble et al., 1980). Eyes were hemisected by a circumferential incision around the pars plana and the anterior segment, lens, and vitreous were removed. After the neural retina was gently peeled away, RPE sheets were isolated from eyecups following incubation at 37 °C with 1% dispase for 30 to 60 min as described previously (Yang et al., 2003). Plasma membrane proteins from bovine RPE sheets and crude membrane proteins from bovine neural retina were isolated as previously described (Zhang et al., 2011).
2.2. RT-PCR
Total RNA was prepared from RPE sheets and neural retina as described previously (Yang et al., 2008) and reverse transcribed with random decamers or oligo(dT) primers using reverse transcriptase (RetroScript, Ambion, Austin, TX) following procedures outlined in the manufacturer’s instructions. Conventional PCR was performed with primer pairs specific for bovine KCNQ1-5 and KCNE1-5 and primers for human GAPDH as a control. Primers (Table 1) were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The PCR products were generated by adding One Taq 2X Master Mix with GC Buffer (New England BioLabs Inc., MA) and cycled 30 (GAPDH) or 40 (KCNQ1-5, KCNE1-5) times for 30 seconds at 94°C, 30 seconds at 50–55°C, and 30 seconds at 68°C, followed by a 10-minute extension at 68°C. DNA sequencing was performed by the DNA Sequencing Core Facility at the University of Michigan.
Table 1.
Target gene primer sequences and expected sizes of RT-PCR products
| Target gene | Primer Sequence | Expected size (bp) | GenBank accession no |
|---|---|---|---|
| KCNE1 | Forward 5′-CTG GGC ATC ATG CTG AGT TA-3′ Reverse 5′-TTC ATC TCG GGA AGG TAT GC-3′ |
203 | NM_001077977.1 |
| KCNE2 | Forward 5′-AAC ACA ACG GTT GAG CAA GA-3′ Reverse 5′-TGT ACT TCC CTT GCC AGT CC-3′ |
205 | NM_001077084.1 |
| KCNE3 | Forward 5′-AAT GCC ACT CTT CAC AGC AA-3′ Reverse 5′-ACA CAT GAT AGG GGT CAC TGC-3′ |
208 | XM_002683752.1 |
| KCNE4 | Forward 5′-CCA CGG CAA CGA ATA CTT CT-3′ Reverse 5′-GCT CTC CTG CAA CAC ATT CA-3′ |
232 | EU177670.1 |
| KCNE5 | Forward 5′-TGA ACT GCA GCG AGA GTC AG-3′ Reverse 5′-AGG CAG GCG TAG AAG ACC AT -3′ |
217 | NM_001077875.1 |
| KCNQ1 | Forward 5′-CTT CGC CAT CTC CTT CTT TG-3′ Reverse 5′-TCT TTA CCA TGG CGG ACT TC-3′ |
257 | NM_001205441.1 |
| KCNQ2 | Forward 5′-GGG GAG AAC GAT CAC TTT GA-3′ Reverse 5′-ACG GTG CTG CTC TTG AAC TT-3′ |
210 | XM_002692250.2 |
| KCNQ3 | Forward 5′-CAT GCT TTC CCG AAT CAA GT-3′ Reverse 5′-CAG TTT CTT CCC CAT GTC GT-3′ |
241 | NM_174374.2 |
| KCNQ4 | Forward 5′-CTT CCC CGA GTA GTG AGC AG-3′ Reverse 5′-CAG CGT CTC CTT GAA TTT CC-3′ |
272 | XM_607172.5 |
| KCNQ5 | Forward 5′-CCG ATG ACG TGT ACG ATG AC-3′ Reverse 5′-CAC GTG TCT GCA GGC TTT TA-3 |
225 | XM_002690016.2 |
| GAPDH | Forward 5′-GTG AAG GTC GGA GTC AAC G-3′ Reverse 5′-GAG ATG ATG ACC CTT TTG GC-3′ |
356 | AF261085 |
2.3. Antibodies
Primary antibodies used in this study are listed in Table 2. Secondary antibodies used include donkey anti-mouse IgG-HRP (cat. no. sc-2314) and donkey anti-rabbit IgG-HRP (cat. no. sc-2313) from Santa Cruz Biotechnology (Santa Cruz, CA) and Alexa Fluor 488 goat anti-mouse (H+L), Alexa Fluor 488 goat anti-rabbit (H+L), and Alex Fluor 555 goat anti-rabbit (H+L) antibodies from Invitrogen Corporation (Camarillo, CA).
Table 2.
Primary Antibodies.
| antibody | source | Catalog number | epitope | type |
|---|---|---|---|---|
| KCNQ1 | Santa Cruz Biotechnology | sc-10646 | C-terminal of human | Rabbit polyclonal |
| KCNQ4 | Neuromab | 75-082 | N-terminal of human | Mouse monoclonal |
| KCNQ5 | Chemicon | AB5599 | N-terminal of human KCNQ5 | Rabbit polyclonal |
| KCNE1 | Sigma-Aldrich | WH0003753M1 | aa 67–130 of human KCNE1 | Mouse monoclonal |
| KCNE1 | Alomone Labs | APC-008 | aa 67–129 of human KCNE1 | Rabbit polyclonal |
| KCNE1 | Santa Cruz Biotechnology | SC-16796 N-16 |
Internal region of human KCNE1 | Goat polyclonal |
| KCNE2 | Alomone Labs | APC-054 | aa 88–107 of rat KCNE2 | Rabbit polyclonal |
| KCNE3 | Alomone Labs | APC-118 | aa 81–93 of human KCNE3 | Rabbit polyclonal |
| KCNE4 | Gift from Dr. A.L. George, Jr in house | aa 73–94 of human KCNE4 | Rabbit polyclonal | |
| Kir7.1 | C-terminal of human Kir7.1 | Rabbit polyclonal | ||
| Na,K-ATPase | Abcam | AB7671 | full-length rabbit α1 subunit Na,K-ATPase | Mouse monoclonal |
| Red/Green Opsin | Millipore | AB5405 | human red/green opsin | Rabbit polyclonal |
| Blue Opsin | Millipore | AB5407 | human blue opsin | Rabbit polyclonal |
Secondary antibodies used include donkey anti-mouse IgG-HRP (cat. no. sc-2314), donkey anti-rabbit IgG-HRP (cat. no. sc-2313) from Santa Cruz Biotechnology (Santa Cruz, CA); Alexa Fluor488 goat anti-rabbit (H+L) and Alexa Fluor555 goat anti-mouse (H+L) (Invitrogen Corporation, Camarillo, CA).
