Abstract
There are currently no effective therapeutic agents for traumatic brain injury (TBI), but drug treatments for TBI can be developed by validation of new drug targets and demonstration that compounds directed to such targets are efficacious in TBI animal models using a clinically relevant route of drug administration. The cysteine protease, cathepsin B, has been implicated in mediating TBI, but it has not been validated by gene knockout (KO) studies. Therefore, this investigation evaluated mice with deletion of the cathepsin B gene receiving controlled cortical impact TBI trauma. Results indicated that KO of the cathepsin B gene resulted in amelioration of TBI, shown by significant improvement in motor dysfunction, reduced brain lesion volume, greater neuronal density in brain, and lack of increased proapoptotic Bax levels. Notably, oral administration of the small-molecule cysteine protease inhibitor, E64d, immediately after TBI resulted in recovery of TBI-mediated motor dysfunction and reduced the increase in cathepsin B activity induced by TBI. E64d outcomes were as effective as cathepsin B gene deletion for improving TBI. E64d treatment was effective even when administered 8 h after injury, indicating a clinically plausible time period for acute therapeutic intervention. These data demonstrate that a cysteine protease inhibitor can be orally efficacious in a TBI animal model when administered at a clinically relevant time point post-trauma, and that E64d-mediated improvement of TBI is primarily the result of inhibition of cathepsin B activity. These results validate cathepsin B as a new TBI therapeutic target.
Key words: : cathepsin B, cysteine protease inhibitor, motor function, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is defined as “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”1 TBI is a major health concern with at least 1.7 million Americans injured each year2 and with young people being the largest patient group3 and military combat casualties adding to those numbers.4 Although much is known about the cellular and molecular mechanisms of TBI,5–8 that knowledge has not, as yet, resulted in a pharmacological therapeutic that improves outcome.9,10 However, a therapeutic may be developed by identifying a molecular target that affects outcome and demonstrating that a compound that affects the target is efficacious in a TBI animal model using protocols that can translate into the clinic.
The cysteine protease, cathepsin B, has been suggested to be such a target because TBI causes a substantial increase in brain cathepsin B protein levels and activity, which correlates with neuronal cell death and behavioral dysfunction.11,12 However, direct validation of the functional role of cathepsin B by gene knockout (KO) studies is lacking; such validation is essential for consideration of cathepsin B as a TBI drug target. Further, parallel comparisons of cathepsin B gene KO and protease inhibitor treatment are necessary for understanding the relative importance of inhibiting cathepsin B versus other proteases in improving outcomes because of the potential for off-target effects by the inhibitors. For these reasons, this study examined the effects of cathepsin B gene KO and chemical inhibition of cathepsin B by E64d (by oral administration) on improving TBI in an animal model.
Cathepsin B KO mice are healthy and generally indistinguishable from wild-type (WT) mice.13 Here, we show, for the first time, that deleting the cathepsin B gene results in substantial improvement in TBI neuromotor dysfunction and brain pathology in an open-skull controlled cortical impact (CCI) TBI mouse model. E64d (aka EST, loxistatin, and L-trans-epoxysuccinyl[OEt]-Leu-3methylbutlamide) is a potent small-molecule inhibitor of papain-like cathepsin cysteine proteases and calcium-activated neutral proteases, which includes cathepsin B and calpain-1.14 For the first time, we demonstrate efficacy of post-trauma E64d treatment, by oral administration, of WT mice in a time frame that has the potential for clinical translation. Further, E64d was administered to cathepsin B KO mice to evaluate the relative importance of inhibiting cathepsin B versus other cysteine proteases in improving outcomes in TBI. The data validate cathepsin B as an important TBI therapeutic target, demonstrate proof of principle that E64d has clinical utility for TBI treatment, and indicate that E64d ameliorates TBI primarily by inhibiting cathepsin B activity.
Methods
Animals
Cathepsin B–deficient (KO) mice were obtained from Professor Christoph Peters at the Albert Ludwig University (Freiburg, Germany), and C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were maintained on a C57BL/6 background. Polymerase chain reaction analysis was utilized to determine the genotype of animals, as previously described.15 All experimental mice were male. Mice were 15–18 weeks of age for these studies and weighed between 20 and 27 g. During experimentation, the animal was placed onto a heated pad and core body temperature was monitored and maintained at 38±0.2°C. Mice were given free access to food and water before and during the experiment. Animal studies were conducted according to regulations by the National Institutes of Health and as approved by the institutional animal care and use committee at the Medical University of South Carolina (Charleston, SC) and Ralph H. Johnson VA Medical Center (Charleston, SC).
TBI injury
The CCI mouse model was used to deliver a controlled, consistent injury to all animals. The procedure requires surgical removal and replacement of a portion of the skullcap so as to be able to directly injure the brain. Adult mice were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) administered by intraperitoneal injection of 0.02 mL of solution per gram of body weight. Degree of anesthesia was assessed by testing corneal reflexes and toe-pinch reflexes. During anesthesia, mice were placed in a stereotaxic frame, with the head positioned in the horizontal plane and the nose bar set at −5. Using sterile procedures (site was shaved and cleaned with Wescodyne before surgical manipulation), the head was positioned in the horizontal plane with the nose bar set at zero. After a mid-line incision exposing the skull, a 3-mm craniotomy was made on the right side of the brain lateral to the sagittal suture and centered between lambda and bregma. The skull at the craniotomy site was removed without disrupting the underlying dura. The exposed cortex was injured using a CCI device (Precision Systems and Instrumentation, Fairfax Station, VA) armed with a 2-mm tip. The CCI device was set at a velocity of 3.5 m/sec and to a depth of 2 mm. After injury, a small, round cover glass was placed on the skull to cover the injury site (EMS no. 72296-05, 5 mm diameter, #1.5 thickness; Electron Microscopy Sciences, Hatfield, PA). The glass was not glued down, and within a few hours, the glass was covered by matrix that prevented movement. The cover glass was autoclaved before use. This helped prevent tearing and sticking of the scalp to the injury site. The cover glass did not affect swelling. The skin was then stapled together, and the animals were placed on a heating pad to recover. Total surgical time was less than 45 min. The survival rate for this procedure was approximately 88%. Animals were returned to their home cages after recovery from anesthesia and monitored daily for any signs of discomfort or other abnormal behavior, and none were observed.
E64d
E64d was manufactured by American Life Science Pharmaceuticals (San Diego, CA) using methods developed and modified from those previously described.16 Purity was determined to be 99% by reverse-phase high-pressure liquid chromatography, which was confirmed with a qualified standard. The identity of the compound was confirmed by proton nuclear magnetic resonance, melting point, elemental analysis, and liquid chromatography/mass spectroscopy (data not shown). It is noted that E64d can also be obtained from commercial sources (e.g., Calbiochem, San Diego, CA).
E64d formulation and oral administration
E64d was formulated at the indicated doses in saline with 1.5% Me2SO (Sigma-Aldrich, St. Louis, MO), and controls received carrier solution (saline with 1.5% Me2SO). A single oral dose of E64d (100 μL) was administered by gavage to animals at different times after TBI. For the initial studies, the dose of the compound was 10 mg/kg immediately after trauma (5–10 min). For dose-response studies, doses were from 0 to 20 mg/kg immediately after trauma. For time-course studies, 10 mg/kg was used and treatment was given at 0–24 h after TBI. Our previous dose-response studies found that a dose of 10 mg/kg of E64d given orally by gavage to guinea pigs for 1 week maximally reduced brain cathepsin B activity by 90%, as assayed upon completion of that dose regimen.17 Thus, there was a reasonable expectation that a single oral administration of that dose would effectively reduce brain cathepsin B in the TBI mouse model, and therefore that dose was used for time-course studies of brain cathepsin B inhibition, behavior, initial brain lesion volume, and initial neuronal cell density. Upon completion of these experiments, dose-response studies using a single oral administration (given 30 min after the time of TBI) were conducted to evaluate effect of lower and higher E64d doses on brain lesion volume and neuronal cell density.
Cathepsin B activity assay
Brain cathepsin B activity was measured 2 h after dosing with E64d (10 mg/kg) or vehicle immediately after trauma (within 5–10 min) using a fluorometric assay kit, as described by the manufacturer (ab65300; Abcam, Cambridge, MA). The 2-h time point was selected for analysis because that is approximately the time of maximum serum-drug concentration after oral administration of E64d,18 and therefore analysis at that time point would likely show maximal inhibition. Briefly, tissues were washed twice in ice-cold phosphate-buffered saline and then homogenized in extraction buffer, as described by the manufacturer. After 10-min incubation on ice, the extract was centrifuged at 10,000g for 5 min and 50 μL of supernatant was mixed with an equal volume of 2×reaction buffer and 2 μL of substrate in a 96-well microplate. Plates were kept in the dark at 37°C for 1 h, and fluorescence was recorded using a FLUOstar Optima plate reader (BMG LABTECH GmbH, Ortenberg, Germany). Protein concentration was determined by the bicinchoninic acid assay method (Bio-Rad, Hercules, CA). Cathepsin B activity was measured in triplicate and was expressed as fluorescent units/mg of protein. For determination of enzyme activity, we isolated the region of trauma for analysis.
Cathepsin B and Bax western blot analyses
Brain cathepsin B, Bax, and actin (control) protein levels were determined 24 h after sham operation or TBI, because cathepsin B and Bax protein levels are known to be significantly increased at that time post-TBI.11 Animals were treated with vehicle or E64d (10 mg/kg) immediately after TBI. Relative levels of cathepsin B, Bax, and actin in the supernatant fraction from the brain extract were determined by western blot (polyclonal antibodies: Cathepsin B, sc-13985; Bax, sc-526; β-actin, sc-130657; Santa Cruz Biotechnology, Santa Cruz, CA), as described previously.15 Relative intensities of western blot bands were assessed by densitometry in triplicate for each sample. Densitometric analysis was done using IQTL software (GE Life Sciences, Piscataway, NJ). For protein studies, the entire lesional area was harvested for western blot analysis. In control or sham animals, a similar region was harvested.
Rotarod assay
An automated rotarod (San Diego Instruments, San Diego, CA) was used to assess the effects on vestibulomotor function of cathepsin B KO and E64d therapeutic intervention (10 mg/kg) given immediately after trauma (5–10 min).19 On the day preceding injury, mice underwent two consecutive conditioning trials at a set rotational speed (16 revolutions per min) for 60 sec, followed by three additional trials with accelerating rotational speeds. The average time to fall from the rotating cylinder in the latter three trials was recorded as baseline latency. After injury, mice underwent consecutive daily testing with three trials of accelerating rotational speed (intertrial interval of 15 min). Average latency to fall from the rod was recorded. Mice unable to grasp the rotating rod were given a latency of 0 sec. The experimenter was blinded as to the groups of animals.
