Abstract
Background/Aims
Chronic kidney disease (CKD) is a progressive deterioration of the kidney function, which may eventually lead to renal failure and the need for dialysis or kidney transplant. Whether initiated in the glomeruli or the tubuli, CKD is characterized by progressive nephron loss, for which the process of tubular deletion is of key importance. Tubular deletion results from tubular epithelial cell death and defective repair, leading to scarring of the renal parenchyma. Several cytokines and signaling pathways, including transforming growth factor-β (TGF-β) and the Fas pathway, have been shown to participate in vivo in tubular cell death. However, there is some controversy about their mode of action, since a direct effect on normal tubular cells has not been demonstrated. We hypothesized that epithelial cells would require specific priming to become sensitive to TGF-β or Fas stimulation and that this priming would be brought about by specific mediators found in the pathological scenario.
Methods
Herein we studied whether the combined effect of several stimuli known to take part in CKD progression, namely TGF-β, tumor necrosis factor-α, interferon-γ (IFN-γ), and Fas stimulation, on primed resistant human tubular cells caused cell death or reduced proliferation.
Results
We demonstrate that these cytokines have no synergistic effect on the proliferation or viability of human kidney (HK2) cells. We also demonstrate that IFN-γ, but not the other stimuli, reduces the proliferation of cycloheximide-primed HK2 cells without affecting their viability.
Conclusion
Our results point at a potentially important role of IFN-γ in defective repair, leading to nephron loss during CKD.
Key Words : Chronic kidney disease, Tubular deletion, Proliferation, Apoptosis, Cytokines, Transforming growth factor-β, Tumor necrosis factor-α, Interferon-γ, Fas
Introduction
Chronic kidney disease (CKD) is a condition, in which the renal excretory function progressively and irreversibly decreases as a consequence of renal tissue injury, nephron loss, and fibrosis [1]. The increasing inability of the kidneys to properly clear the blood of waste products eventually results in the need for dialysis (or kidney transplant) in order to prevent azotemia, systemic organ damage, and death [2,3]. During CKD, progressive nephron loss may start by damage to the glomerulus (glomerular diseases) or to the tubule (tubulointerstitial diseases) [4]. In both cases, affected nephrons eventually degenerate and their space is occupied by scar-like tissue. In this process, tubular deletion is thought to result mainly from tubular cell death (i.e. apoptosis) [5]. It has also been suggested that the epithelial-to-mesenchymal transition of tubular cells plays a role but to what extent has not yet been determined [6]; there is, however, disagreement about this [7]. In addition, reduced tubular cell proliferation has been proposed to play a critical role in tubular deletion by impeding tubular regeneration [5]. Several mediators have been suggested to be responsible for inducing apoptosis in tubular cells, including Fas ligand [8], transforming growth factor-β (TGF-β) [9], and tumor necrosis factor-α (TNF-α) [10].
Inhibition of TGF-β action reduces tubular deletion in animal models of CKD [11]. The blockade of the Fas pathway also reduces tubular deletion in vivo [12]. However, it is not yet clear whether these cytokines have the ability to induce apoptosis directly on tubular cells or whether their alleged function results from an indirect effect. In fact, it has been shown that, in general, epithelial cells (including renal tubular cells) are resistant to the action of Fas stimulation [13,14], a classic cell death mechanism [15]. Activation of Fas, in other cells, leads to the assembly of the death-inducing signaling complex, which facilitates the activation of the zimogen procaspase 8 and of the extrinsic apoptotic pathway. However, in normal epithelial cells, the Fas pathway is usually uncoupled by basally expressed inhibitors of the death-inducing signaling complex, such as FLICE-like inhibitory protein (FLIP). Under certain pathological circumstances, epithelial cells become sensitive to Fas activation by the downregulation of FLIP expression [16,17,18]. How pathological scenarios specifically prime epithelial cells for apoptosis is at present unknown. In the case of TGF-β, in vitro studies with tubular cells usually fail to demonstrate a direct proapoptotic effect of this cytokine [reviewed in [5]]. In this sense, hypotheses have been proposed that either (1) the proapoptotic effect of TGF-β only takes place under pathological circumstances with the cooperation of other mediators or cytokines or (2) amplifying mechanisms of damage are present only in vivo, but are absent in in vitro systems [1]. On these grounds, we investigated whether different cytokines sensitize tubular cells to the action of TGF-β and whether they induce synergistic effects among them. These cytokines included TGF-β, TNF-α, and interferon-γ (IFN-γ), and the stimulation of the Fas receptor was also included.