2.4. Western blot analysis
Western blot analysis was performed using the techniques described previously (Zhang et al., 2011). Briefly, proteins were mixed in Laemmli sample buffer (62.5 mM Tris [pH 6.8], 25% glycerol 2% SDS, 0.01% bromophenol blue, and 5% 2-mercaptoethanol; Bio-Rad, Hercules, CA) at room temperature for 20 min and centrifuged at 20,000 g for 10 min. Ten to twenty μg of proteins were applied to a 4% to 20% linear gradient Tris-HCl gel (Bio-Rad). After electrophoresis, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) at a constant current of 350 mA for 90 minutes at 4 °C in a solution containing 25 mM Tris, 193 mM glycine, and 10% methanol. The membrane was then incubated at room temperature, first with Tris-buffered saline containing 5% nonfat dried milk and 0.1% Tween 20 and then with primary antibodies (working dilutions: KCNQ1, 1:500; KCNQ4, 1:500; KCNQ5, 1:2000; KCNE1, 1:1000; KCNE2, 1:1000; KCNE3, 1:500; KCNE4, 1:1000; and Na,K-ATPase, 1:2000). Immune complexes were detected with horseradish peroxidase–conjugated secondary antibodies at a dilution of 1:2500, enhanced with chemiluminescent substrate (Pierce, Rockford, IL), and imaged by exposing the membrane to X-ray film. In experiments testing antibody specificity, blots probed with antibody alone and with antibody preabsorbed with antigenic peptide (1:20) were processed in parallel.
Protein molecular weights were calculated from FASTA sequences using Protein Calculator v3.3 (http://www.scripps.edu/~cdputnam/software/software.html#calc).
2.5. Immunohistochemistry
Pieces of bovine retina-RPE-choroid were fixed by immersion for 1 hour in freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer (PB) and then washed in chilled PB (3x, 20 min). To cryoprotect before freezing, tissues were incubated in successive 1 hour incubations in 5% and 10% sucrose solutions in PB, then in 20% sucrose in PB overnight at 4°C. Tissues were embedded in 20% sucrose in optimal cutting temperature embedding medium (Tissue-Tek; Sakura Finetek, Inc., Torrance, CA) and frozen in liquid nitrogen. Cryosections (10 μm) were cut, collected on glass slides, dried at room temperature, and stored at −80 °C until use. For indirect immunofluorescence labeling, cryosections were blocked with phosphate-buffered saline (PBS) containing 10% normal goat serum, 1% BSA and 0.3% Triton X-100 and then incubated overnight at 4°C in PBS containing primary antibodies (anti-Kir7.1, 1:100 dilution; anti-KCNE2, anti-KCNE3, and anti-KCNE4, 1:200 dilutions; anti-KCNE1 anti-blue opsin, and anti-red/green opsin, 1:500 dilutions) plus 2% normal goat serum and 0.2% Triton X-100. Sections were washed eight times and incubated at room temperature in the dark for 1 hour with the secondary antibodies diluted in 0.2% Triton X-100 and 2% normal goat serum in PBS to a final dilution of 1:500. After washing eight times in the dark, sections were covered in mounting medium (Gel Mount; Biomeda, Foster City, CA) and secured with a coverslip.
Specimens were analyzed on a scanning laser confocal microscope (Leica SP5, Mannheim, Germany). Digital images were collected at 16 bit resolution in 0.29-0.5 micron z-sections and analyzed by image-analysis software (Leica LAS AF). The confocal immunofluorescence images in Fig. 5 and 6 are 3-D projections generated from 4 to 8 consecutive z-sections. Files were exported for additional processing by Photoshop CS2 (Adobe, San Jose, CA).
Figure 5.
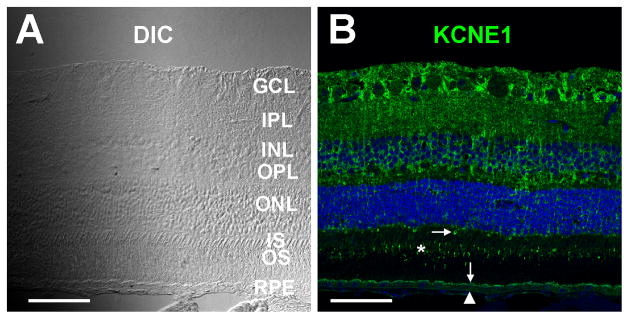
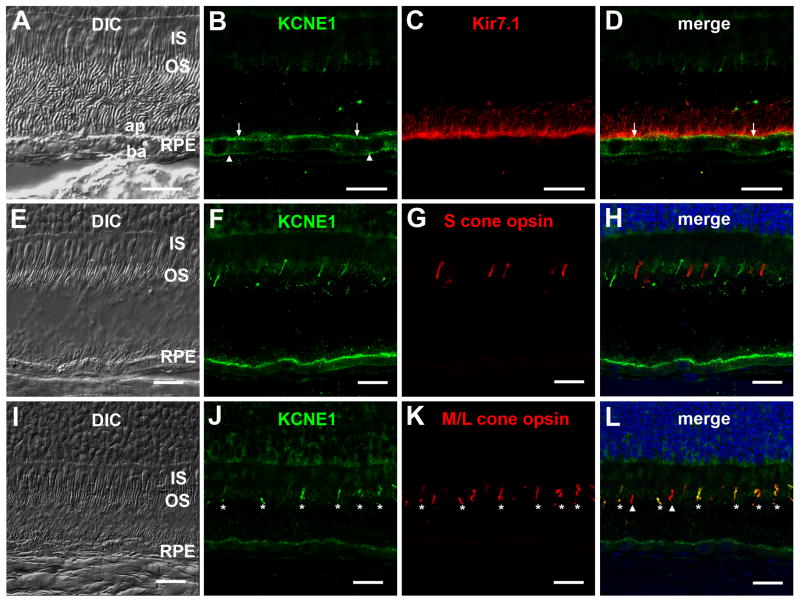
Immunofluorescence localization of KCNE1 in bovine retina. Differential interference contrast (DIC, A) and confocal immunofluorescence images (B) of an oblique section of frozen bovine neural retina-RPE-choroid labeled with anti-KCNE1 antibody (green). KCNE1 labeling is present in the ganglion cell layer (GCL), the inner (IPL) and outer (OPL) plexiform layers, the inner (INL) and outer (ONL) nuclear layers, cone outer segments (asterisk), the proximal region of some cone inner segments (horizontal arrow) and the RPE apical (vertical arrow) and basal (arrowhead) membranes. Scale bars in A and B, 50 microns.