Brain lesion volume analysis
Histological analysis occurred on the last day of the behavioral assay (day 7 post-TBI mice) to allow correlation of behavior with pathology. Mice were anesthetized and transcardially perfused with saline and 10% buffered formalin phosphate solution containing 4% paraformaldehyde (PFA). Brains were removed, postfixed in PFA for 24 h, and protected in 30% sucrose. Frozen brain sections (30 μm) were cut on a cryostat and mounted onto glass slides. Every fourth section was processed for immunohistochemical analysis beginning from a random start point. Thirty-micron sections were stained with hematoxylin and eosin (H&E), dehydrated, and mounted for analysis. Lesion volume in each section was determined with a computer-assisted image analysis system, consisting of a Power Macintosh computer (Apple Inc., Cupertino, CA) equipped with a QuickCapture frame grabber card, Hitachi CCD camera (Hitachi Kokusai Electric Inc., Tokyo, Japan) mounted on an Olympus microscope (Olympus, Tokyo, Japan), and camera stand. Images were captured and total area of damage was determined over sections using National Institutes of Health (NIH) Image Analysis Software (v. 1.55; NIH, Bethesda, MD) conducted by a single operator blinded to treatment status for analyses of all measurements.
Neuronal cell density determination
Cell counting was conducted using a Nikon Eclipse E800 light microscope (Nikon Imaging Japan Inc., Tokyo, Japan) interfaced with the StereoInvestigator software package (MicroBrightField, Williston, VT). Neuronal density was calculated as the number of stained neurons per volume of hippocampus determined using the optical fractionator method, as previously described.20,21 Before counting, all slides were coded to avoid bias. As determined by StereoInvestigator, three sections (40 μm) spaced eight sections apart along the hippocampal formation were selected by systematic random sampling. On each section, the hippocampal area was delineated. Only cells within the counting frame or overlapping the right or superior border of the counting frame, and for which nuclei came into focus while focusing down through the dissector height, were counted. Tissue generated and H&E labeled for the brain lesion volume analysis was used for the neuronal cell-density determination.
Statistic analysis
Experiments consisted of 10 mice in each group. Statistical analyses and data graphing were conducted utilizing computer software designed for scientific data analysis (GraphPad Prism 4; GraphPad Software Inc., La Jolla, CA). Quantitative data were displayed as the mean with standard error of the mean and differences among means determined by one-way analysis of variance (p<0.0001) and either a Bonferroni's or Dunnett's multiple comparison test for the data of Figures 1 through 6 and of Figures 7 and 8, respectively (p<0.05).
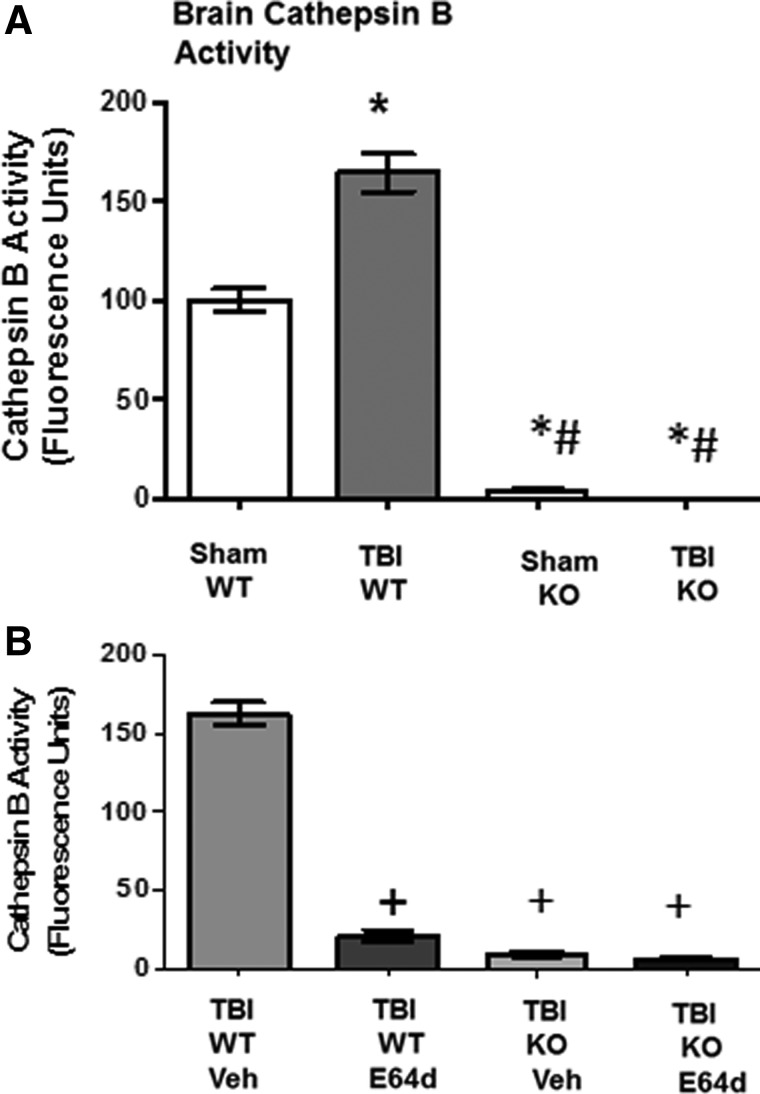
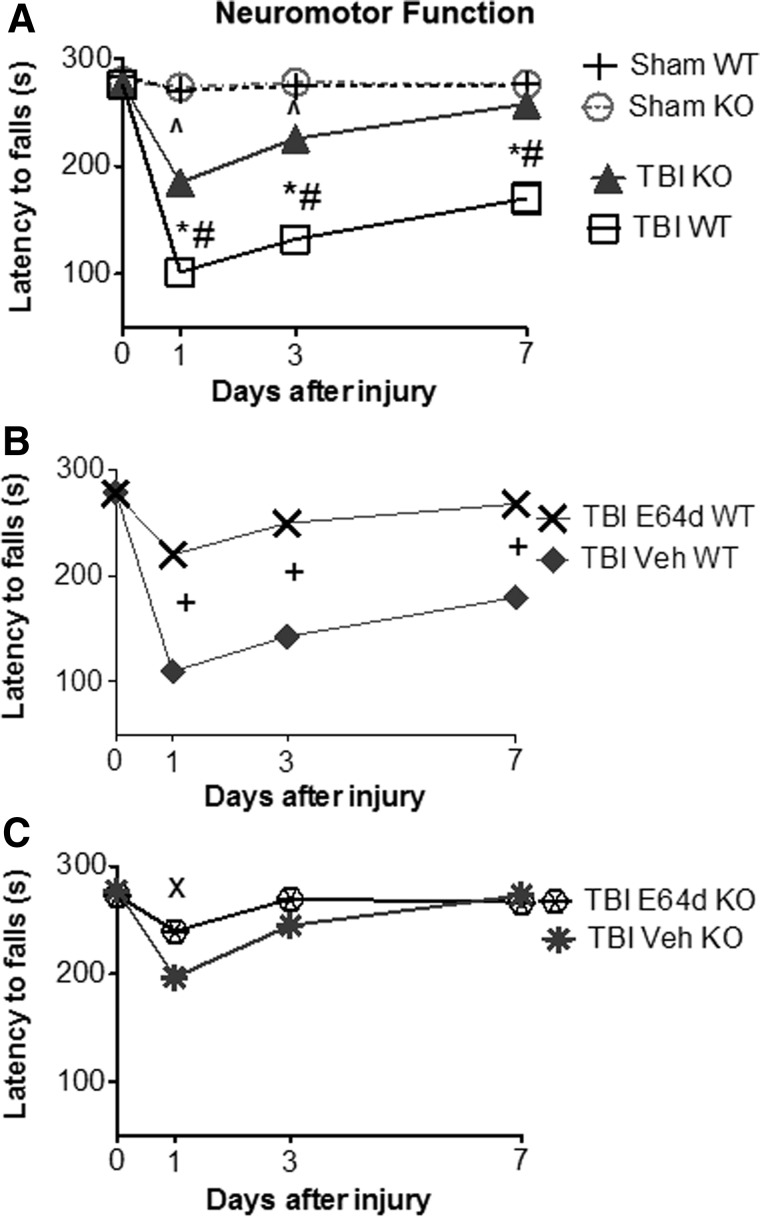
FIG. 1.
TBI increases brain cathepsin B activity, which is eliminated by cathepsin B gene KO and is reduced by oral E64d administration. Brain cathepsin B activities were determined 2 h after sham operation and TBI trauma, expressed as fluorescent units per mg protein. (A) Brain cathepsin B activities for sham and TBI conditions in WT and cathepsin B KO animals are shown. Mean cathepsin B activities differ among groups, with TBI WT mice having a higher brain cathepsin B activity than all other groups (*) and sham KO and TBI KO mice having a lower brain cathepsin B activity than sham WT mice (#). (B) Brain cathepsin B activities of TBI WT and cathepsin B KO mice treated orally with either E64d (10 mg/kg) or Veh immediately after TBI trauma (5–10 min) are shown. Mean cathepsin B activities differ among groups, with E64d TBI WT, vehicle-treated TBI KO, and E64d-treated KO mice having lower brain cathepsin B activities than TBI WT vehicle-treated mice (+) (N=10 animals/group; significant differences, p<0.05, Bonferroni's multiple comparison test). TBI, traumatic brain injury; KO, knockout; WT, wild type; Veh, vehicle.