Materials and Methods
All reagents were purchased from Sigma (Madrid, Spain), except where otherwise indicated.
Cell Culture
Human kidney (HK2) cells were used. They were grown in RPMI 1640 (Bio Whittaker Labs) supplemented with 10% FCS, 1 mM L-glutamine, 0.66 mg/ml penicillin, 60 mg/ml streptomycin sulfate, 5 mg/ml insulin, 5 mg/ml transferrin, and 5 ng/ml selenium, in an atmosphere of 95% air/5% CO2 at 37°C. Cells were seeded in 100-mm Petri dishes (Nunc, Roskilde, Denmark). HK2 cells were treated during 1 and 2 days with TGF-β1 (1 ng/ml; Upstate-Millipore, Madrid, Spain), TNF-α (25 ng/ml; Hycult Biotech, Uden, The Netherlands), IFN-γ (50 ng/ml; Hycult Biotech), stimulatory anti-Fas antibody (0.5 μg/ml; clone CH11, Millipore, Madrid, Spain), and cycloheximide (100 µM).
MTT Assay
The viable cell number was determined by incubating cell cultures with 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 4 h. Then, 10% sodium dodecyl sulfate in 0.01 M HCl was added 1:1 (vol/vol) and left overnight at 37°C. Finally, absorbance was measured at 570 nm.
DNA Fragmentation
DNA fragmentation was determined by ELISA with the commercial kit Cell Death Detection Plus (Roche Diagnostics, Barcelona, Spain) according to the manufacturer's instructions. Ten micrograms of protein were used from each cell extract.
Cell Cycle Analysis
Thirty minutes before harvest, 10 µM 5-bromo-2-deoxyuridine (BrdU, Sigma Aldrich) were added to the cell culture. Subsequently, cells were fixed in ice-cold 70% ethanol overnight and stained with 0.01 mg/ml anti-BrdU-FITC antibody (Abcam, Cambridge, UK). Then, 0.01 mg/ml propidium iodide was added to cell suspensions and 1 h later they were analyzed by flow cytometry (FACScalibur, BD Pharmingen) with the CellQuest software. Singlet discrimination was done by means of an FL2-A versus FL2-W representation.
Results
Absence of Cooperation among Cytokines on the Proliferation and Viability of HK2 Cells