Figure 6.
Subcellular localization of KCNE1 immunoreactivity in bovine outer retina. RPE: Differential interference contrast (DIC, A) and confocal immunofluorescence images of bovine neural retina-RPE-choroid cryosection immunolabeled with anti-KCNE1 (green; B) and anti-Kir7.1 (red; C) antibodies. KCNE1 immunolabeling was present in the RPE basal membrane (arrowhead) as well as the apical surface (arrow), where it co-localized with Kir7.1 (D). Note the absence of KCNE1 immunoreactivity in the apical processes, which were immunolabeled with anti-Kir7.1 antibody. Cone photoreceptors: DIC (E, I) and confocal immunofluorescence images of bovine RPE-choroid sections co-immunolabeled with anti-KCNE1 antibody (green, F, J) and anti-S opsin antibody (red; G) or anti-M/L opsin antibody (red; K). Merged images show that KCNE1 immunostaining was absent in cone outer segments positive for S opsin (H) but that it co-localized in most but not all cone outer segments immunolabeled with M/L opsin (L). Asterisk: cone photoreceptor outer segments co-labeled with KCNE1 and M/L opsin; arrowheads: cone photoreceptor outer segments immunolabeled with M/L opsin but not KCNE1. Scale bars: 20 microns.
3. RESULTS
3.1. KCNQ subunits
3.1.1. RT-PCR
Previously, we showed that human and monkey RPE express KCNQ1, KCNQ4, and KCNQ5 transcripts, whereas human and monkey neural retina express all five KCNQ family members (Zhang et al., 2011). Using bovine KCNQ subunit specific primer pairs, we found a similar expression pattern in bovine RPE and neural retina (Fig. 1). Positive PCR fragments were sequenced and comparison of sequenced products with published bovine sequences confirmed their identities.
Figure 1.

KCNQ subunit mRNA expression in bovine RPE and neural retina. Total RNA from bovine RPE and neural retina (ret) was used in conjunction with KCNQ subunit-specific primer pairs (Table 1) for RT-PCR analysis of KCNQ transcript expression.
3.1.2. Western blot analysis
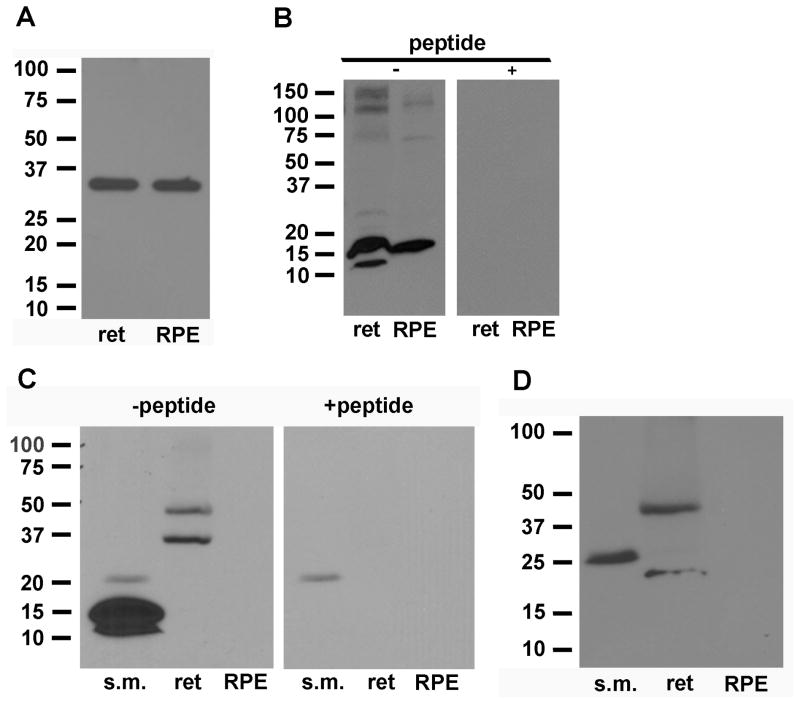
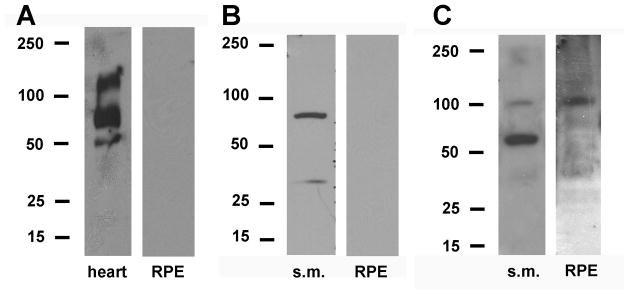
In Western blots of monkey RPE plasma membrane proteins, we previously detected KCNQ5 protein but neither KCNQ1 nor KCNQ4. Using a commercially available rabbit antibody against KCNQ1, we detected ~78 and ~155 kDa bands in blots of bovine heart plasma membrane proteins, consistent with the detection of KCNQ1 monomers (74 kDa) and dimers (148 kDa), respectively (Fig. 2A, left panel). On the other hand, we did not detect KCNQ1 in blots of either bovine RPE plasma membrane proteins (Fig. 2A, right panel) or bovine retina crude membrane proteins (results not shown). Re-probing with anti-Na,K-ATPase antibody after stripping the blots confirmed protein loading (results not shown).
Figure 2.
KCNQ subunit protein expression in bovine RPE. A. Western blot analysis of bovine heart sarcolemma proteins (left panel) and bovine RPE plasma membrane proteins (right panel) using antibodies against KCNQ1 (Table 2). B. Western blot analysis of bovine skeletal muscle sarcolemma proteins (s.m., left panel) and bovine RPE plasma membrane proteins (right panel) using antibodies against KCNQ4 (Table 2). C. Western blot analysis of bovine skeletal muscle sarcolemma proteins (s.m., left panel) and bovine RPE plasma membrane proteins (right panel) using antibodies against KCNQ5 (Table 2).
