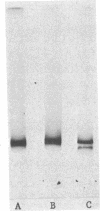
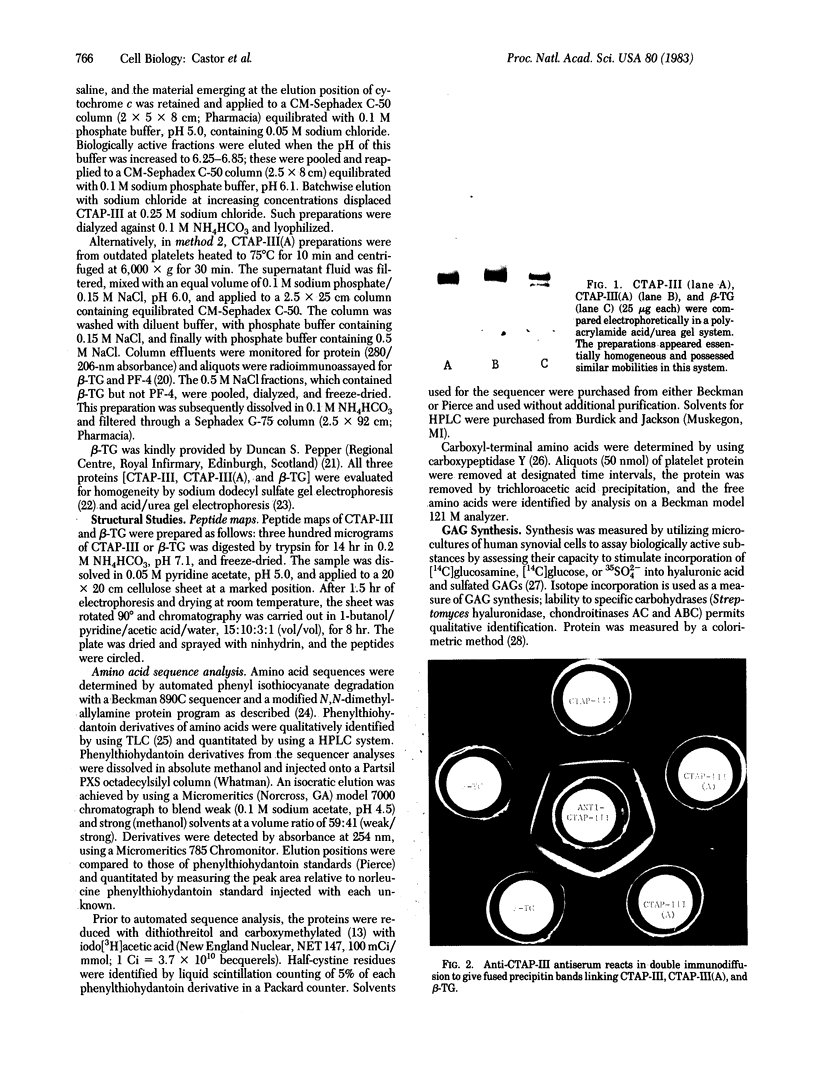
Abstract
Connective tissue activating peptides (CTAPs) extracted from leukocytes and platelets stimulate glycolysis and synthesis of glycosaminoglycan and DNA in cultured human connective tissue cells. CTAP-III, isolated from fresh or outdated human platelets, is a low molecular weight single-chain protein with an isoelectric point of 8.5 that markedly stimulates DNA synthesis and multiple aspects of glycosaminoglycan and proteoglycan metabolism. This report presents a definitive comparison of CTAP-III prepared by two methods [one designated (A), alternative] with similar platelet proteins described by others, beta-thromboglobulin (beta-TG) and low-affinity platelet factor 4 (LA-PF-4). CTAP-III, CTAP-III(A), LA-PF-4, and beta-TG have common antigenic determinants documented by immunoprecipitation and radioimmunoassay. CTAP-III, CTAP-III(A), and LA-PF-4 are biologically active in that they stimulate DNA and glycosaminoglycan synthesis by human synovial cells; beta-TG is inactive. Carboxyl-terminal digestion gave identical terminal sequences for CTAP-III, CTAP-III(A), and beta-TG. Amino-terminal sequence data indicate that CTAP-III and CTAP-III(A) (also LA-PF-4) are identical and differ from beta-TG only by an additional amino-terminal tetrapeptide (Asn-Leu-Ala-Lys-). The biologically active molecule, CTAP-III, may be proteolytically converted to its inactive degradation product (beta-TG) in the course of platelet aging, platelet storage, release from the platelets, or initiation of biological activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Feb;68(2):271–275. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg G. S., Pepper D. S., Chesterman C. N., Morgan F. J. Complete covalent structure of human beta-thromboglobulin. Biochemistry. 1978 May 2;17(9):1739–1744. doi: 10.1021/bi00602a024. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Ho T. T., Walz D. A. Improved radioimmunoassay of platelet factor 4 and beta-thromboglobulin in plasma. Clin Chim Acta. 1980 Feb 28;101(2-3):225–233. doi: 10.1016/0009-8981(80)90247-8. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Bignall M. C., Hossler P. A., Roberts D. J. Connective tissue activation. XXI. Regulation of glycosaminoglycan metabolism by lymphocyte (CTAP-I) and platelet (CTAP-III) growth factors. In Vitro. 1981 Sep;17(9):777–785. doi: 10.1007/BF02618444. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Cobel-Geard S. R., Hossler P. A., Kelch R. P. Connective tissue activation. XXII. A platelet growth factor (connective tissue-activating peptide-III) in human growth hormone-deficient patients. J Clin Endocrinol Metab. 1981 Jan;52(1):128–132. doi: 10.1210/jcem-52-1-128. [DOI] [PubMed] [Google Scholar]