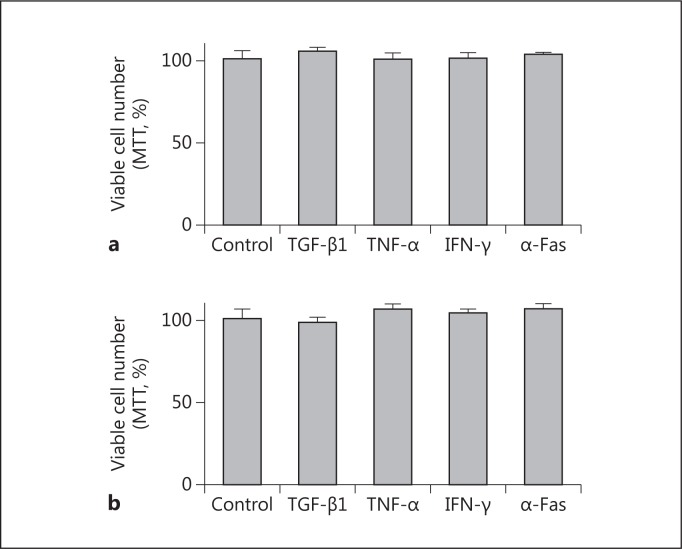
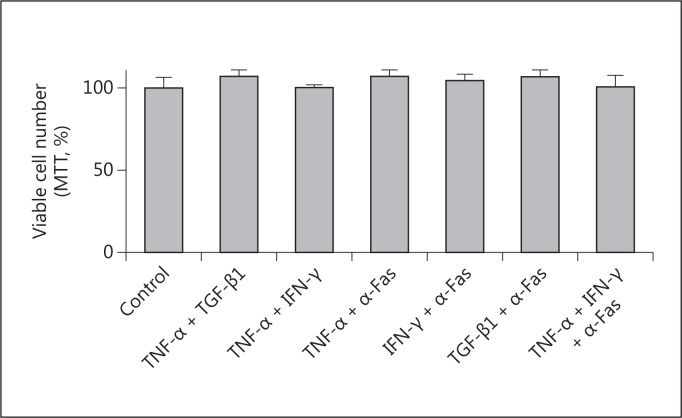
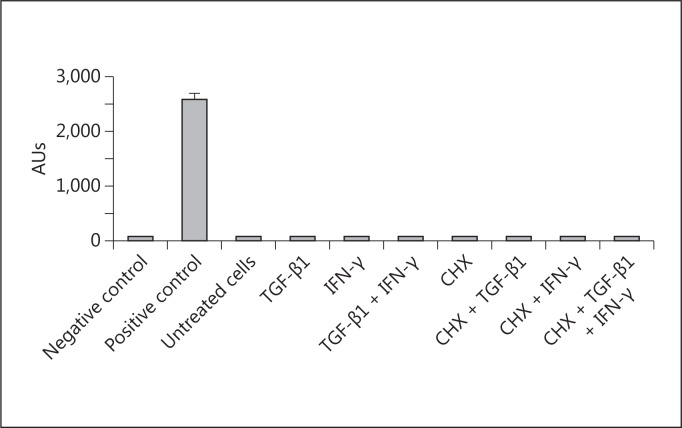
As shown in figure 1, TGF-β1, TNF-α, IFN-γ, and anti-Fas failed to modify the number of proliferating HK2 cells after 24 and 48 h of treatment. Combinations of these stimuli also failed to modify the proliferation and viability of HK2 cells (fig. 2). In fact, no evidence of apoptosis was detected by evaluating internucleosomal DNA fragmentation (fig. 3), a hallmark of this mode of cell death. By visual inspection (data not shown), cells in all conditions appeared to be indistinguishable from control cells (i.e. treated with the vehicle) and also from the regular culture. No signs of cytotoxicity were evident.
Fig. 1.
Effect of different cytokines on the proliferation of HK2 cells. Cells were treated for 24 h (a) or 48 h (b) with 1 ng/ml TGF-β1, 25 ng/ml TNF-α, 50 ng/ml IFN-γ, 0.5 μg/ml anti-Fas antibody (α-Fas), or vehicle (as control). After the indicated periods of time, an MTT assay was performed. Data represent the average ± SEM of 4 independent experiments performed in triplicate.
Fig. 2.
Effect of different cytokines on the proliferation of HK2 cells. Cells were treated for 48 h with different combinations of 1 ng/ml TGF-β1, 25 ng/ml TNF-α, 50 ng/ml IFN-γ, 0.5 μg/ml anti-Fas antibody (α-Fas), or vehicle (as control). At the end, an MTT assay was performed. Data represent the average ± SEM of 3 independent experiments performed in triplicate.
Fig. 3.
Effect of different cytokines on the internucleosomal DNA fragmentation of HK2 cells. Cells were treated for 48 h with different combinations of 1 ng/ml TGF-β1, 50 ng/ml IFN-γ, or cycloheximide (CHX). At the end, DNA fragmentation (as an index of apoptosis) was measured by ELISA. Data represent the average ± SEM of 3 independent experiments performed in duplicate. A positive control was included containing an extract of apoptotic cells. AUs = Arbitrary units.