Figure 2B shows blots of bovine skeletal muscle (s.m.) proteins and bovine RPE plasma membrane proteins probed with a mouse monoclonal antibody against human KCNQ4. The antibody labeled a ~77 kDa band in skeletal muscle (left pane), consistent with the detection of a KCNQ4 monomer (predicted molecular weight of human KCNQ4 variant 1, 77 kDa), but nothing in RPE (right panel) or neural retina (results not shown). Re-probing with anti-Na,K-ATPase antibody after stripping the blots confirmed protein loading. The failure to detect KCNQ4 in bovine RPE is similar to our previous finding in monkey using a different anti-KCNQ4 antibody.
Figure 2C shows Western blots of plasma membrane proteins from bovine skeletal muscle and bovine RPE probed with a rabbit anti-KCNQ5 antibody. The antibody labeled 100 kDa bands in both skeletal muscle and RPE, consistent with the detection of KCNQ5 monomers (predicted molecular weight of unglycosylated protein, 91 kDa). Unfortunately, antigenic peptide was not available to determine antibody specificity. When applied in Western blots of RPE total protein or retina crude membrane proteins, multiple bands of various molecular masses (including 100 kDa bands) were observed (results not shown).
3.1.3. Immunohistochemistry
To determine subcellular localization of KCNQ4 and KCNQ5 subunits in bovine RPE, we performed indirect immunofluorescence microscopy in frozen sections of bovine retina using the same antibodies employed in Western blot analysis. Neither the anti-KCNQ4 antibody nor the anti-KCNQ5 antibody yielded specific labeling in the RPE or neural retina (results not shown).
3.2. KCNE subunits
3.2.1. RT-PCR
To identify KCNE subunits expressed in bovine RPE, we performed conventional RT-PCR analysis using bovine KCNE subunit specific primer pairs (Table 1). Reactions using mRNA from neural retina served as positive controls. Figure 3 shows that KCNE1, E2, E3, and E4, but not E5, are expressed in bovine RPE, whereas all five KCNE subtypes are expressed in bovine neural retina. Positive PCR fragments were sequenced and comparison of sequenced products with published bovine sequences confirmed their identities.
Figure 3.
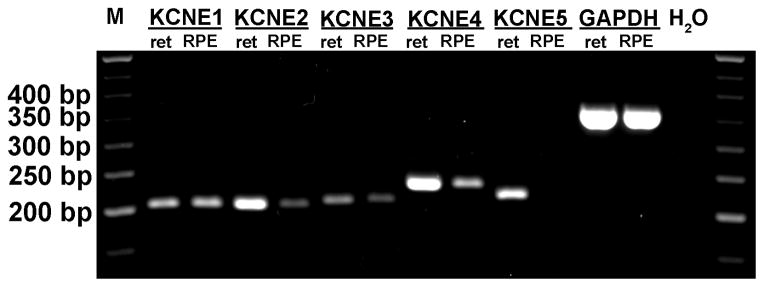
KCNE subunit mRNA expression in bovine RPE and neural retina. Total RNA from bovine RPE and neural retina was used in conjunction with KCNE subunit-specific primer pairs (Table 1) for RT-PCR analysis of KCNE transcript expression.
3.2.2. Western blot analysis
To examine the expression of KCNE1 protein, we tested three commercially-available antibodies: a mouse monoclonal antibody raised against the C-terminus of human KCNE1 (Sigma-Aldrich), a rabbit polyclonal antibody raised against the C-terminus of human KCNE1 (Alomone Labs), and a goat polyclonal antibody against an internal region of human KCNE1 (Santa Cruz Biotechnology) (Table 2). The mouse monoclonal antibody recognized a ~35 kDa band in RPE plasma membrane proteins and in retina total membrane proteins (Fig. 4A); the unavailability of antigenic peptide, however, precluded our testing of antibody specificity. Experiments using rabbit or goat polyclonal antibodies detected ~35 kDa bands as well as ~60 kDa bands in RPE plasma membrane and, in both cases, the two bands were eliminated after preabsorbing the antibodies with the corresponding antigenic peptide (results not shown). Similar results were obtained in Western blot experiments using a crude membrane protein fraction from neural retina. As the expected molecular mass of unglycosylated KCNE1 is 15 kDa, the presence of the ~35 kDa bands suggests that KCNE1 in the RPE and neural retina is glycosylated, as has been shown for KCNE1 in heart (Chandrasekhar et al., 2006). The identity of the ~60 kDa band is unclear, but it could represent KCNE1 complexed with another protein.
Figure 4.
KCNE subunit protein expression in bovine RPE and neural retina. A. Western blot analysis of bovine retinal crude membrane proteins (ret; left panel) and RPE plasma membrane proteins (right panel) using antibodies against KCNE1 (Table 2). B. Western blot analysis of bovine retinal crude membrane proteins and RPE plasma membrane proteins (left panel) using antibodies against KCNE2 (Table 2). Bands were eliminated when the antibodies were preabsorbed with antigenic peptide (right panel). C. Western blot analysis of bovine skeletal muscle sarcolemma proteins (s.m.), retinal crude membrane proteins, and RPE plasma membrane proteins (left panel) using antibodies against KCNE3 (Table 2). Bands were eliminated when the antibodies were preabsorbed with antigenic peptide (right panel). D. Western blot analysis of bovine skeletal muscle sarcolemma proteins, retinal crude membrane proteins, and RPE plasma membrane proteins using antibodies against KCNE4 (Table 2).
Using a rabbit polyclonal antibody against rat KCNE2, ~16 kDa bands were detected in Western blots of bovine RPE plasma membrane and retinal crude membrane proteins (Fig. 4B). This apparent molecular mass is similar to that predicted for unglycosylated human KCNE2 (15 kDa). A fainter ~12 kDa band also detected in retinal proteins may represent a protein degradation product. The specificity of immunoreactivity with bovine KCNE2 protein was confirmed by elimination of the signals after pre-absorption of antibody with antigenic peptide (Fig. 4B, right panel).