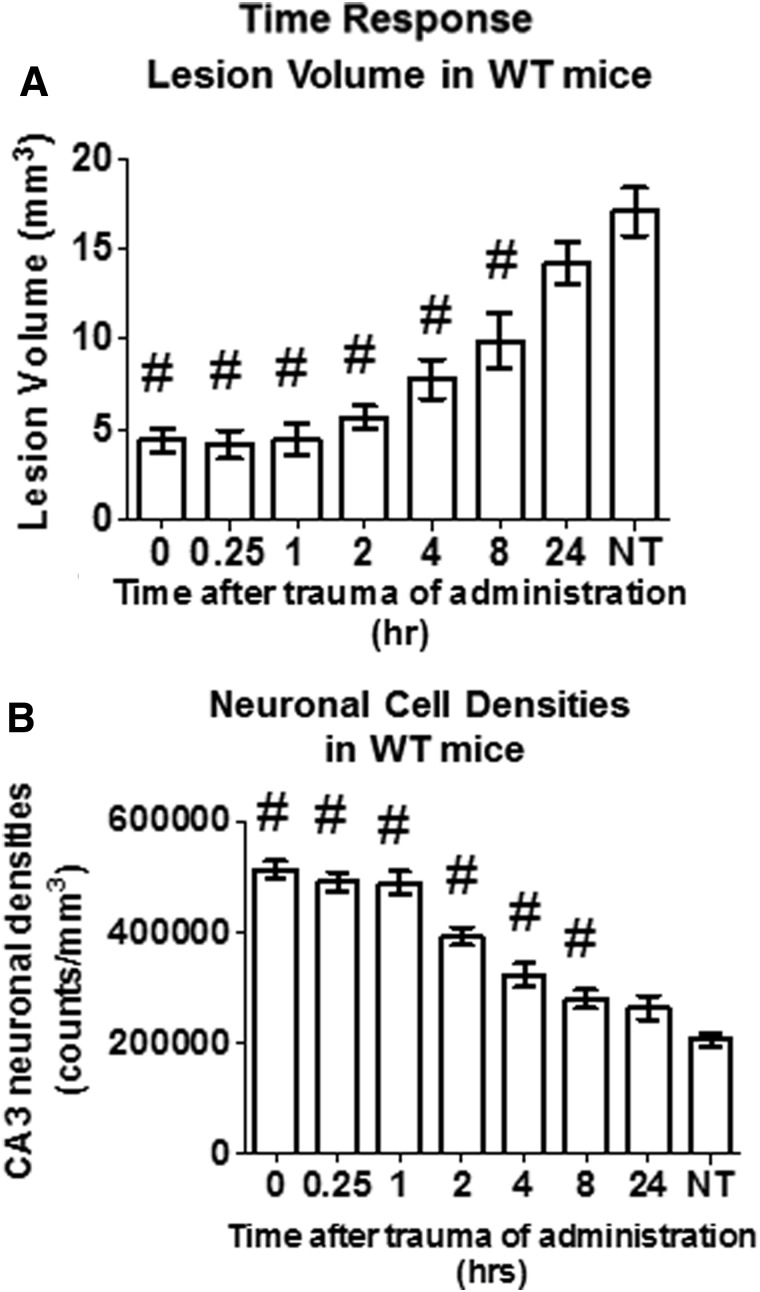
FIG. 7.
Effectiveness of a single oral E64d dose is inversely proportional to the time of administration after TBI trauma and is effective up to 8 h post-trauma at reducing brain lesion volume and increasing brain neuronal cell density. Effects of administering a single oral E64d dose (10 mg/kg) to WT mice at various times after trauma on brain lesion volume and CA3 hippocampal neuronal cell density are shown. Experimental animals and those that received no treatment (NT) were sacrificed 7 days post-trauma and compared. (A) Brain lesion volumes for groups receiving E64d at increasing times after trauma and for the NT group are shown. Lesion volume was significantly reduced for all groups receiving E64d up to 8 h post-trauma, relative to that of the NT group (#). (B) Neuronal cell densities for groups receiving E64d at increasing times after trauma and for the NT group are shown. Density was significantly increased for all groups receiving E64d up to 8 h post-trauma, relative to that of the NT group (#) (N=10 animals/group; significant differences, p<0.05, Dunnett's multiple comparison test). WT, wild type.
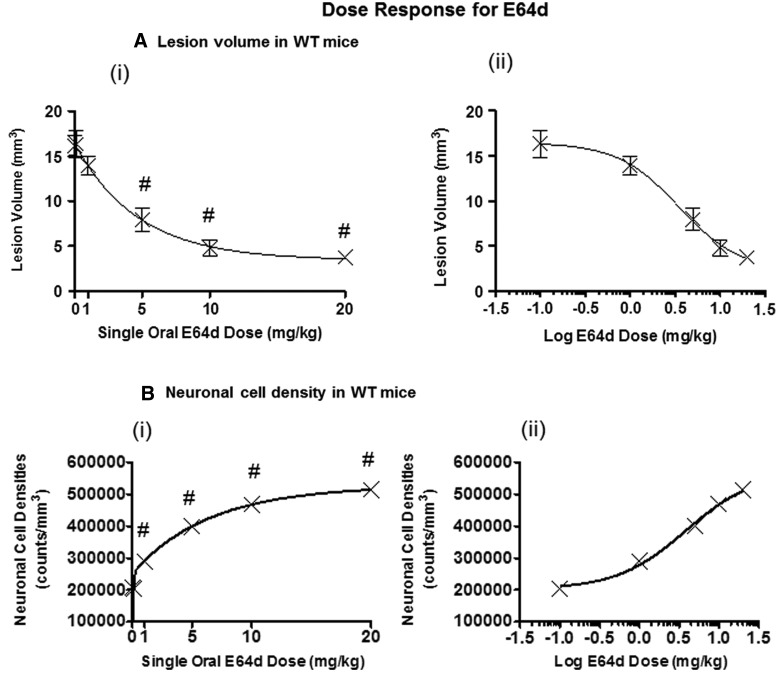
FIG. 8.
Oral E64d dose-response effects on brain lesion volume and neuronal cell density. Effects of a single oral E64d dose given to WT mice 0.5 h after trauma on brain lesion volume and CA3 hippocampal neuronal cell density were determined. (A) (i) Brain lesion volume versus the E64d dose is plotted on a linear-linear scale and fitted to a decreasing biphasic exponential curve. A steep reduction in brain lesion volume occurred for 0- to 5-mg/kg doses, followed by a gradual reduction for 5- to 20-mg/kg doses. Doses at or above 5 mg/kg resulted in significant reductions in brain lesion volumes, relative to no dose (#). (A) (ii) Brain lesion volume versus the E64d dose is plotted on a linear-logarithmic scale and fitted to a decreasing sigmoidal curve. (B) (i) Neuronal density versus E64d dose is plotted on a linear-linear scale and fitted to an increasing biphasic exponential scale. A steep increase in neuronal cell density occurred for 0- to 5-mg/kg doses, followed by a gradual reduction for 5- to 20-mg/kg doses is shown. Doses at or above 1 mg/kg resulted in significant increase in neuronal cell densities, relative to no dose (#). (B) (ii) Neuronal cell density versus the E64d dose is plotted on a linear-logarithmic scale and fitted to an increasing sigmoidal curve (N=10 animals/group; significant differences, p<0.05, Dunnett's multiple comparison test). WT, wild type.
Results
Brain cathepsin B activity
The mouse CCI model of TBI was evaluated for brain cathepsin B activity 2 h after trauma in cathepsin B KO and WT mice. Traumatized WT mice had a brain cathepsin B activity of 164.2±9.5 fluorescence units (FUs; per mg protein), which was a significant 64% higher than the 100.1±5.5 FUs of sham WT mice (Fig. 1A). Cathepsin B gene deletion reduced brain cathepsin B activities to background levels with TBI KO and sham KO mice having 0.0 and 3.6±0.6 FUs, respectively, which were a significant 100% lower than TBI WT and sham WT levels.
To assess the effects of E64d, brain cathepsin B activity was measured in TBI WT and KO mice 2 h after E64d (10 mg/g, orally) or vehicle administration, which occurred immediately after trauma (5–10 min; Fig. 1B). E64d TBI WT mice had a brain cathepsin B activity of 20.7±3.6 FUs, which was a significant 88% lower than the 162.4±7.3 FUs of vehicle-treated TBI WT mice. E64d- and vehicle-treated TBI KO mice had 5.8±1.2 and 9.2±1.9 FUs, respectively, which were a significant 95% lower than vehicle treated TBI WT mice, but not different from each other or TBI KO mice.
Brain cathepsin B protein level
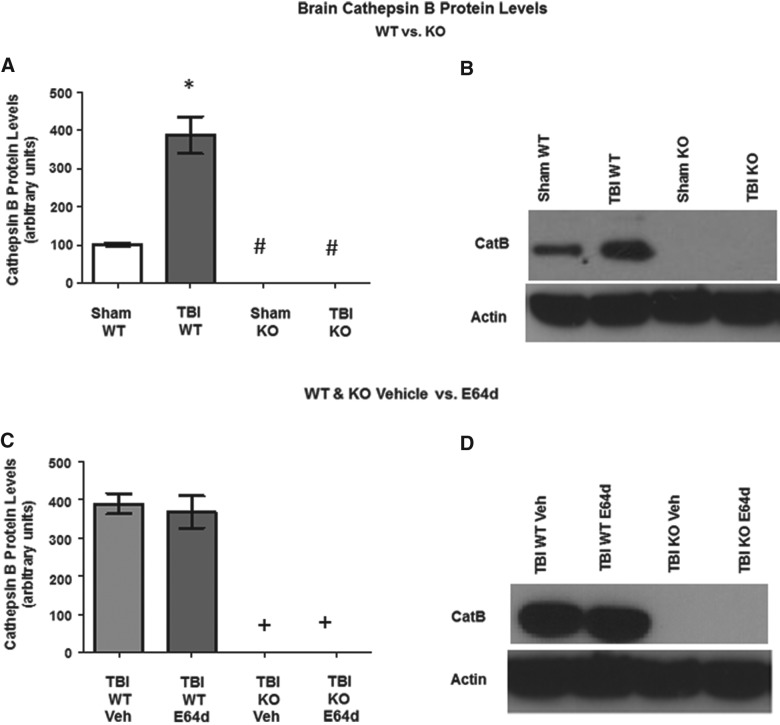
Cathepsin B protein levels were determined 24 h after TBI trauma (Fig. 2). TBI WT mice had brain cathepsin B levels of 387.8±48.7 relative units (RUs), which was a significant 380% higher level, as compared to the 102.3±3.8 RUs for sham WT mice (Fig. 2A). As shown by exemplary western blots, cathepsin B KO resulted in the absence of cathepsin B protein in TBI or sham animals (Fig. 2B).
FIG. 2.
TBI increases brain cathepsin B protein levels, which is eliminated by cathepsin B gene KO and is not affected by E64d treatment. Brain cathepsin B protein levels were determined 24 h after sham operation or TBI trauma. (A) Quantitative analysis of brain cathepsin B protein levels of sham and TBI conditions in WT and cathepsin B KO mice are shown. TBI WT mice had higher mean cathepsin B levels than all other groups (*), and sham KO and TBI KO mice had lower cathepsin B levels than sham WT mice (#). (B) An exemplary Western blot of brain cathepsin B protein levels of sham and TBI conditions in WT and KO mice is shown. (C) Quantitative comparisons of brain cathepsin B protein levels of the TBI condition in WT and KO mice treated with vehicle or E64d (10 mg/kg) immediately after trauma. TBI KO mice treated with either vehicle or E64d had lower levels than TBI WT mice treated with either vehicle or E64d (+). (D) An exemplary Western blot of brain cathepsin B levels of the TBI condition in WT and KO mice, treated with vehicle or E64d (N=10 animals/group; significant differences, p<0.05, Bonferroni's multiple comparison test). TBI, traumatic brain injury; KO, knockout; WT, wild type; Veh, vehicle.
WT mice treated immediately after trauma with E64d (10 mg/kg) or vehicle had brain cathepsin B protein levels of 367.9±41.8 and 389±26.9 RUs, respectively, which were not significantly different from each other (Fig. 2C). Cathepsin B protein was absent in cathepsin B KO mice (with E64d or vehicle treatment), illustrated by exemplary western blots (Fig. 2D).