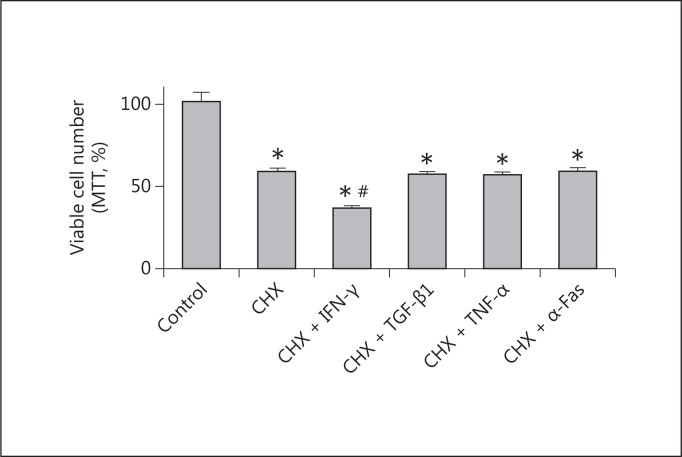
IFN-γ Reduces the Proliferation of Primed HK2 Cells
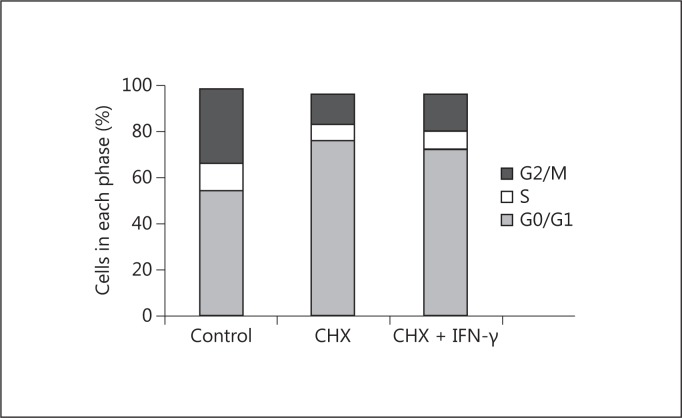
Cycloheximide is an inhibitor of protein biosynthesis in eukaryotic cells, which acts by impeding translational elongation. At low concentration, it has been used to inhibit the expression of proteins with a high turnover, including those protecting from apoptosis such as FLIP [16,17,18]. When we primed HK2 cells with cycloheximide, IFN-γ, but not TGF-β1, TNF-α, or anti-Fas, induced a reduction of cell proliferation (fig. 4). This reduction in proliferation did not correlate with the induction of apoptosis (fig. 3) or with an accumulation in specific phases of the cell cycle (fig. 5). In fact, IFN-γ induced per se a redistribution of cells through the phases of the cell cycle with no effect on net proliferation. In addition, despite reducing the proliferation of cycloheximide-primed cells (over the effect of cycloheximide), it did not further alter the cell cycle profile significantly with respect to the effect of cycloheximide (fig. 5).
Fig. 4.
Effect of priming cells with cycloheximide (CHX) on the action of different cytokines on the proliferation of HK2 cells. Cells were treated for 24 h with 100 µM cycloheximide or vehicle (as control), and then for 24 h with 1 ng/ml TGF-β1, 25 ng/ml TNF-α, 50 ng/ml IFN-γ, or 0.5 μg/ml anti-Fas antibody (α-Fas). At the end, an MTT assay was performed. Data represent the average ± SEM of 4 independent experiments performed in triplicate. * p < 0.05 with respect to the control; # p < 0.05 with respect to cycloheximide.
Fig. 5.
Effect of cycloheximide (CHX) and IFN-γ on the distribution of HK2 cells through the cell cycle phases. Cells were treated for 24 h with 100 µM cycloheximide, and then for 24 h with 50 ng/ml IFN-γ or vehicle (as control). At the end, cells were double-stained for BrdU incorporation and DNA content, and the number of cells in each phase of the cell cycle was measured by flow cytometry. Data are representative of 3 independent experiments.