Western blot of bovine skeletal muscle proteins (positive control) using rabbit polyclonal antibodies against a C-terminal peptide in human KCNE3 revealed a prominent 15 kDa band (Fig. 4C, left panel), which is slightly higher than the molecular mass predicted by the KCNE3 open reading frame (11 kDa). The anti-KCNE3 antibody labeled 36 kDa and 54 kDa bands in blots of retinal crude membrane proteins. The specificity of the immunolabeling of bands in blots of skeletal muscle and retinal proteins was confirmed by diminishment of band intensity after pre-absorption of the antibody with antigenic peptide (Fig. 4C, right panel). No band was detected in RPE plasma membrane proteins. Re-probing the blot with anti-Na,K-ATPase antibody confirmed RPE plasma membrane protein loading (results not shown).
A polyclonal antibody against a peptide in the C-terminus of human KCNE4 revealed a prominent 28 kDa band in Western blots of bovine skeletal muscle proteins (Fig 4D). This is larger than the predicted molecular mass (~18 kDa) but consistent with results from immunoblots of lysates from KCNE4-transfected COS cells and various human tissues using the same antibody (Manderfield and George, 2008). Nothing was detected in RPE plasma membrane proteins and, again, re-probing the blot with anti-Na,K-ATPase antibody confirmed protein loading. In neural retina, 35 kDa and 46 kDa bands were detected, but, unfortunately, no antigenic peptide was available with which to test antibody specificity.
Because KCNE5 transcripts were not detected in bovine RPE, no attempt was made to investigate KCNE5 protein expression.
3.2.3. Immunohistochemistry
Figures 5 and 6 show the expression pattern of KCNE1 in cryosections of bovine retina obtained with the mouse anti-KCNE1 antibody. KCNE1 immunolabeling (green) was present in the inner retina, where it had a radial pattern reminiscent of Muller cells. In the outer retina, KCNE1 immunoreactivity was distributed in the outer nuclear layer, the proximal region of some cone inner segments (Fig. 5B, horizontal arrow), and cone outer segments (Fig. 5B, asterisk; Fig. 6, F and J). KCNE1 immunolabeling was also present in the RPE apical (Figs. 5B and 6B, vertical arrows) and basolateral (Figs. 5B and 6B, arrowheads) membranes. Double-labeling with rabbit anti-Kir7.1 antibodies (Yang et al., 2003) revealed that KCNE1 immunostaining at the RPE apical membrane was prominent near the cell surface, but its distribution pattern differed from that of Kir7.1 immunolabeling, which lay more proximally and extended into apical processes (Fig. 6, B and D). To confirm KCNE1 labeling of cone outer segments, we performed double-labeling experiments using antibodies against human S (blue) opsin and human M/L (red/green) opsin (Fig. 6). Figure 6, F, G, and H, shows that there was no overlap in the immunolabeling by anti-KCNE1 and anti-S opsin antibodies, indicating that KCNE1 is not expressed in S cone photoreceptors. Double-labeling with anti-KCNE1 and anti-M/L opsin antibodies revealed that the majority of cone photoreceptor outer segments expressed both KCNE1 and M/L opsin (Fig. 6, J,K, and L, asterisks), but interestingly, a minority of M/L opsin-positive cells appeared to lack KCNE1 immunostaining (Fig. 6L, arrowheads).
Experiments using the rabbit anti-KCNE2 antibody resulted in faint immunostaining of the RPE cytoplasm but no labeling of either the apical or basolateral membrane; in the neural retina, KCNE2 immunolabeling was present primarily in the outer plexiform layer (results not shown). In immunohistochemistry experiments with anti-KCNE3 and anti-KCNE4 antibodies, there was perinuclear and punctate nuclear labeling, respectively, but no labeling of RPE plasma membrane (results not shown).
4. Discussion
4.1. KCNQ subunits
Previously, we showed that KCNQ1, KCNQ4, and KCNQ5 transcripts are expressed in monkey RPE but detected only KCNQ5 at the protein level (Zhang et al., 2011). Additionally, we presented pharmacological evidence that heterologous KCNQ4/5 channels underlie the M-type K+ current in these cells (Pattnaik and Hughes, 2012). The purpose of the present study was two-fold: 1) to confirm the expression of KCNQ subunits in bovine RPE, which also exhibits an M-type K+ current (Takahira and Hughes, 1997), and 2) to evaluate the expression of KCNE β subunits, which can potentially interact with KCNQ4/5 and other voltage-gated K+ channels to affect their surface expression and functional properties.
We determined that bovine RPE has an expression pattern of KCNQ subunits that is identical to that observed previously in monkey RPE (Zhang et al., 2011). Of the five members of the KCNQ family, transcripts for KCNQ1, KCNQ4, and KCNQ5 were detected in bovine RPE and all were found to be present in the neural retina. Multiple splice variants for KCNQ1, KCNQ4 and KCNQ5 have been annotated and, in monkey, RPE KCNQ4 variant 1 and KCNQ5 variant 2 predominate (Zhang et al., 2011). There is no information available about alternative splice variation of bovine KCNQ4 and KCNQ5.
At the protein level, we detected the expression of KCNQ5 in bovine RPE plasma membranes but neither KCNQ1 nor KCNQ4. Again, these results are similar to those obtained previously in monkey RPE (Zhang et al., 2011). The failure to detect KCNQ1 and KCNQ4 suggests that these proteins, if expressed, are present in the plasma membranes at low levels or are located primarily in intracellular membranes. We cannot, however, exclude the possibility that the negative results are related to the relatively poor reactivity of the antibodies with bovine proteins, despite our positive controls. In a previous study on monkey retina, we used a rabbit polyclonal antibody to immunolocalize KCNQ5 to the RPE basolateral membrane as well as to cone photoreceptor inner segments (Zhang et al., 2011). However, this particular antibody did not react with bovine proteins (not shown). In the present study, we used a different antibody that recognized bovine KCNQ5 protein in Western blots (Fig. 2C), but, unfortunately, it did not give specific membrane staining in indirect immunofluorescence microscopy experiments.
4.2. KCNE subunits
Using subunit-specific primer pairs, we performed RT-PCR on mRNA from freshly isolated bovine RPE and neural retina. In bovine RPE, we detected appropriately-sized bands for KCNE1, E2, E3, and E4; in neural retina, we found all five KCNE subtypes to be expressed.