Neurological motor function
Neuromotor function of mice after TBI was determined using the rotarod assay by measuring the length of time animals were able to stay on a rotating rod before falling off (latency to fall time); a shorter time reflected poorer neuromotor function. Latency times to fall were measured just before trauma on day 0 (before injury) and on days 1, 3, and 7 after injury (Fig. 3). At day 0, all groups had equivalent latency to fall times with the mean value for all groups being 277.5±1.5 sec, showing that all groups began the experiment having the same ability (Fig. 3A). Sham WT and sham KO mice had equivalent latency times within and between all days, with the mean for both groups on all days being 276.2±1.4 sec, which demonstrates that deletion of the cathepsin B gene did not affect performance and that the ability of nontraumatized WT and KO sham controls remained constant throughout the experiment (Fig. 3A).
FIG. 3.
TBI causes severe neuromotor dysfunction, which is improved by cathepsin B gene KO and by oral E64d treatment. Neuromotor dysfunction was assessed at different time points during the week after TBI trauma using the rotarod assay by measuring latency to fall time, with a shorter time reflecting a greater dysfunction. (A) Latency to fall times for sham and TBI conditions in WT and cathepsin B KO mice are shown. At day 0 (pretrauma), there was no difference in performance among groups. At all days post-trauma, there was no difference between sham WT and sham KO mice. In contrast, there were significant differences in latency to fall times among groups on days 1, 3, and 7 post-trauma. TBI WT mice had shorter times than for sham WT mice (*) on days 1, 3, and 7 post-trauma, showing that TBI caused significant impairment and a failure to recover within the measured time period. TBI KO mice had shorter times than sham KO mice on days 1 and 3 post-trauma (^), but by day 7, TBI KO mice regained the function of the sham animals and thus had recovered within the measured time period. Importantly, cathepsin B KO mice had longer times than TBI WT mice on days 1, 3, and 7 post-trauma (#), showing that cathepsin B gene KO reduced the severity of motor dysfunction on all days post-trauma. (B) Latency to fall times for TBI WT mice treated with E64 (10 mg/kg) or Veh immediately after trauma are shown. E64d treatment of TBI WT mice resulted in significantly improved motor performances on all days post-trauma, compared to Veh-treated animals (+). TBI WT Veh-treated and TBI WT mice (from panel A) had equivalent performances. (C) Latency to fall times for TBI KO mice treated with E64d (10 mg/kg) or Veh immediately after trauma are shown. E64d treatment of TBI KO mice resulted in longer latency time on day 1, relative to Veh treatment (×), but on days 3 and 7, there was no significant difference in times for E64d- and Veh-treated animals. No significant difference was observed in mean performances on all days between TBI KO Veh-treated mice and TBI KO mice (from panel A) or between the TBI KO E64d-treated and TBI WT E64d-treated mice (from panel B) (N=10 animals/group; significant differences, p<0.05, Bonferroni's multiple comparison test). TBI, traumatic brain injury; KO, knockout; WT, wild type; Veh, vehicle.
The neuromotor function of TBI WT and KO mice are shown in Figure 3A. On day 1 post-trauma, WT and KO mice had latency times of 102.1±8.6 and 185.2±9.7 sec, respectively, which were 63 and 33% shorter times, respectively, than sham controls with the TBI WT mice, having a significant 30% shorter time than the TBI KO mice. Thus, whereas both TBI groups had poorer neuromotor function, relative to controls, TBI WT mice had significantly poorer performance than the TBI KO mice. On day 3 post-TBI, WT and KO mice latency times were 132.3±11.0 and 225.6±4.3 sec, respectively, which were 56 and 18% shorter times, respectively, than sham controls, with TBI WT mice having a 38% shorter time than TBI KO animals. Again, both groups performed significantly worse than controls, with TBI WT mice performing worse than TBI KO mice. By day 7, latency times for TBI WT and TBI KO mice were 170.2±14.2 and 257.7±3.8 sec, respectively, with TBI WT mice having a 38% shorter time than control, whereas TBI KO mice had an equivalent time to that of the control. Thus, the performance of TBI WT and TBI KO mice were poorer and the same, respectively, as the sham control. In summary, TBI KO mice suffered less impairment and recovered faster than TBI WT mice.
Neuromotor performance of TBI WT mice treated with E64d (10 mg/kg) or vehicle immediately after TBI trauma (5–10 min) is shown in Figure 3B. In brief, E64d-treated TBI WT animals had significantly less neuromotor dysfunction and recovered faster than did vehicle-treated TBI WT mice. Specifically, on day 1, latency times were 109.8±10.2 and 219.6±8.2 sec for TBI WT vehicle and E64d mice, respectively, with TBI WT vehicle-treated mice having a significant 50% shorter time than that of the E64d-treated animals. Latency times on day 3 for TBI WT vehicle- and E64d-treated mice were 142.7±10.8 and 249.2±6.0 sec, respectively, with vehicle treatment resulting in a 43% shorter time than that of E64d treatment and, on day 7, TBI WT vehicle- and E64d-treated mice had latency times of 179.5±11.2 and 267.3±5.1 sec, respectively, with vehicle-treated animals having a 33% shorter time than E64d-treated animals. Thus, E64d treatment significantly improved performance on all days post-trauma.
Motor performance was also evaluated for vehicle- and E64d-treated TBI KO mice using the methods described above (Fig. 3C). In general, E64d treatment caused no improvement in rotarod performance, relative to vehicle, in these animals, except on day 1 post-trauma, when vehicle- and E64d-treated KO animals had latency times of 197.0±6.5 and 239.6±8.6 sec, respectively, which showed that vehicle-treated animals had a significant 17% shorter time than E64d-treated TBI KO animals. On day 3, vehicle- and E64d-treated TBI KO mice had latency times of 244.8±5.4 and 269.5±5.7 sec, respectively, which were not statistically different. Similarly, on day 7, there was no difference between vehicle and E64d TBI KO mice latency times, which were 273.1±4.7 and 266.5±6.0 sec, respectively.
Importantly, these data show that deleting the cathepsin B gene or administering E64d significantly reduced the severity of neuromotor dysfunction resulting from TBI (at 1 and 3 days post-TBI), with nearly full recovery of motor function at 7 days after TBI trauma. Results also show that at day 1 post-trauma, E64d treatment of TBI KO mice caused a slight improvement in motor performance, relative to TBI vehicle-treated KO mice.
Brain lesion volume
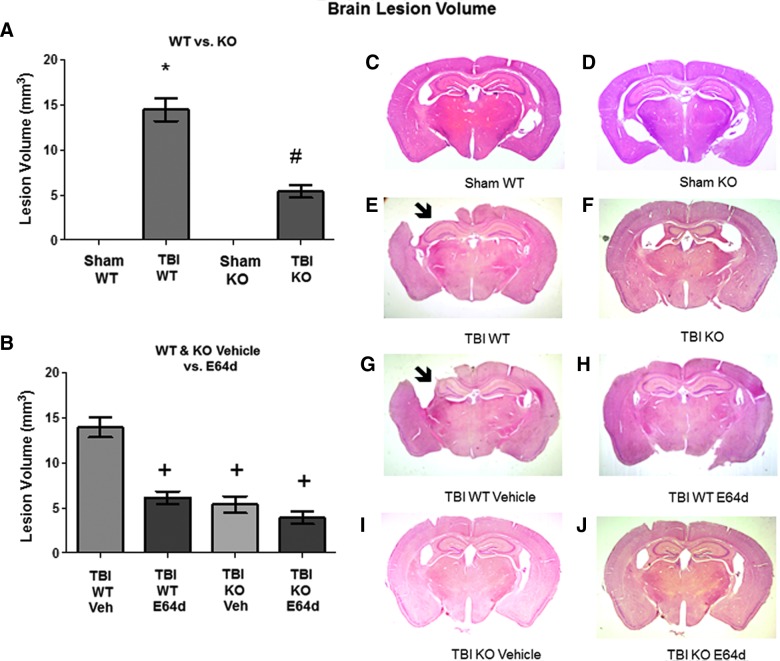
Animals tested in the rotarod assay described above were sacrificed on the seventh day post-trauma, and their brains were histopathologically evaluated for brain lesion volume with quantitation (Fig. 4). Brain lesions were absent in sham WT and sham KO mice, but TBI WT and TBI KO mice had lesion volumes of 14.5±1.3 and 5.4±0.7 cubic millimeters (mm3), respectively (Fig. 4A). Importantly, TBI KO mice had lesion volumes that were approximately one third that of TBI WT mice. These data indicate that the increased cathepsin B activity in TBI WT mice (from Fig. 1) resulted in substantial brain tissue loss at the cortical impact site, because eliminating that activity by deleting the cathepsin B gene greatly reduced that loss.
FIG. 4.
Brain lesion caused by TBI is reduced by cathepsin B gene KO and by oral E64d treatment. Animals tested in the rotarod assay were sacrificed at day 7 post-TBI and their were brains analyzed by quantitative histology to determine brain lesion volume at the impact site. (A) Quantitative brain lesion volumes for sham and TBI conditions in WT and KO mice are shown. TBI WT mice had a significantly larger lesion volume than sham WT, sham KO, and TBI KO mice (*), which had a larger volume than either sham groups (#). (B) Quantitative brain lesion volumes for the TBI condition in WT and KO mice treated with vehicle or E64d are shown. E64d-treated TBI WT, Veh-treated TBI KO, and E64d-treated TBI KO mice had significantly lower lesion volumes than Veh-treated TBI WT mice (+). There was no difference in volumes between Veh-treated or untreated TBI WT mice or between E64d- and Veh-treated TBI KO mice and untreated TBI KO mice (A). (C–J) Exemplary brain micrographs from each group are shown. Note the absence of brain tissue in the upper-left quadrant of (E) and (G), which are from TBI WT and TBI WT vehicle-treated animals, respectively (arrow; full picture width, 10 mm; N=10 animals/group; significant differences, p<0.05, Bonferroni's multiple comparison test). TBI, traumatic brain injury; KO, knockout; WT, wild type; Veh, vehicle. Color image is available online at www.liebertpub.com/neu
In the TBI condition, brain lesion volumes of WT mice treated with E64d or vehicle were 6.1±0.7 and 13.9±1.1 mm3, respectively (Fig. 4B). Notably, E64d-treated WT mice had less than half the lesion of vehicle-treated WT mice, indicating that E64d provided protection from TBI lesion. The E64d reduced brain lesion volume correlated with the reduced brain cathepsin B activity caused by E64d treatment post-trauma (data of Fig. 1). Treatment of TBI KO mice with E64d or vehicle resulted in lesion volumes of 3.9±0.7 and 5.4±0.9 mm3, respectively, which were not significantly different from each other or from the lesion volume of untreated KO animals. These results showed that reduction in brain lesion volume occurring with E64d treatment was primarily the result of inhibition of cathepsin B activity.