Discussion
During CKD progression, tubuli become atrophied and eventually disappear, leading to nephron loss. Epithelial tubular cell death (mainly by apoptosis) and defective repair are two key events underlying this process. When tubuli are injured by epithelial destruction, repair mechanisms exist that rebuild the damaged structures and regain function. This is exemplified by most cases of acute kidney injury, in which tubuli regenerate when the insult ceases [19]. This happens not only for mild renal injuries, but for massive tubular necrosis (as in experimental models) as well. It tells us that the kidneys have the ability to regenerate, even after severe damage. An important question is why these repair mechanisms do not work under CKD conditions, in which damage occurs more progressively. On these grounds, we hypothesized elsewhere [5] that yet undetermined pathophysiological circumstances alter the correct repair process, leading to tubule degeneration. The normal repair process involves dedifferentiation of any remaining epithelial cells, proliferation, migration to damaged areas, and redifferentiation into epithelial cells. Pathological circumstances may involve an imbalance between pro- and antiproliferative signals (e.g. cytokines), which would (1) induce cell dedifferentiation, (2) enforce the proliferative status of previously dedifferentiated cells, and (3) maintain their undifferentiated state and the excessive synthesis and deposition of extracellular matrix components. In this scenario, repair would be distorted, tubules would not regenerate, and their space would be replaced by scar-like tissue.
Another key aspect of progressive tubule degeneration is epithelial cell death. These cells are, under normal circumstances, insensitive to a potential proapoptotic action purportedly assigned to diverse cytokines, including TGF-β, and Fas receptor stimulation. It was also hypothesized that hitherto undetermined mediators present under pathological conditions of CKD would sensitize epithelial cells to the deadly action of these cytokines. In fact, all these cytokines (including TGF-β, TNF-α, and IFN-γ) and the Fas pathway have been involved in the pathophysiology of CKD [3,4].
Our results indicate that none of these cytokines alter the viability of HK2 cells and that combinations of two or three of these cytokines also fail to sensitize to cell death. However, our results additionally indicate that IFN-γ might be involved in the interference with the process of tubular repair. In fact, IFN-γ seems to be able to reduce tubular cell proliferation in previously primed cells. This indicates that IFN-γ, and not TNF-α, TGF-β, or Fas stimulation, might contribute to skewing epithelial cell substitution with the necessary help of unidentified mediators (mimicked experimentally by cycloheximide) that prime the cells to the action of IFN-γ. In fact, in other cell types, IFN-γ may induce both apoptosis and cell cycle arrest [20,21]. The innate inflammatory response is, in principle, a protective reaction of the organism against damage, caused either by exogenous or endogenous stimuli [22]. However, overactivation or persistence of the inflammatory response may, under various circumstances, turn deleterious and damage the inflamed tissue [23]. The mechanisms underlying this conversion are, however, poorly understood. Interestingly, IFN-γ may provide a mechanistic link between the inflammatory response and the distortion of the repair response, leading to progressive degeneration. Further research is necessary to unravel the identity of these mediators and to further ascertain the role of IFN-γ in tubular degeneration.
Additionally, IFN-γ might be targeted to prevent the progression of CKD. In fact, patients with progressive diabetic nephropathy, the leading cause of end-stage renal disease in developed countries, respond with higher IFN levels when challenged with tuberculosis antigens. This might indicate that these patients have an overreactive inflammatory response that might damage their kidneys [24]. Furthermore, advanced CKD patients are often treated with erythropoietin. Patients who respond well to the treatment are associated with low levels of inflammatory cytokines including IFN-γ, whereas those failing to respond are associated with high levels of these cytokines [25]. In other words, the study of IFN-γ can deepen our understanding of the mechanisms involved in CKD progression, and IFN-γ also presents a potential target for the treatment of this disease.
In conclusion, IFN-γ, but not TNF-α, TGF-β, or Fas stimulation, reduces the proliferation of cycloheximide-primed HK2 cells without affecting their viability. Our results suggest that IFN-γ has an important role in defective repair, leading to a loss of nephrons during CKD and that it can potentially be targeted when treating this disease.