- Castor C. W. Connective tissue activation. I. The nature, specificity, measurement and distribution of connective tissue activating peptide. Arthritis Rheum. 1971 Jan-Feb;14(1):41–54. doi: 10.1002/art.1780140107. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Pek S. Connective tissue activation. XX. Stimulation of prostaglandin secretion by mediators from lymphocytes (CTAP-I) and platelets (CTAP-III). Arthritis Rheum. 1981 Mar;24(3):504–509. doi: 10.1002/art.1780240309. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Ritchie J. C., Scott M. E., Whitney S. L. Connective tissue activation. XI. Stimulation of glycosaminoglycan and DNA formation by a platelet factor. Arthritis Rheum. 1977 Apr;20(3):859–868. doi: 10.1002/art.1780200316. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Ritchie J. C., Williams C. H., Jr, Scott M. E., Whitney S. L., Myers S. L., Sloan T. B., Anderson B. E. Connective tissue activation. XIV. Composition and actions of a human platelet autacoid mediator. Arthritis Rheum. 1979 Mar;22(3):260–272. doi: 10.1002/art.1780220308. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Partial purification and characterization of platelet factors stimulating the multiplication of normal human glial cells. Exp Cell Res. 1977 Oct 15;109(2):429–437. doi: 10.1016/0014-4827(77)90023-4. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. C., Niewiarowski S. Conversion of low-affinity platelet factor 4 to beta-thromboglobulin by plasmin and trypsin. Biochim Biophys Acta. 1980 Oct 1;632(2):284–289. doi: 10.1016/0304-4165(80)90086-0. [DOI] [PubMed] [Google Scholar]
- MacCarter D. K., Hossler P. A., Castor C. W. Connective tissue activation. XXIII. Increased plasma levels of a platelet growth factor (CTAP-III) in patients with rheumatic diseases. Clin Chim Acta. 1981 Sep 10;115(2):125–134. doi: 10.1016/0009-8981(81)90068-1. [DOI] [PubMed] [Google Scholar]
- Myers S. L., Castor C. W. Connective tissue activation. XV. Stimulation of glycosaminoglycan and DNA synthesis by a polymorphonuclear leukocyte factor. Arthritis Rheum. 1980 May;23(5):556–563. doi: 10.1002/art.1780230506. [DOI] [PubMed] [Google Scholar]
- Myers S. L., Hossler P. A., Castor C. W. Connective tissue activation XIX. Plasma levels of the CTAP-III platelet antigen in rheumatoid arthritis. J Rheumatol. 1980 Nov-Dec;7(6):814–819. [PubMed] [Google Scholar]
- Niewiarowski S. Proteins secreted by the platelet. Thromb Haemost. 1977 Dec 15;38(4):924–938. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Rucinski B., Niewiarowski S., James P., Walz D. A., Budzynski A. Z. Antiheparin proteins secreted by human platelets. purification, characterization, and radioimmunoassay. Blood. 1979 Jan;53(1):47–62. [PubMed] [Google Scholar]
- Sisson J. C., Castor C. W., Klavons J. A. Connective tissue activation. XVIII. Stimulation of hyaluronic acid synthetase activity. J Lab Clin Med. 1980 Aug;96(2):189–197. [PubMed] [Google Scholar]
- Walz D. A., Hewett-Emmett D., Seegers W. H. Amino acid sequence of human prothrombin fragments 1 and 2. Proc Natl Acad Sci U S A. 1977 May;74(5):1969–1972. doi: 10.1073/pnas.74.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz D. A., Reuterby J. Improved thin-layer chromatography technique for the identification of phenylthiohydantoin amino acids. J Chromatogr. 1975 Jan 29;104(1):180–183. doi: 10.1016/s0021-9673(01)85506-0. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Wu V. Y., de Lamo R., Dene H., McCoy L. E. Primary structure of human platelet factor 4. Thromb Res. 1977 Dec;11(6):893–898. doi: 10.1016/0049-3848(77)90117-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]