Exemplary histopathological brain micrographs from each of the animal groups tested are shown in Figure 4C–J. Of note is the large cortical brain area missing in the upper-left quadrant of TBI WT and TBI vehicle-treated WT mouse brain sections (Fig. 4E and 4G, respectively), demonstrating that the TBI impact caused severe brain lesions. In contrast, micrographs from TBI KO mice and TBI E64d-treated WT mice show relatively intact cortical brain tissue with minimal lesion in the corresponding upper-left quadrant (Fig. 4F and 4H).
Hippocampal neuronal cell density
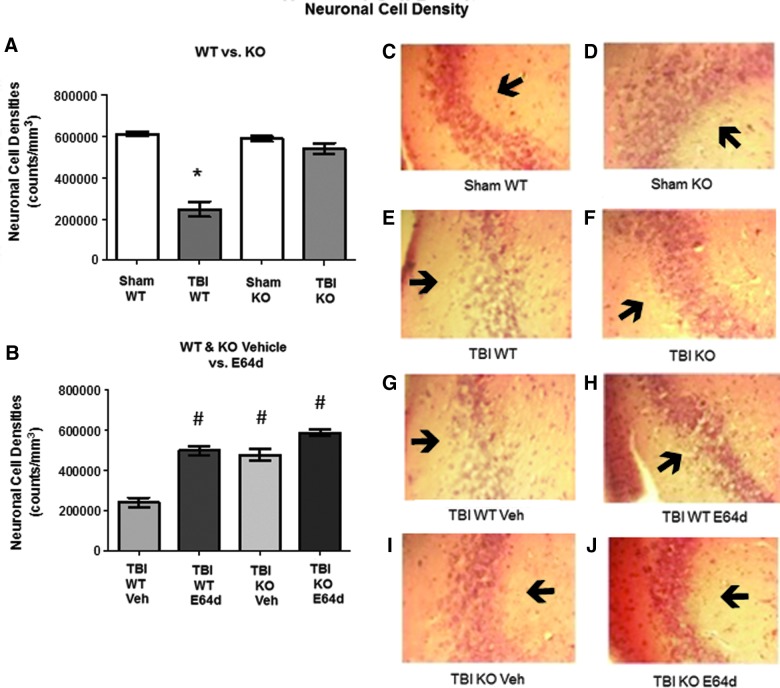
Mouse brains from animals tested in the rotarod assays described above were evaluated for neuronal cell densities in the CA3 region of the hippocampus by quantitative histopathology image analysis (Fig. 5). It has previously been shown that the hippocampus is particularly vulnerable to TBI and that this may contribute to subsequent cognitive impairment.22,23 Therefore, we used the CA3 region as a marker for neuronal cell loss correlated to behavioral changes. The prelesional cortex was not chosen because of the massive damage and difficulty in counting the cells.
FIG. 5.
TBI-mediated reduction of hippocampal neuronal densities in WT mice is restored by cathepsin B gene KO and by oral E64d treatment. Brains of animals analyzed in the rotarod assay were also evaluated at day 7 post-TBI for neuronal cell densities in the CA3 region of the hippocampus by quantitative histological methods. (A) Quantitative neuronal cell densities for sham and TBI conditions in WT and KO mice are shown. TBI WT mice had significantly less neuronal cell density than all other groups (*). (B) Quantitative neuronal cell densities for the TBI condition of WT and KO mice treated with E64d or Veh treatment are shown. E64d-treated WT and KO mice and vehicle-treated KO mice had increased neuronal cell densities, compared to Veh-treated TBI WT mice (#). E64d- and vehicle-treated TBI KO mice and E64d-treated WT mice had the same densities as the untreated TBI KO and sham KO and WT mice (A). (C–J) Exemplary micrographs of the hippocampal region from each group are shown. Dark spots within micrographs are neurons, which are aggregated together to form the dark band of the CA3 layer (arrow). Note that the density of the layer is much less in the TBI WT micrograph than in the TBI KO micrograph (E and F) and is much less in the TBI WT Veh vs. TBI WT E64d micrographs (I and H) (full picture width, 0.1 mm; N=10 animals/group; significant differences, p<0.05, Bonferroni's multiple comparison test). TBI, traumatic brain injury; KO, knockout; WT, wild type; Veh, vehicle. Color image is available online at www.liebertpub.com/neu
Neuronal cell densities from sham WT, sham KO, and TBI KO mice were not significantly different from each other, with mean values of 609,797±10,648, 586,744±12,457, and 540,444±25,831 counts/mm3, respectively (Fig. 5A). In contrast, TBI WT mice had a neuronal cell density of 246,968±36,759 counts/mm3, which was a significant 60% lower than sham WT cell density (Fig. 5A). Thus, these data show that cathepsin B gene KO protects against neuronal loss induced by TBI because eliminating the increased cathepsin B activity caused by TBI (data of Fig. 1) prevented that loss.
In the TBI condition, E64d or vehicle treatment of WT mice resulted in neuronal cell densities of 495,143±21,667 and 239,906±23,507 counts/ mm3, respectively, which showed that E64d treatment resulted in a significant 50% increase in density, relative to vehicle-treated animals (Fig. 5B). Densities for E64d-treated WT mice and TBI KO mice (vehicle) were not statistically different. These data and that of Figure 1 show that the beneficial effect of E64d on increasing neuronal cell density correlates with the inhibition of cathepsin B activity.
TBI KO mice treated with E64d or vehicle had neuronal densities of 583,669±15,498 and 474,713±29,178 counts/ mm3, respectively, with E64d- and vehicle-treated KO animals having significantly higher cell densities than vehicle-treated TBI WT mice (Fig. 5B). There was no difference between densities of E64d-treated KO mice and untreated KO mice or E64d-treated WT mice.
Exemplary micrographs of the CA3 hippocampus from the experimental groups are shown in Figure 5C–J. Note within the micrographs the dark bands (arrows), which are composed of CA3 neurons, and that the bands are less dense in TBI WT and TBI vehicle-treated WT mice (Fig. 5E and 5G, respectively) than in TBI KO and TBI E64d-treated WT mice (Fig. 5F and 5H, respectively).
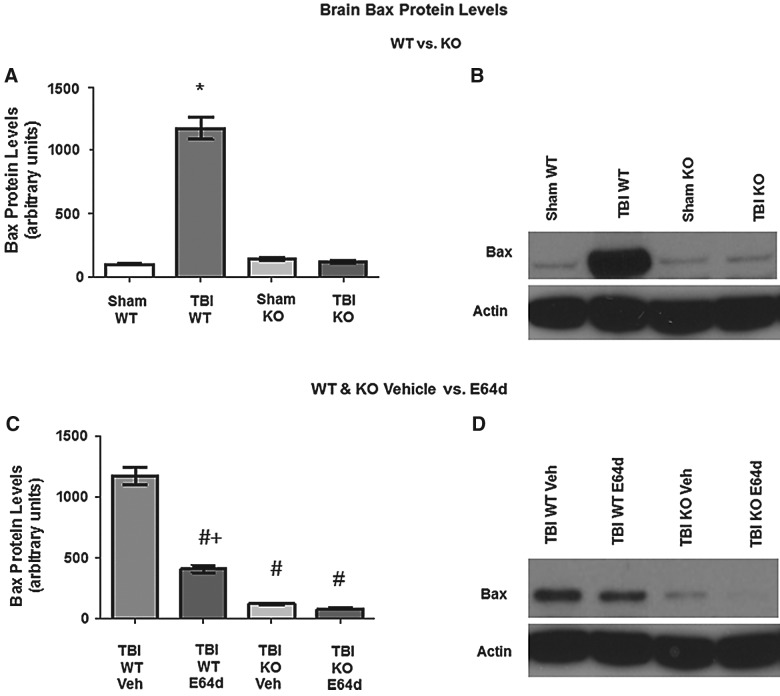
Bax protein levels
Brains of animals evaluated for cathepsin B activity and protein levels (Fig. 2) were also evaluated for proapoptotic Bax protein 24 h after trauma (Fig. 6). Western blot data showed that sham WT, sham KO, and TBI KO mice had equivalent Bax levels of 98.5±6.2, 139.2±9.9, and 113.0±12.6 RUs, respectively, whereas TBI WT mice had Bax expression levels that were a significant 10-fold greater than that, at 1175±87.5 RUs (Fig. 6A). Importantly, KO of the cathepsin B gene blocked TBI induction of Bax protein (Fig. 6B). These findings and that of Figure 1 indicate that the increased Bax expression caused by TBI is mediated by cathepsin B activity, because TBI induction of Bax was blocked by cathepsin B gene KO.
FIG. 6.
TBI-induced elevation of Bax levels in brains is reduced by cathepsin B KO and E64d treatment. Brains of animals used to measure cathepsin B protein levels (Fig. 2) were also used to measure proapoptotic Bax protein levels by quantitative densitometry of western blots. (A) Brain Bax levels of sham and TBI conditions in WT and KO mice are shown. Mean levels were different among the groups, with TBI WT mice having increased Bax levels relative to all other groups (*). (B) An exemplary western blot of brain Bax protein levels from sham and TBI conditions in WT and KO mice is shown. (C) Brain Bax levels are shown for the TBI condition in WT and KO mice treated with E64d (10 mg/kg) or Veh immediately after trauma. Mean levels in TBI WT Veh-treated mice were higher level than all other groups (#), indicating that E64d and cathepsin B KO reduced the TBI-induced increase in Bax levels. E64d TBI WT mice had higher levels than either treatment of the KO mice (+). (E64d- or Veh-treated TBI KO mice had the same densities as untreated sham and TBI KO mice and sham WT mice [A].) (D) An exemplary western blot is shown of the TBI condition in WT and KO mice, with E64d or Veh treatment (N=10 animals/group; significant differences, p<0.05, Bonferroni's multiple comparison test). TBI, traumatic brain injury; KO, knockout; WT, wild type; Veh, vehicle.
E64d and vehicle treatment of TBI WT mice resulted in Bax levels of 410±31.1 and 1172±72.2 RUs, respectively, showing that E64d treatment reduced Bax levels by a significant 65%, relative to vehicle treatment (Fig. 6C). In TBI KO mice, E64d and vehicle treatment resulted in Bax levels of 84.8±8.5 and 122.0±9.3 RUs, respectively, which were approximately 90% less than the Bax level of vehicle-treated TBI WT mice and approximately 75% less than the level of E64d treated TBI WT mice. Thus, E64d significantly reduced TBI-induced elevation of Bax levels in WT mice.