Guided by the results of our RT-PCR experiments, we performed Western blot analysis using commercially-available antibodies against KCNE1, E2, E3, and E4 on plasma membrane proteins from bovine RPE and total membrane proteins from bovine neural retina. In blots of crude retinal membrane proteins, we detected KCNE1, E2, E3, and E4 proteins. In RPE plasma membrane proteins, however, only KCNE1 and KCNE2 were detected. As KCNE5 transcript was not detected in the RPE, we did not perform experiments with anti-KCNE5 antibodies.
Using indirect immunofluorescence confocal microscopy, we localized KCNE1 in the RPE apical and basal membranes and, in the outer retina, in cone outer segments, the proximal region of some cone inner segments, and the outer nuclear layer. The distribution of KCNE1 at the RPE apical surface did not overlap with that of Kir7.1, an apical membrane marker. This raises the possibility that KCNE1 near the apical membrane may be predominantly in sub-membrane vesicles. Interestingly, KCNE1 immunoreactivity in cone outer segments was observed only in a subset of M/L cone photoreceptors. This finding is surprising because cows are dichromatic mammals, possessing only short-wavelength (S) and medium-wavelength (M/L) cone photoreceptors with spectral peaks of 455 nm and 554 nm, respectively (Jacobs et al., 1998). Our finding of differential KCNE1 immunoreactivity in M/L cone outer segments suggests some degree of heterogeneity within this class of cone photoreceptor.
KCNE1 immunoreactivity was also present in the inner retina, extending from the internal limiting membrane to the outer plexiform layer in a pattern reminiscent of Muller cells. Additional experiments with cell-specific markers are needed to confirm this possibility and to determine whether the KCNE1 immunostaining in the outer nuclear layer reflects labeling of cone and/or rod photoreceptor soma or Muller cells.
In immunohistochemical experiments, we used the same rabbit anti-KCNE2 antibodies that detected KCNE2 in RPE plasma membrane proteins in Western blots and found that although there was faint KCNE2 immunostaining in the RPE cytoplasm, neither the RPE apical or basal membrane was immunolabeled. Likewise, immunohistochemical experiments using KCNE3 and KCNE4 antibodies yielded no immunolabeling of RPE plasma membranes but did reveal immunostaining within the cytoplasm. Future studies incorporating the use of markers of the secretory and endocytic pathways are needed to determine if KCNE subunits might play a role in the intracellular processing of KCNQ and other K+ channel alpha subunits.
4.3. Physiological significance
Our finding that KCNE1 is localized to the basolateral membrane of the RPE suggests that it may serve as an accessory subunit to K+ channels residing in that membrane. Previously, we localized KCNQ5 protein to the basolateral membrane of monkey RPE (Zhang et al., 2011). Although we were not able to determine the subcellular localization of KCNQ4, pharmacological studies on the M-type K+ current in isolated monkey RPE cells suggested that the underlying channel is likely composed of KCNQ4/Q5 heteromers (Pattnaik and Hughes, 2012). Because bovine RPE cells exhibit M-type K+ currents similar to those in monkey RPE (Takahira and Hughes, 1997), it is reasonable to expect that the channel composition in this species is similar. In the present study, we detected KCNQ5 but not KCNQ4 in Western blots of bovine RPE plasma membrane protein. Unfortunately, the anti-KCNQ5 antibody was not effective in immunohistochemistry experiments on bovine retinal sections.
KCNE1 is known to interact with both homomeric KCNQ4 and KCNQ5 channels. Strutz-Seebohm and colleagues (Strutz-Seebohm et al., 2006) investigated functional interactions between KCNE subunits and KCNQ4 expressed in Xenopus oocytes and found that all KCNEs modulated KCNQ4 voltage dependence, protein stability, or ion selectivity. Co-expression of either KCNE1 or KCNE2 with KCNQ4 did not markedly affect KCNQ4 surface expression but produced a moderate increase in KCNQ4 current amplitude and shifted the voltage for half-maximal activation in the negative direction. KCNE3 and KCNE4 had the greatest effects on KCNQ4, with KCNE3 strongly suppressing KCNQ4 currents and KCNE4 increasing them. Interestingly, KCNE4 co-expression decreased KCNQ4 K+ selectivity. In another study using the HEK-293 cell expression system, Roura-Ferrer and colleagues (Roura-Ferrer et al., 2009) investigated whether co-expression of KCNE subunits modifies KCNQ5 channel function and found that only KCNE1 and KCNE3 had effects, with KCNE1 increasing current amplitude and KCNE3 producing strong current suppression. Subsequently, the same group determined that KCNQ5 specifically forms oligomeric channels with KCNE1 and KCNE3 but not with KCNE2 (Roura-Ferrer et al., 2012). Although interactions between KCNE subunits and heteromeric KCNQ4/5 channels have not been determined, it seems possible that KCNE1 co-assembles with KCNQ4/5 channels and modifies channel properties to increase current amplitude. If so, this modulation might increase the capacity for K+ efflux across the RPE basolateral membrane and, thus, K+ absorption. Additional experiments involving functional co-expression in a mammalian system and co-immunoprecipitation of KCNQ4, KCNQ5, and KCNE1 subunits from RPE basolateral membrane proteins are needed to confirm this hypothesis.
In addition to KCNQ4 and KCNQ5, a number of other voltage-gated K+ channel subunits have been identified in mammalian RPE at the transcript or protein level (Klumpp et al., 1995). Studies in rat RPE indicated that Kv1.3 channels are the likely basis for the delayed rectifier current (Strauss et al., 2002; Wollmann et al., 2006), an outward K+ current that is also expressed in bovine RPE cells (Takahira and Hughes, 1997). Other studies suggest that the transient A-type seen in the RPE of some species (Tao et al., 1994; Wen et al., 1993) may be mediated by Kv1.4 and/or Kv4.2 channels (Klumpp et al., 1995; Pinto and Klumpp, 1998). KCNE1 slows the rates of Kv4.2 activation and inactivation (Zhang et al., 2001), but KCNE4 suppresses Kv1.3 currents by affecting membrane trafficking currents by affecting membrane trafficking and channel gating (Sole et al., 2009) and both KCNE1 and KCNE2 suppress currents generated by Kv1.4 channels by trapping them in the secretory pathway (Kanda et al., 2011). Thus, interactions of KCNE subunits with Kv1.3, Kv1.4, and Kv4.2 α-subunits may influence the amplitudes and kinetics of delayed rectifier and A-type K+ currents in the RPE.
