Abstract
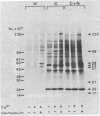
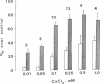
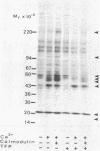
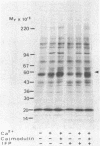
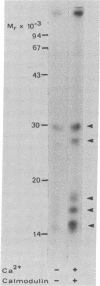
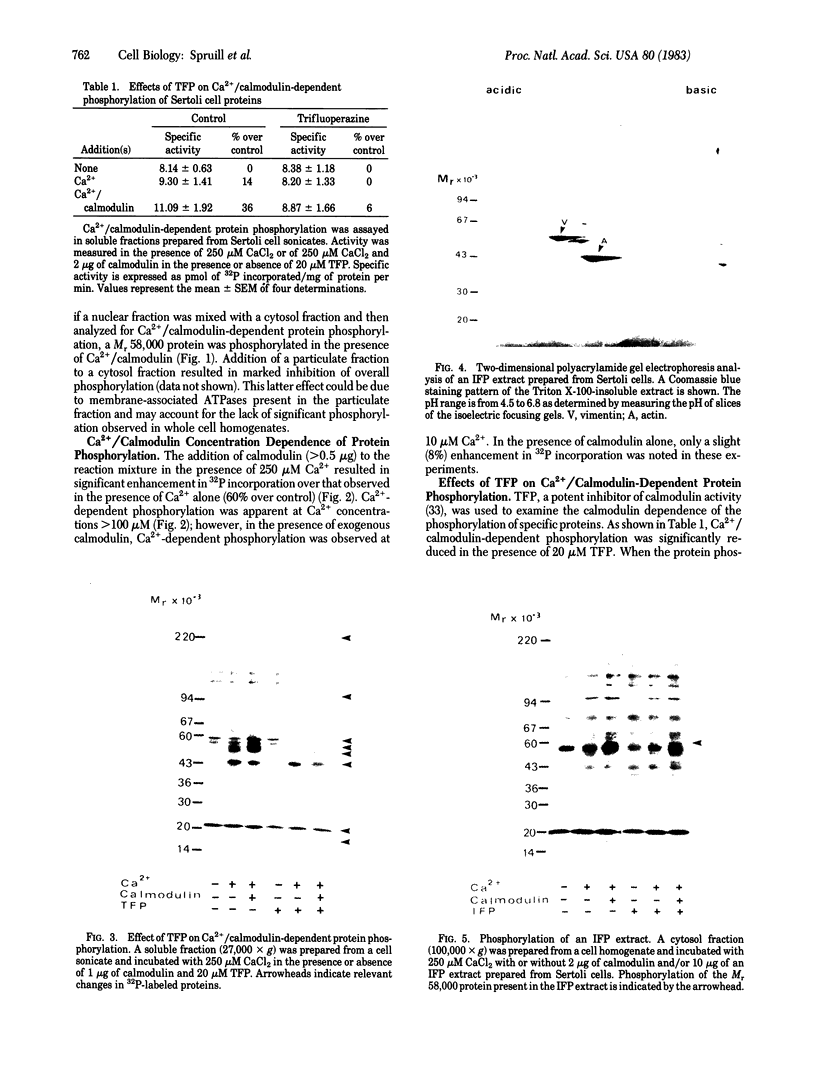
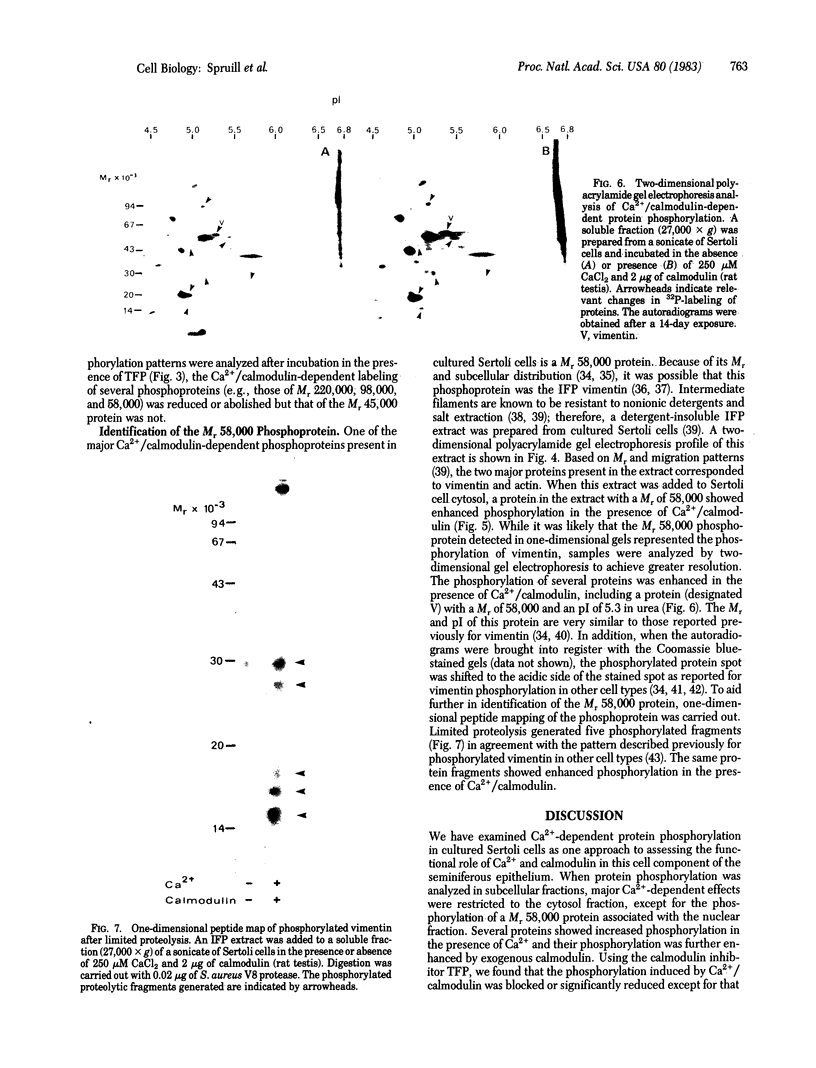
Ca2+-dependent protein phosphorylation and the role of calmodulin in this process was investigated in subcellular fractions of primary cultures of rat Sertoli cells. Significant Ca2+/calmodulin-dependent protein phosphorylation in Sertoli cells was restricted to the cytosol fraction. The calmodulin dependence of these effects was confirmed by using the calmodulin inhibitor trifluoperazine. One of the Ca2+/calmodulin-dependent phosphoproteins was identified as the intermediate filament protein vimentin, based on the following criteria: (i) migration pattern in two-dimensional polyacrylamide gels, (ii) Ca2+/calmodulin-dependent phosphorylation of a 58-kilodalton protein present in detergent-insoluble intermediate filament protein extract of Sertoli cells, and (iii) peptide mapping of the phosphoprotein. These data support a role for Ca2+/calmodulin-dependent protein phosphorylation in the modulation of Sertoli cell cytoskeletal components.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browning E. T., Sanders M. M. Vimentin: a phosphoprotein under hormonal regulation. J Cell Biol. 1981 Sep;90(3):803–808. doi: 10.1083/jcb.90.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CABAUD P. G., WROBLEWSKI F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Pathol. 1958 Sep;30(3):234–236. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M. Phosphorylation of the 10-nm filament protein from Chinese hamster ovary cells. J Biol Chem. 1979 Jul 25;254(14):6203–6206. [PubMed] [Google Scholar]
- Cabral F., Willingham M. C., Gottesman M. M. Ultrastructural localization to 10 nm filaments of an insoluble 58K protein in cultured fibroblasts. J Histochem Cytochem. 1980 Jul;28(7):653–662. doi: 10.1177/28.7.7391554. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Lin Y. M. Purification and characterization of cyclic 3',5'-nucleotide phosphodiesterase from bovine brain. Methods Enzymol. 1974;38:223–239. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- DIXON T. F., PURDOM M. Serum 5-nucleotidase. J Clin Pathol. 1954 Nov;7(4):341–343. doi: 10.1136/jcp.7.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePhilip R. M., Kierszenbaum A. L. Hormonal regulation of protein synthesis, secretion, and phosphorylation in cultured rat Sertoli cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6551–6555. doi: 10.1073/pnas.79.21.