Time course of E64d effect on brain lesion volume and neuronal cell density
Effects of a single oral E64d dose (10 mg/kg) given at progressively longer times after TBI were evaluated. Treated and untreated animals were sacrificed 7 days postinjury and compared. Our previous dose-response studies found that an oral 10-mg/kg E64d dose given by gavage to guinea pigs for 1 week maximally reduced brain cathepsin B activity by 90%, as assayed upon completion of the dose regimen.17 Thus, a 10-mg/kg E64d dose was selected because there was a reasonable expectation that a single administration of that dose may reduce brain cathepsin B in the TBI model.
The effect on brain lesion volume of progressively delaying E64d treatment post-trauma is shown in Figure 7A. A delay in dosing generally resulted in increased lesion volumes. Significant reductions in lesion volume occurred in animals treated with E64d up to 8 h post-trauma, compared to the no-treatment (NT) TBI animals, with the dosing at 8 h after trauma resulting in a brain lesion volume of 9.9±1.5 mm3, which was a significant 40% lower than the 17.1±1.3 mm3 volume of NT TBI mice.
The effect on neuronal cell densities as a function of time after TBI injury of E64d administration is shown in Figure 7B. Neuronal cell densities were significantly increased with E64d treatment, relative to the NT TBI group, for administration up to 8 h post-trauma, with administration of a dose at 8 h resulting in a density of 280,096±16,595 counts/ mm3, which was a significant 35% higher than the 207,811±11,483 counts/ mm3 of NT TBI animals.
E64d dose response for brain lesion volume and neuronal cell density
The dose-response relationship and minimum effective single oral E64d dose was determined for brain lesion volume and CA3 hippocampal neuronal cell density. E64d was administered to WT mice 0.5 h after injury, and animals were sacrificed 7 days later. This protocol was selected because a maximum effect was obtained with this regimen in the time-course studies. A dose range of 0–20 mg/kg was studied because it encompassed doses that maximally inhibited brain cathepsin B activity, because a single oral 10-mg/kg E64d dose resulted in a 90% reduction in brain cathepsin B activity (Fig. 1B). Thus, the selected dose range predicted that a dose-response effect would be observed.
The decreasing lesion volumes as a function of increasing doses of E64d (oral administration) after trauma to WT mice are shown in Figure 8A(i) and 8A(ii). The relationship of increasing E64d doses providing decreased lesion volumes can be fitted to a two-phase exponential decay curve (Fig. 8A(i)). The curve has a sharp downward slope for doses between 0 and approximately 5 mg/kg body weight, followed by a more-gradual reduction for doses between 5 and 20 mg/kg, which produced significant reductions in brain lesion, relative to no treatment. These data indicated a minimal effective dose for this paradigm of approximately 5 mg/kg. Figure 8A(ii) is a logarithmic plot of the E64d dose versus lesion volume and shows that the data fit a decreasing sigmoidal curve.
The increasing neuronal cell densities resulting from increasing doses of E64d administered after trauma to WT mice is shown in Figure 8B(i) and 8B(ii). Figure 8B(i) shows a two-phase increasing exponential relationship, and that the minimum effective dose was approximately 1 mg/kg. The logarithmic dose response for an oral E64d dose to neuronal cell density is displayed in Figure 8B(ii) and shows an increasing sigmoidal relationship.
Discussion
The major new finding of this study is that deleting the cathepsin B gene alleviates the behavioral dysfunction and pathology of TBI, thus validating cathepsin B as a target for TBI drug development. Data showed that KO of the cathepsin B gene resulted in significant improvement in neuromotor dysfunction induced by TBI in the CCI mouse model at days 1 and 3 postinjury. By 7 days postinjury, cathepsin B KO mice showed full recovery of motor function, but WT mice still showed neuromotor dysfunction. Cathepsin B gene deletion prevented the TBI-induced increase in cathepsin B activity that participates in neuromotor dysfunction. Increased cathepsin B activity was also a significant cause of brain pathology, illustrated by comparison of WT and cathepsin B KO mice, showing that traumatized WT mice had three times the brain lesion volume and less than half the CA3 hippocampal cell density, compared to TBI cathepsin B KO animals. These cathepsin B KO animal studies unambiguously illustrate the central role of cathepsin B in the neuromuscular dysfunction and brain pathology of TBI.
Notably, oral treatment of TBI WT mice with the cysteine protease inhibitor, E64d, post-trauma produced substantially the same outcomes as deleting the cathepsin B gene for improving neuromotor dysfunction and brain pathology post-trauma. This result is all the more impressive given that only a single E64d dose was administered. Moreover, treatment affected outcomes when E64d was administered up to 8 h after trauma, which is within the time frame for clinical intervention.9 These data establish proof of principle that cysteine protease inhibitors have TBI therapeutic potential and provide strong motivation to develop therapeutic methods of using cysteine protease inhibitors to treat TBI.
Based on previous work, it was not at all obvious that a cathepsin B inhibitor treatment, which could translate into the clinic, would be efficacious in a TBI animal model. The closest previous studies showed that prophylactic intracerebroventricular (i.c.v.) infusion of E64d or CA074Me, which is a compound that also inhibits cathepsin B, to CCI TBI mice is efficacious.11,12 Neither prophylactic nor i.c.v. administration is a practical method for treating TBI. Moreover, because the cathepsin B inhibitors were administered directly to the brain, the compounds did not have to penetrate the blood–brain barrier and thus it was not clear, from those studies, whether a sufficient amount of E64c, which is the systemically active form of E64d, would reach the brain to be effective. As it turned out, oral administration of E64d was very effective at reducing brain cathepsin B (up to 90%), whereas i.c.v. administration of cathepsin B inhibitors resulted in substantially less brain cathepsin B inhibition (less than 20% inhibition). That is likely a result of the systemic dosing (by the oral route) causing the drug to penetrate cells throughout the brain, whereas i.c.v. drug distribution is limited by diffusion to cells at the surface of the ventricles.24 Another significant problem in developing a practical TBI treatment is in finding a cathepsin B inhibitor capable of sufficient brain cell penetration for effective target engagement. For example, intravenous (i.v.) administration of CA074, which is a selective cathepsin B inhibitor, in a spinal trauma model did not inhibit cathepsin B activity in the damaged tissue, likely because its charged nature did not allow it to penetrate into cells.24 An inability to reach the target proteases inside cells may also have been the case for the cathepsin B and calpain inhibitor, MDL28170, because i.v. administration of that compound to a TBI animal model did not inhibit endogenous brain calpain activity.25 Thus, the demonstration of E64d efficacy, when used in a clinically relevant manner, in a TBI model is a significant new result.
E64d is particularly well suited for clinical advancement because of its earlier safe use in humans. Originally developed in the 1980s for treatment of muscular dystrophy, E64d (also known as EST and Loxistatin) completed phase III trials, but did not advance as a drug because of insufficient efficacy for treating muscular dystrophy.26 Nonetheless, clinical data show that E64d could be safely administered to adult volunteers and pediatric patients.18,27,28 E64d has a very wide toxic-therapeutic window,29–34 no mutagenic effect,35 and wide dose window for any reproductive effects36–39 as well as good oral bioavailability.18,40–42 In those studies, an oral E64d dose of approximately 5 mg/kg was chronically administered to pediatric patients and the toxicity data suggest that higher oral doses may be tolerated, especially on a short-term basis. The single oral E64d doses of 1–10 mg/kg, shown here to improve behavior and pathology in mice, are within a dose range that can likely be safely administered to TBI patients. Thus, E64d, or an E64d derivative, has good potential for clinical advancement as a TBI therapeutic.
An interesting aspect of this study is the comparison of cathepsin B KO mice with or without E64d treatment. E64d treatment of TBI WT mice had essentially the same beneficial outcomes as E64d treatment of cathepsin B KO mice, showing that effects of E64d are primarily the result of inhibition of cathepsin B. To the best of our knowledge, this is the first evaluation of a protease inhibitor in an animal model lacking the protease gene targeted by the inhibitor, after inducing TBI. This strategy assessed the relative importance of E64d inhibition of the protease of interest, compared with other proteases, in improving TBI. E64d inhibits papain-like cathepsin cysteine proteases and calcium-activated neutral proteases14 and thus can have multiple protease targets. The similar effects observed in E64d, cathepsin B KO, and E64d-cathepsin B KO mice indicate that E64d likely improves TBI outcomes through inhibiting cathepsin B.
However, an exception was on day 1 post-trauma, when E64d treatment of cathepsin B KO mice resulted in a modest 20% shorter latency to fall time than occurred for cathepsin B KO mice in the rotarod assay. These data suggest that E64d may have some off-target effects during early post-trauma. A likely off-target candidate may be inhibition of the calpains, which are known TBI drug targets,43 because calpain-1 KO studies have validated as such.44 Brain calpain activity spikes within 24 h of trauma,45–47 and E64d administration has been shown to reduce calpain activity and provide neuroprotection after trauma.48,49 Thus, whereas the cathepsin B KO studies here show that E64d acts primarily by inhibition of cathepsin B, some additional benefits may occur in TBI treatment through E64d inhibition of other proteases, especially calpains.
A further benefit of inhibiting cathepsin B and calpain is that these proteases regulate matrix metallopeptidase-9 (MMP-9), which participates in TBI dysfunction.50 The role of MMP-9 in TBI is shown by MMP-9 gene KO studies, which results in improved motor function and reduced brain lesion volumes.50 Increased cathepsin B and calpain up-regulate MMP-9,51 and thus E64d treatment may also improve TBI outcomes through cathepsin B and calpain inhibition reducing MMP-9 activity, as occurs with E64d treatment in an ischemic animal model.52
Under normal conditions, cathepsin B is sequestered in lysosomes at mM concentrations and is not found in the cytosol. Though cathepsin B has maximum activity in the acidic environment of lysosomes, it can function with more modest activity in the neutral pH environment of the cytoplasm; to prevent that cytoplasmic activity, cytoplasmic stefins (also known as cystatins) inhibit cytoplasmic cathepsin B activity under normal conditions.53 However, upon injury, lysosomal membranes can be compromised or ruptured and large amounts of cathepsin B can be released into the cystosol, which can overwhelm the cystatin control and cause necrotic and apoptotic cell death.54 Ischemic injury has long been known to cause lysosomal cathepsin B leakage and brain neuronal cell death.55 Also, recent data show that TBI also causes lysosomal membrane permeability and cathepsin B leakage into the cytoplasm of brain neuronal cells.56 Moreover, cathepsin B leakage from lysosomes to the cytoplasm has been shown to occur in an in vitro neuronal cell system, which models TBI injury.57 Thus, TBI-induced lysosomal leakage of cathepsin B into the cytoplasm may be a key event in causing neuronal cell death.
Earlier inhibitor studies suggested that inhibiting cathepsin B may reduce necrotic cell death after TBI.11,12 This study provides direct evidence that cathepsin B KO animals have reduced brain lesion volume after TBI and thus validates cathepsin B as a key factor in causing TBI necrotic cell death.