Delayed rectifier currents have been recorded in human rods and cones lacking outer segments (Kawai et al., 2005; Yagi and Macleish, 1994), but there are no reports of K+ currents originating from cone or rod outer segments. Thus, the significance of KCNE1 immunoreactivity in cone outer segments is unclear. The presence of KCNE1 immunoreactivity in the proximal portion of cone inner segments and in the outer nuclear layer, however, raises the possibility that this subunit associates with voltage-gated K+ channels elsewhere in cone and rod photoreceptors. Transcripts for the gene KCNV2, which encodes the K+ channel subunit Kv8.2, were detected in the inner segments of human rod and cone photoreceptors (Wu et al., 2006). Interestingly, Kv8.2 does not form functional homotetrameric channels but does form conducting heterotetrameric channels with several other K+ channel subunits; this includes Kv2.1 (Ottschytsch et al., 2002), which, incidentally, is expressed in photoreceptor inner segments (Hwang et al., 1993; Klumpp et al., 1995). Co-expression of Kv8.2 shifted the activation of Kv2.1 to more negative potentials and gave rise to transient hyperpolarizing overshoots in current clamp experiments, properties that are similar to those of IKx (Czirjak et al., 2007). Both KCNE1 and KCNE2 reduced Kv2.1 current density and slowed activation and deactivation kinetics (McCrossan et al., 2009). It will be interesting to learn whether KCNE1 interacts with Kv2.1/Kv8.2 channels and, if so, how it affects channel function.
5. Conclusions
We determined that bovine RPE has an expression pattern of KCNQ subunits that is similar to that previously reported for monkey RPE, namely the expression of KCNQ1, KCNQ4, and KCNQ5 at the transcript level and KCNQ5 at the protein level. In addition, we determined that bovine RPE cells express KCNE1 auxiliary subunits in both the apical and basal membranes. We conclude that KCNE1 may modify the surface expression and functional properties of KCNQ4/5 channels in the RPE basolateral membrane as well as other voltage-gated K+ channels in the RPE and neural retina.
The expression of KCNQ and KCNE K+ channel subunits in native bovine RPE was investigated.
Transcripts for KCNQ1, Q4, and Q5 and KCNE1, E2, E3, and E4 were found to be present.
KCNQ5, KCNE1, and KCNE2 were detected in RPE membrane proteins.
KCNE1 was localized to the RPE apical and basal membranes and in the neural retina.
KCNE1 β-subunits may contribute to KCNQ and other K+ channels in the RPE and retina.
Acknowledgments
The authors wish to thank Mr. Mitchell Gilette for assistance with retinal cryosectioning and Connor Baharozian for careful reading of this manuscript. This work was supported by NIH Grant EY08850, Core Grant EY07703, and an RPB unrestricted grant to the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott GW, Goldstein SA. Potassium channel subunits encoded by the KCNE gene family: physiology and pathophysiology of the MinK-related peptides (MiRPs) Mol Interv. 2001;1:95–107. [PubMed] [Google Scholar]
- Chandrasekhar KD, Bas T, Kobertz WR. KCNE1 subunits require co-assembly with K+ channels for efficient trafficking and cell surface expression. J Biol Chem. 2006;281:40015–40023. doi: 10.1074/jbc.M604398200. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Toth ZE, Enyedi P. Characterization of the heteromeric potassium channel formed by Kv2.1 and the retinal subunit Kv8.2 in Xenopus oocytes. J Neurophysiol. 2007;98:1213–1222. doi: 10.1152/jn.00493.2007. [DOI] [PubMed] [Google Scholar]
- Deschenes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528:183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- Galante P, Mosthaf L, Kellerer M, Berti L, Tippmer S, Bossenmaier B, Fujiwara T, Okuno A, Horikoshi H, Haring HU. Acute hyperglycemia provides an insulin-independent inducer for GLUT4 translocation in C2C12 myotubes and rat skeletal muscle. Diabetes. 1995;44:646–651. doi: 10.2337/diab.44.6.646. [DOI] [PubMed] [Google Scholar]
- Gordon E, Roepke TK, Abbott GW. Endogenous KCNE subunits govern Kv2.1 K+ channel activation kinetics in Xenopus oocyte studies. Biophys J. 2006;90:1223–1231. doi: 10.1529/biophysj.105.072504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BA, Takahira M, Segawa Y. An outwardly rectifying K+ current active near resting potential in human retinal pigment epithelial cells. Am J Physiol. 1995;269:C179–187. doi: 10.1152/ajpcell.1995.269.1.C179. [DOI] [PubMed] [Google Scholar]
- Hwang PM, Cunningham AM, Peng YW, Snyder SH. CDRK and DRK1 K+ channels have contrasting localizations in sensory systems. Neuroscience. 1993;55:613–620. doi: 10.1016/0306-4522(93)90427-h. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF, 2nd, Neitz J. Photopigment basis for dichromatic color vision in cows, goats, and sheep. Vis Neurosci. 1998;15:581–584. doi: 10.1017/s0952523898153154. [DOI] [PubMed] [Google Scholar]
- Kanda VA, Abbott GW. KCNE regulation of K+ channel trafficking - a sisyphean task? Front Physiol. 2012;3:231. doi: 10.3389/fphys.2012.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda VA, Lewis A, Xu X, Abbott GW. KCNE1 and KCNE2 inhibit forward trafficking of homomeric N-type voltage-gated potassium channels. Biophys J. 2011;101:1354–1363. doi: 10.1016/j.bpj.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F, Horiguchi M, Ichinose H, Ohkuma M, Isobe R, Miyachi E. Suppression by an h current of spontaneous Na+ action potentials in human cone and rod photoreceptors. Invest Ophthalmol Vis Sci. 2005;46:390–397. doi: 10.1167/iovs.04-0724. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Song EJ, Pinto LH. Identification and localization of K+ channels in the mouse retina. Vis Neurosci. 1995;12:1177–1190. doi: 10.1017/s0952523800006805. [DOI] [PubMed] [Google Scholar]