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedman J. R., Brinkley B. R., Means A. R. Regulation of microfilaments and microtubules by calcium and cyclic AMP. Adv Cyclic Nucleotide Res. 1979;11:131–174. [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardino J. J., Harrison D. E., Christie M. R., Gagliardino E. E., Ashcroft S. J. Evidence for the participation of calmodulin in stimulus-secretion coupling in the pancreatic beta-cell. Biochem J. 1980 Dec 15;192(3):919–927. doi: 10.1042/bj1920919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Bell P. B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Bretscher A., Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6458–6462. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. Function of a calmodulin in postsynaptic densities. II. Presence of a calmodulin-activatable protein kinase activity. J Cell Biol. 1981 Jun;89(3):440–448. doi: 10.1083/jcb.89.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Browning E. T. Norepinephrine-dependent protein phosphorylation in intact C-6 glioma cells. Analysis by two-dimensional gel electrophoresis. Mol Pharmacol. 1980 Nov;18(3):427–437. [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Li A. P., Hsie A. W. Studies on adenosine 3',5'-phosphate-binding and adenosine 3',5-phosphate-dependent protein kinase activities associated with subcellular fractions of Chinese hamster ovary cells. Biochim Biophys Acta. 1977 Nov 7;500(1):140–151. doi: 10.1016/0304-4165(77)90054-x. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin in endocrine cells and its multiple roles in hormone action. Mol Cell Endocrinol. 1980 Sep;19(3):215–227. doi: 10.1016/0303-7207(80)90052-0. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R., Tash J. S., Tindall D. J., van Sickle M., Welsh M. J. Regulation of the testis sertoli cell by follicle stimulating hormone. Annu Rev Physiol. 1980;42:59–70. doi: 10.1146/annurev.ph.42.030180.000423. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Purification of the intermediate filament protein vimentin from Ehrlich ascites tumor cells. J Biol Chem. 1982 May 25;257(10):5536–5543. [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan J. P., Dunn L. A., Lovenberg W. Calcium-regulated phosphorylation in synaptosomal cytosol: dependence on calmodulin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5812–5816. doi: 10.1073/pnas.77.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. M., Balzer D. R., Jr, Lazarides E. Phosphorylation of subunit proteins of intermediate filaments from chicken muscle and nonmuscle cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):819–823. doi: 10.1073/pnas.76.2.