Previous studies also suggested that inhibiting cathepsin B may reduce apoptotic cell death in TBI, because administration of cysteine protease inhibitors to TBI animals improved brain neuronal cell survival and reversed the TBI-induced reduction in antiapoptotic B-cell lymphoma 2 levels and reversed the increase in proapoptotic Bax levels, Bid cleavage, cytochrome c levels, and caspase3 activation.11,12 TBI KO mice of this study had no brain Bax expression and an insignificant loss of hippocampal neuronal cell density, and thus these data directly demonstrate that cathepsin B is essential for increases in proapoptotic cell death protein Bax and neuronal cell death resulting from TBI. The data are consistent with previous data showing that deleting the cathepsin B gene also reduced brain neuronal apoptotic cell death in a myoclonus epileptic mouse model.58 In addition, E64d treatment of WT mice resulted in reduced levels of brain Bax levels and increased neuronal cell density post-trauma, which confirms the findings of previous cathepsin B TBI-inhibitor studies.11,12 Thus, inhibiting brain cathepsin B is an important means for reducing proapoptotic Bax levels and improving neuronal cell survival after TBI.
Tumor necrosis factor alpha (TNF-α) is an apoptotic cell death ligand that is increased by TBI.59–61 Hepatocytes from cathepsin B KO mice or fibrosarcoma cells treated with cathepsin B inhibitors resist TNF-α-induced apoptotic death because cathepsin B lysosomal leakage into the cytoplasm is required for that death pathway to occur.62,63 Moreover, cathepsin B–deficient macrophages produce less TNF-α in response to lipopolysaccharide activation.64 Thus, reducing the elevated cathepsin B activity after TBI may result in neuroprotection, in part, by preventing TNF-α-induced apoptosis.
Interestingly, cathepsin B is not critical for the essential apoptosis that occurs in development because cathepsin B–deficient mice develop normally.13 That is thought to be because the closely related protease, cathepsin L, likely provides that function in development and that hypothesis is supported by the fact that cathepsin B and cathepsin L double KO mice exhibit selective neuronal vulnerability and fail to develop normally.65 On the other hand, cathepsin L does not seem to be able to provide the pathological function of cathepsin B, because cathepsin L did not do so in the cathepsin B KO mice.
E64d treatment does not cause neuronal pathology, even though it inhibits both cathepsin B and cathepsin L, probably because E64d only partially attenuates the activities of these two proteases and thus does not eliminate their functions. Moreover, E64d spares essential lysosomal protease activity because E64c, which is the biologically active form of E64d, concentrates in the cytoplasm and not in lysosomes.66 Because cell death results from cathepsin B leakage into the cytoplasm, the concentration of E64c in the cytoplasm makes E64d a uniquely well-suited compound for reducing cell death.
TBI patients are at increased risk of developing Alzheimer's disease (AD),67 and TBI often induces AD-like amyloid-β (Aβ) plaque shortly after injury and elevates Aβ in injured brain neurons long after the trauma has occurred.68,69 Our previous work in transgenic AD mice showed that deleting the cathepsin B gene or treating them with E64d (oral administration) causes major reductions in brain Aβ, brain amyloid plaque, and improves memory deficits.15,17,70,71 Thus, treating TBI patients with a cathepsin B inhibitor may also reduce the subsequent long-term risk of AD.
In summary, gene KO studies here establish that cathepsin B is an important new TBI drug target. The demonstration that a clinically viable protocol using E64d is efficacious in TBI animal models enables the development of E64d for TBI treatment. Initiation of E64d treatment at ∼8 h after TBI is clinically feasible to improve TBI brain damage. This study illustrates that E64d produces beneficial outcomes in TBI primarily through inhibiting cathepsin B.
Acknowledgments
The authors thank Christoph Peters, MD, of Albert Ludwig University (Freiburg, Germany) for providing the cathepsin B–deficient mice and Lawrence Marshall, MD, for reviewing the manuscript. This work was supported, in part, by NIH grants R44AG032784 (to American Life Science Pharmaceuticals; ALSP) and 5R01ES016774-02 (to M.S.K.) and VA Merit Review 1I01RX000331-01 (to M.S.K.).
Author Disclosure Statement
G.H., N.S., and M.P. have equity interests in ALSP. V.H. is the chair of ALSP's Scientific Advisory Board and holds equity in ALSP, and the relationship has been disclosed to the University of California, San Diego (San Diego, CA).
References
- 1.Menon D.K., Schwab K., Wright D.W., and Maas A.I. (2010). Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1637–1640 [DOI] [PubMed] [Google Scholar]
- 2.Faul M., Xu L.X., Wald M., and Coronado V. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Intjury Prevention and Control: Atlanta, GA [Google Scholar]
- 3.Rutland-Brown W., Langlois J.A., Thomas K.E., and Xi Y.L. (2006). Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 21, 544–548 [DOI] [PubMed] [Google Scholar]
- 4.Warden D. (2006). Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 21, 398–402 [DOI] [PubMed] [Google Scholar]
- 5.Park E., Bell J.D., and Baker A.J. (2008). Traumatic brain injury: can the consequences be stopped? CMAJ 178, 1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veenith T., Goon S., and Burnstein R.M. (2009). Molecular mechanisms of traumatic brain injury: the missing link in management. World J. Emerg. Surg. 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner C., and Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9 [DOI] [PubMed] [Google Scholar]
- 8.McAllister T.W. (2011). Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 13, 287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauchamp K., Mutlak H., Smith W.R., Shohami E., and Stahel P.F. (2008). Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 14, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R., Choi S.C., Clifton G.L., Contant C.F., Coplin W.M., Dietrich W.D., Ghajar J., Grady S.M., Grossman R.G., Hall E.D., Heetderks W., Hovda D.A., Jallo J., Katz R.L., Knoller N., Kochanek P.M., Maas A.I., Majde J., Marion D.W., Marmarou A., Marshall L.F., McIntosh T.K., Miller E., Mohberg N., Muizelaar J.P., Pitts L.H., Quinn P., Riesenfeld G., Robertson C.S., Strauss K.I., Teasdale G., Temkin N., Tuma R., Wade C., Walker M.D., Weinrich M., Whyte J., Wilberger J., Young A.B., and Yurkewicz L. (2002). Clinical trials in head injury. J. Neurotrauma 19, 503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo C.L., Chen X.P., Yang R., Sun Y.X., Li Q.Q., Bao H.J., Cao Q.Q., Ni H., Qin Z.H., and Tao L.Y. (2010). Cathepsin B contributes to traumatic brain injury-induced cell death through a mitochondria-mediated apoptotic pathway. J. Neurosci. Res. 88, 2847–2858 [DOI] [PubMed] [Google Scholar]
- 12.Yang R. (2010). E64d attenuating nerve cell plasmolemma permeability and neurological dysfunction through apoptotic and autophagic pathways after traumatic brain injury [Master's thesis]. Soochow University: Suzhou, China [Google Scholar]
- 13.Reinheckel T., Deussing J., Roth W., and Peters C. (2001). Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol. Chem. 382, 735–741 [DOI] [PubMed] [Google Scholar]
- 14.Tamai M., Matsumoto K., Omura S., Koyama I., Ozawa Y., and Hanada K. (1986). In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J. Pharmacobiodyn. 9, 672–677 [DOI] [PubMed] [Google Scholar]
- 15.Hook V.Y., Kindy M., Reinheckel T., Peters C., and Hook G. (2009). Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem. Biophys. Res. Commun. 386, 284–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamai M., Yokoo C., Murata M., Oguma K., Sota K., Sato E., and Kanaoka Y. (1987). Efficient synthetic method for ethyl (+)-(2S,3S)-3-[(S)-3-methyl- 1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarb oxylate (EST), a new inhibitor of cysteine proteinases. Chem. Pharm. Bull. (Tokyo) 35, 1098–1104 [PubMed] [Google Scholar]
- 17.Hook G., Hook V., and Kindy M. (2011). The cysteine protease inhibitor, E64d, reduces brain amyloid-beta and improves memory deficits in Alzheimer's disease animal models by inhibiting cathepsin B, but not BACE1, beta-secretase activity. J. Alzheimers Dis. 26, 387–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imahori K., Sugita H., Miyatake T., Ishihara T., Miyatani N., and Kuwabara T. (1985). Pharmacokinetics and safety of thiol protease inhibitor EST in patients with muscle disease. Rinsho Yakuri 16, 749–757 [Google Scholar]
- 19.Hamm R.J., Pike B.R., O'Dell D.M., Lyeth B.G., and Jenkins L.W. (1994). The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 11, 187–196 [DOI] [PubMed] [Google Scholar]
- 20.West M.J., Ostergaard K., Andreassen O.A., and Finsen B. (1996). Estimation of the number of somatostatin neurons in the striatum: an in situ hybridization study using the optical fractionator method. J. Comp. Neurol. 370, 11–22 [DOI] [PubMed] [Google Scholar]
- 21.West M.J., Slomianka L., and Gundersen H.J. (1991). Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497 [DOI] [PubMed] [Google Scholar]
- 22.Ariza M., Serra-Grabulosa J.M., Junque C., Ramirez B., Mataro M., Poca A., Bargallo N., and Sahuquillo J. (2006). Hippocampal head atrophy after traumatic brain injury. Neuropsychologia 44, 1956–1961 [DOI] [PubMed] [Google Scholar]
- 23.Hicks R.R., Smith D.H., Lowenstein D.H., Saint Marie R., and McIntosh T.K. (1993). Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J. Neurotrauma 10, 405–414 [DOI] [PubMed] [Google Scholar]
- 24.Ellis R.C. (2004). Characterization of cathepsin B mRNA and protein expression, enzymatic activity and cellular localization following contusion spinal cord injury in rats [Ph.D. dissertation]. University of Florida:,Gainesville, FL [Google Scholar]
- 25.Thompson S.N., Carrico K.M., Mustafa A.G., Bains M., and Hall E.D. (2010). A pharmacological analysis of the neuroprotective efficacy of the brain- and cell-permeable calpain inhibitor MDL-28170 in the mouse controlled cortical impact traumatic brain injury model. J. Neurotrauma 27, 2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoyoshi E. (1992). Therapeutic trials on progressive muscular dystrophy. Intern. Med. 31, 841–846 [DOI] [PubMed] [Google Scholar]
- 27.Miyahara T., Shimojo S., Toyohara K., Imai T., Miyajima M., Honda H., Kamegai M., Ohzeki M., and Kokatsu J. (1985). Clinical phase I trial of thiol protease inhibitor (report 2): safety and pharmacokinetics in continuous administration. Rinsho Yakuri 16, 537–546 [Google Scholar]