References
- 1.López-Hernández FJ, López-Novoa JM. Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012;347:141–154. doi: 10.1007/s00441-011-1275-6. [DOI] [PubMed] [Google Scholar]
- 2.De Vecchi AF, Dratwa M, Wiedemann ME. Healthcare systems and end-stage renal disease (ESRD) therapies – an international review: costs and reimbursement/funding of ESRD therapies. Nephrol Dial Transplant. 1999;14(suppl 6):31–41. doi: 10.1093/ndt/14.suppl_6.31. [DOI] [PubMed] [Google Scholar]
- 3.López-Novoa JM, Martínez-Salgado C, Rodríguez-Peña AB, López Hernández FJ. Common pathophysiological mechanisms of chronic kidney disease: therapeutic perspectives. Pharmacol Ther. 2010;128:61–81. doi: 10.1016/j.pharmthera.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 4.López-Novoa JM, Rodríguez-Peña AB, Ortiz A, Martínez-Salgado C, López Hernández FJ. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications. J Transl Med. 2011;9:13. doi: 10.1186/1479-5876-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Sánchez O, López-Hernández FJ, López-Novoa JM. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010;77:950–955. doi: 10.1038/ki.2010.88. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 7.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorz C, Benito-Martin A, Justo P, Sanz AB, Sanchez-Niño MD, Santamaria B, Egido J, Ortiz A. Modulation of renal tubular cell survival: where is the evidence? Curr Med Chem. 2006;13:449–454. doi: 10.2174/092986706775527956. [DOI] [PubMed] [Google Scholar]
- 9.Chea SW, Lee KB. TGF-β mediated epithelial-mesenchymal transition in autosomal dominant polycystic kidney disease. Yonsei Med J. 2009;50:105–111. doi: 10.3349/ymj.2009.50.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol. 2012;36:261–270. doi: 10.1159/000342333. [DOI] [PubMed] [Google Scholar]
- 11.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301–2313. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 12.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol. 2003;284:F955–F965. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 13.Khan S, Koepke A, Jarad G, Schlessman K, Cleveland RP, Wang B, Konieczkowski M, Schelling JR. Apoptosis and JNK activation are differentially regulated by Fas expression level in renal tubular epithelial cells. Kidney Int. 2001;60:65–76. doi: 10.1046/j.1523-1755.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 14.Jarad G, Wang B, Khan S, DeVore J, Miao H, Wu K, Nishimura SL, Wible BA, Konieczkowski M, Sedor JR, Schelling JR. Fas activation induces renal tubular epithelial cell beta 8 integrin expression and function in the absence of apoptosis. J Biol Chem. 2002;277:47826–47833. doi: 10.1074/jbc.M204901200. [DOI] [PubMed] [Google Scholar]
- 15.Peter ME, Krammer PH. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 16.Ogura H, Tsukumo Y, Sugimoto H, Igarashi M, Nagai K, Kataoka T. Ectodomain shedding of TNF receptor 1 induced by protein synthesis inhibitors regulates TNF-alpha-mediated activation of NF-kappaB and caspase-8. Exp Cell Res. 2008;314:1406–1414. doi: 10.1016/j.yexcr.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Fischer-Posovszky P, Keuper M, Nagel S, Hesse D, Schürmann A, Debatin KM, Strauss G, Wabitsch M. Downregulation of FLIP by cycloheximide sensitizes human fat cells to CD95-induced apoptosis. Exp Cell Res. 2011;317:2200–2209. doi: 10.1016/j.yexcr.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Bonventre JV. Pathophysiology of AKI: injury and normal and abnormal repair. Contrib Nephrol. 2010;165:9–17. doi: 10.1159/000313738. [DOI] [PubMed] [Google Scholar]
- 20.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 21.Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts) Clin Ter. 2006;157:377–386. [PubMed] [Google Scholar]
- 22.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 23.Anders HJ, Vielhauer V, Schlöndorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- 24.Lane C, Ashcroft A, Bothamley G, Yaqoob MM, Fan SL. Accelerated decline of GFR in diabetic nephropathy predicted by interferon release assay to tuberculosis antigens. Nephron Clin Pract. 2011;117:c266–c269. doi: 10.1159/000320753. [DOI] [PubMed] [Google Scholar]
- 25.Macdougall IC. Could anti-inflammatory cytokine therapy improve poor treatment outcomes in dialysis patients? Nephrol Dial Transplant. 2004;19(suppl 5):V73–V78. doi: 10.1093/ndt/gfh1060. [DOI] [PubMed] [Google Scholar]