- Manderfield LJ, George AL., Jr KCNE4 can co-associate with the IKs (KCNQ1-KCNE1) channel complex. FEBS J. 2008;275:1336–1349. doi: 10.1111/j.1742-4658.2008.06294.x. [DOI] [PubMed] [Google Scholar]
- McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- McCrossan ZA, Lewis A, Panaghie G, Jordan PN, Christini DJ, Lerner DJ, Abbott GW. MinK-related peptide 2 modulates Kv2.1 and Kv3.1 potassium channels in mammalian brain. J Neurosci. 2003;23:8077–8091. doi: 10.1523/JNEUROSCI.23-22-08077.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrossan ZA, Roepke TK, Lewis A, Panaghie G, Abbott GW. Regulation of the Kv2.1 potassium channel by MinK and MiRP1. J Membr Biol. 2009;228:1–14. doi: 10.1007/s00232-009-9154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ. Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci U S A. 2002;99:7986–7991. doi: 10.1073/pnas.122617999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik BR, Hughes BA. Effects of KCNQ channel modulators on the M-type potassium current in primate retinal pigment epithelium. Am J Physiol Cell Physiol. 2012;302:C821–833. doi: 10.1152/ajpcell.00269.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LH, Klumpp DJ. Localization of potassium channels in the retina. Prog Retin Eye Res. 1998;17:207–230. doi: 10.1016/s1350-9462(97)00011-6. [DOI] [PubMed] [Google Scholar]
- Roura-Ferrer M, Etxebarria A, Sole L, Oliveras A, Comes N, Villarroel A, Felipe A. Functional implications of KCNE subunit expression for the Kv7.5 (KCNQ5) channel. Cell Physiol Biochem. 2009;24:325–334. doi: 10.1159/000257425. [DOI] [PubMed] [Google Scholar]
- Roura-Ferrer M, Sole L, Oliveras A, Villarroel A, Comes N, Felipe A. Targeting of Kv7.5 (KCNQ5)/KCNE channels to surface microdomains of cell membranes. Muscle Nerve. 2012;45:48–54. doi: 10.1002/mus.22231. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Sole L, Roura-Ferrer M, Perez-Verdaguer M, Oliveras A, Calvo M, Fernandez-Fernandez JM, Felipe A. KCNE4 suppresses Kv1.3 currents by modulating trafficking, surface expression and channel gating. J Cell Sci. 2009;122:3738–3748. doi: 10.1242/jcs.056689. [DOI] [PubMed] [Google Scholar]
- Strauss O, Rosenthal R, Dey D, Beninde J, Wollmann G, Thieme H, Wiederholt M. Effects of protein kinase C on delayed rectifier K+ channel regulation by tyrosine kinase in rat retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:1645–1654. [PubMed] [Google Scholar]
- Strutz-Seebohm N, Seebohm G, Fedorenko O, Baltaev R, Engel J, Knirsch M, Lang F. Functional coassembly of KCNQ4 with KCNE-beta- subunits in Xenopus oocytes. Cell Physiol Biochem. 2006;18:57–66. doi: 10.1159/000095158. [DOI] [PubMed] [Google Scholar]
- Takahira M, Hughes BA. Isolated bovine retinal pigment epithelial cells express delayed rectifier type and M-type K+ currents. Am J Physiol. 1997;273:C790–803. doi: 10.1152/ajpcell.1997.273.3.C790. [DOI] [PubMed] [Google Scholar]
- Tao Q, Rafuse PE, Kelly ME. Potassium currents in cultured rabbit retinal pigment epithelial cells. J Membr Biol. 1994;141:123–138. doi: 10.1007/BF00238246. [DOI] [PubMed] [Google Scholar]
- Trumble WR, Sutko JL, Reeves JP. ATP-dependent calcium transport in cardiac sarcolemmal membrane vesicles. Life Sci. 1980;27:207–214. doi: 10.1016/0024-3205(80)90139-3. [DOI] [PubMed] [Google Scholar]
- Um SY, McDonald TV. Differential association between HERG and KCNE1 or KCNE2. PLoS One. 2007;2:e933. doi: 10.1371/journal.pone.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R, Lui GM, Steinberg RH. Whole-cell K+ currents in fresh and cultured cells of the human and monkey retinal pigment epithelium. J Physiol. 1993;465:121–147. doi: 10.1113/jphysiol.1993.sp019669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Lenzner S, Berger W, Rosenthal R, Karl MO, Strauss O. Voltage-dependent ion channels in the mouse RPE: comparison with Norrie disease mice. Vision Res. 2006;46:688–698. doi: 10.1016/j.visres.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Wu H, Cowing JA, Michaelides M, Wilkie SE, Jeffery G, Jenkins SA, Mester V, Bird AC, Robson AG, Holder GE, Moore AT, Hunt DM, Webster AR. Mutations in the gene KCNV2 encoding a voltage-gated potassium channel subunit cause “cone dystrophy with supernormal rod electroretinogram” in humans. Am J Hum Genet. 2006;79:574–579. doi: 10.1086/507568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Macleish PR. Ionic conductances of monkey solitary cone inner segments. J Neurophysiol. 1994;71:656–665. doi: 10.1152/jn.1994.71.2.656. [DOI] [PubMed] [Google Scholar]
- Yang D, Pan A, Swaminathan A, Kumar G, Hughes BA. Expression and localization of the inwardly rectifying potassium channel Kir7.1 in native bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3178–3185. doi: 10.1167/iovs.02-1189. [DOI] [PubMed] [Google Scholar]
- Yang D, Swaminathan A, Zhang X, Hughes BA. Expression of Kir7.1 and a novel Kir7.1 splice variant in native human retinal pigment epithelium. Exp Eye Res. 2008;86:81–91. doi: 10.1016/j.exer.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El-Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R. MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res. 2001;88:E84–87. doi: 10.1161/hh1201.093511. [DOI] [PubMed] [Google Scholar]
- Zhang M, Jiang M, Tseng GN. minK-related peptide 1 associates with Kv4.2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel? Circ Res. 2001;88:1012–1019. doi: 10.1161/hh1001.090839. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hughes BA. Expression of KCNQ and KCNE proteins in bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50 ARVO E-Abstract 2923. [Google Scholar]
- Zhang X, Yang D, Hughes BA. KCNQ5/Kv7.5 potassium channel expression and subcellular localization in primate retinal pigment epithelium and neural retina. Am J Physiol Cell Physiol. 2011;301:C1017–1026. doi: 10.1152/ajpcell.00185.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]