- 28.Miyahara T., Shimojo S., Toyohara K., Imai T., Miyajima M., Honda H., Kamegai M., Ohzeki M., and Kokatsu J. (1985). Phase I clinical trial of thiol protease inhibitor EST (report 1): safety and pharmacokinetics with single administration. Rinsho Yakuri 16, 357–365 [Google Scholar]
- 29.Ohshima T., Watanabe T., Nagato C., Kimura M., Tsuchida T., and Nakane S. (1986). Toxicological studies on ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report VI): chronic toxicity study in rats. Iyakuhin Kenkyu 17, 781–801 [Google Scholar]
- 30.Abe S., Aida S., and Nakane S. (1986). Toxicological studies on ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report X): effect on vision and hearing in rats. Iyakuhin Kenkyu 17, 826–834 [Google Scholar]
- 31.Kimura M., Yagi K., Fujinuma S., Tsuchida T., Tarumoto Y., Noda K., and Nakane S. (1986). Toxicological studies on ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (Report III) Subacute toxicity in rats. Iyakuhin Kenkyu 17, 744–767 [Google Scholar]
- 32.Setoyama K., Koike M., Abe S., Tsutsui Y., Tarumoto Y., and Nakane S. (1986). Toxicological studies of Ethyl (+)-(2S,3S)-3-[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report 1): acute toxicity studies of EST and metabolite and by-product of EST. Iyakuhin Kenkyu 17, 736–743 [Google Scholar]
- 33.Ueki S., Watanabe S., Fujiwara M., Shibata S., Shi-yu L., Iwazaki K., Ohta H., and Shimazoe T. (1986). Effect of cysteine proteinase inhibitor EST on central nervous system. Yakuri Chiryo 14, 725–735 [Google Scholar]
- 34.Tarumoto Y., Sakagawa T., Tsutsui Y., Kawanishi M., Kimura M., and Nakane S. (1986). Toxicological studies on ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report V): subacute toxicity in dogs. Iyakuhin Kenkyu 17, 768–780 [Google Scholar]
- 35.Yasui H., Goto H., Suzuki H., Sakai S., Takamura T., and Nakane S. (1986). Toxicological studies on ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report IX): mutagenicity study. Iyakuhin Kenkyu 17, 815–825 [Google Scholar]
- 36.Yamada T., Nishiyama T., and Nakane S. (1986). Reproduction studies of ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report I): study of administration to rats prior to and in early stage of gestation. Iyakuhin Kenkyu 17, 609–616 [Google Scholar]
- 37.Yamada T., Nishiyama T., Ohno H., and Nakane S. (1986). Reproduction studies of ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report III): study of administration to rabbits during organogenesis. Iyakuhin Kenkyu 17, 632–638 [Google Scholar]
- 38.Yamada T., Uchida H., Inoue T., Ohba Y., and Nakane S. (1986). Reproduction studies of ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report II): study of administration to rats during organogenesis. Iyakuhin Kenkyu 14, 617–631 [Google Scholar]
- 39.Yamada T., Uchida H., Ohno H., Matsuzawa N., and Nakane S. (1986). Reproduction studies of ethyl(+)-(2S,2S)-3[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarboxylate (EST) (report IV): study of administration to rats during perinatal and postnatal periods. Iyakuhin Kenkyu 17, 639–651 [Google Scholar]
- 40.Fukushima K., Kono Y., Osabe W., Shinozaki F., Kudo K., Arai M., Taigawa K., and Suwa T. (1986). Pharmacokinetics of EST (report 2): tissue Distribution of 14C-EST. Kiso Rinsho 20, 328–341 [Google Scholar]
- 41.Fukushima K., Yoshida H., Osabe W., Shinozaki F., Kudo K., Arai M., and Suwa T. (1986). Pharmacokinetics of EST (report 1): absorption and excretion of 14C-EST. Kiso Rinsho 20, 319–327 [Google Scholar]
- 42.Watanabe T., Fukushima K., Ushiyama Y., Noda K., and Suwa T. (1986). Pharmacokinetics of EST (report 5): pharmacokinetics of EST in humans. Kiso Rinsho 20, 362–366 [Google Scholar]
- 43.Saatman K.E., Creed J., and Raghupathi R. (2010). Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics 7, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada K.H., Kozlowski D.A., Seidl S.E., Lance S., Wieschhaus A.J., Sundivakkam P., Tiruppathi C., Chishti I., Herman I.M., Kuchay S.M., and Chishti A.H. (2012). Targeted gene inactivation of calpain-1 suppresses cortical degeneration due to traumatic brain injury and neuronal apoptosis induced by oxidative stress. J. Biol. Chem. 287, 13182–13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Posmantur R., Kampfl A., Siman R., Liu J., Zhao X., Clifton G.L., and Hayes R.L. (1997). A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience 77, 875–888 [DOI] [PubMed] [Google Scholar]
- 46.Deng Y., Thompson B.M., Gao X., and Hall E.D. (2007). Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 205, 154–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newcomb J.K., Kampfl A., Posmantur R.M., Zhao X., Pike B.R., Liu S.J., Clifton G.L., and Hayes R.L. (1997). Immunohistochemical study of calpain-mediated breakdown products to alpha-spectrin following controlled cortical impact injury in the rat. J. Neurotrauma 14, 369–383 [DOI] [PubMed] [Google Scholar]
- 48.Saatman K.E., Murai H., Bartus R.T., Smith D.H., Hayward N.J., Perri B.R., and McIntosh T.K. (1996). Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc. Natl. Acad. Sci. U. S. A. 93, 3428–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colak A., Kaya M., Karaoglan A., Sagmanligil A., Akdemir O., Sahan E., and Celik O. (2009). Calpain inhibitor AK 295 inhibits calpain-induced apoptosis and improves neurologic function after traumatic spinal cord injury in rats. Neurocirugia (Astur.) 20, 245–254 [PubMed] [Google Scholar]
- 50.Wang X., Jung J., Asahi M., Chwang W., Russo L., Moskowitz M.A., Dixon C.E., Fini M.E., and Lo E.H. (2000). Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J. Neurosci. 20, 7037–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanamandra N., Gumidyala K.V., Waldron K.G., Gujrati M., Olivero W.C., Dinh D.H., Rao J.S., and Mohanam S. (2004). Blockade of cathepsin B expression in human glioblastoma cells is associated with suppression of angiogenesis. Oncogene 23, 2224–2230 [DOI] [PubMed] [Google Scholar]
- 52.Tsubokawa T., Solaroglu I., Yatsushige H., Cahill J., Yata K., and Zhang J.H. (2006). Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke 37, 1888–1894 [DOI] [PubMed] [Google Scholar]
- 53.Turk V., Turk B., and Turk D. (2001). Lysosomal cysteine proteases: facts and opportunities. EMBO J. 20, 4629–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guicciardi M.E., Leist M., and Gores G.J. (2004). Lysosomes in cell death. Oncogene 23, 2881–2890 [DOI] [PubMed] [Google Scholar]
- 55.Yamashima T. (2000). Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 62, 273–295 [DOI] [PubMed] [Google Scholar]
- 56.Lafrenaye A.D., McGinn M.J., and Povlishock J.T. (2012). Increased intracranial pressure after diffuse traumatic brain injury exacerbates neuronal somatic membrane poration but not axonal injury: evidence for primary intracranial pressure-induced neuronal perturbation. J. Cereb. Blood Flow Metab. 32, 1919–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo C.L., Chen X.P., Li L.L., Li Q.Q., Li B.X., Xue A.M., Xu H.F., Dai D.K., Shen Y.W., Tao L.Y., and Zhao Z.Q. (2013). Poloxamer 188 attenuates in vitro traumatic brain injury-induced mitochondrial and lysosomal membrane permeabilization damage in cultured primary neurons. J. Neurotrauma 30, 597–607 [DOI] [PubMed] [Google Scholar]
- 58.Houseweart M.K., Pennacchio L.A., Vilaythong A., Peters C., Noebels J.L., and Myers R.M. (2003). Cathepsin B but not cathepsins L or S contributes to the pathogenesis of Unverricht-Lundborg progressive myoclonus epilepsy (EPM1). J. Neurobiol. 56, 315–327 [DOI] [PubMed] [Google Scholar]
- 59.Goodman J.C., Robertson C.S., Grossman R.G., and Narayan R.K. (1990). Elevation of tumor necrosis factor in head injury. J. Neuroimmunol. 30, 213–217 [DOI] [PubMed] [Google Scholar]
- 60.Csuka E., Morganti-Kossmann M.C., Lenzlinger P.M., Joller H., Trentz O., and Kossmann T. (1999). IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J. Neuroimmunol. 101, 211–221 [DOI] [PubMed] [Google Scholar]
- 61.Bermpohl D., You Z., Lo E.H., Kim H.H., and Whalen M.J. (2007). TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 27, 1806–1818 [DOI] [PubMed] [Google Scholar]
- 62.Guicciardi M.E., Deussing J., Miyoshi H., Bronk S.F., Svingen P.A., Peters C., Kaufmann S.H., and Gores G.J. (2000). Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106, 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foghsgaard L., Wissing D., Mauch D., Lademann U., Bastholm L., Boes M., Elling F., Leist M., and Jaattela M. (2001). Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ha S.D., Martins A., Khazaie K., Han J., Chan B.M., and Kim S.O. (2008). Cathepsin B is involved in the trafficking of TNF-alpha-containing vesicles to the plasma membrane in macrophages. J. Immunol. 181, 690–697 [DOI] [PubMed] [Google Scholar]
- 65.Felbor U., Kessler B., Mothes W., Goebel H.H., Ploegh H.L., Bronson R.T., and Olsen B.R. (2002). Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc. Natl. Acad. Sci. U. S. A. 99, 7883–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishiura S., Hanada K., Tamai M., Kashiwagi K., and Sugita H. (1981). The effect of an in vivo-injected thiol protease inhibitor, E-64-c, on the calcium-induced degeneration of myofilaments. J. Biochem. 90, 1557–1560 [DOI] [PubMed] [Google Scholar]
- 67.Gentleman S.M., Graham D.I., and Roberts G.W. (1993). Molecular pathology of head trauma: altered beta APP metabolism and the aetiology of Alzheimer's disease. Prog. Brain Res. 96, 237–246 [DOI] [PubMed] [Google Scholar]
- 68.Roberts G.W., Gentleman S.M., Lynch A., Murray L., Landon M., and Graham D.I. (1994). Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 57, 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X.H., Johnson V.E., Uryu K., Trojanowski J.Q., and Smith D.H. (2009). A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 19, 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kindy M.S., Yu J., Zhu H., El-Amouri S.S., Hook V., and Hook G.R. (2012). Deletion of the cathepsin B gene improves memory deficits in a transgenic Alzheimer's disease mouse model expressing AβPP containing the wild-type β-secretase site sequence. J. Alzheimers Dis. 29, 827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hook V.Y., Kindy M., and Hook G. (2008). Inhibitors of cathepsin B improve memory and reduce Abeta in transgenic Alzheimer's Disease mice expressing the wild-type, but not the Swedish mutant, beta -secretase APP site. J. Biol. Chem. 283, 7745–7753 [DOI] [PubMed] [Google Scholar]