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. M., Gard D. L., Lazarides E. Phosphorylation of intermediate filament proteins by cAMP-dependent protein kinases. Cell. 1981 Jan;23(1):135–143. doi: 10.1016/0092-8674(81)90278-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Prashad N., Rosenberg R. N., Wischmeyer B., Ulrich C., Sparkman D. Induction of adenosine 3',5'-monophosphate binding proteins by N6,O2'-dibutyryladenosine 3',5'-monophosphate in mouse neuroblastoma cells. Analysis by two-dimensional gel electrophoresis. Biochemistry. 1979 Jun 26;18(13):2717–2725. doi: 10.1021/bi00580a005. [DOI] [PubMed] [Google Scholar]
- Schubart U. K., Erlichman J., Fleischer N. The role of calmodulin in the regulation of protein phosphorylation and insulin release in hamster insulinoma cells. J Biol Chem. 1980 May 10;255(9):4120–4124. [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W., Theoharides T. C., Alper S. L., Douglas W. W., Greengard P. Calcium-dependent protein phosphorylation during secretion by exocytosis in the mast cell. Nature. 1978 Sep 28;275(5678):329–331. doi: 10.1038/275329a0. [DOI] [PubMed] [Google Scholar]
- Spruill W. A., White M. G., Steiner A. L., Tres L. L., Kierszenbaum A. L. Temporal sequence of cell shape changes in cultured rat sertoli cells after experimental elevation of intracellular cAMP. Exp Cell Res. 1981 Jan;131(1):131–148. doi: 10.1016/0014-4827(81)90414-6. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Intermediate filament systems are collapsed onto the nuclear surface after isolation of nuclei from tissue culture cells. Exp Cell Res. 1982 Mar;138(1):207–214. doi: 10.1016/0014-4827(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Van den Berg G. B., Van Berkel T. J., Koster J. F. The role of Ca2+ and cyclic AMP in the phosphorylation of rat-liver soluble proteins by endogenous protein kinases. Eur J Biochem. 1980 Dec;113(1):131–140. doi: 10.1111/j.1432-1033.1980.tb06147.x. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Waisman D. M. Calmodulin and its role in the second-messenger system. Curr Top Cell Regul. 1979;15:47–107. doi: 10.1016/b978-0-12-152815-7.50006-5. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Most of the Ca2+-dependent endogenous phosphorylation of rat brain cytosol proteins requires Ca2+-dependent regulation protein. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1172–1178. doi: 10.1016/0006-291x(79)91160-4. [DOI] [PubMed] [Google Scholar]
- Yerna M. J., Dabrowska R., Hartshorne D. J., Goldman R. D. Calcium-sensitive regulation of actin-myosin interactions in baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):184–188. doi: 10.1073/pnas.76.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]