Abstract
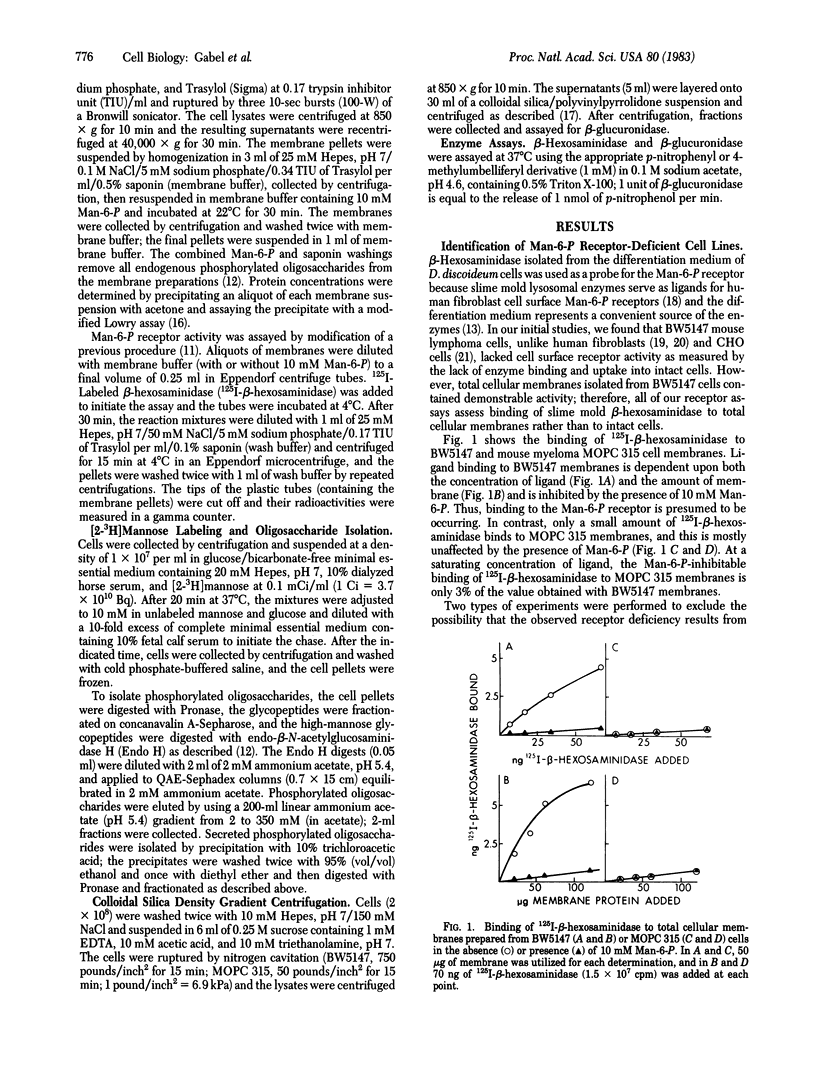
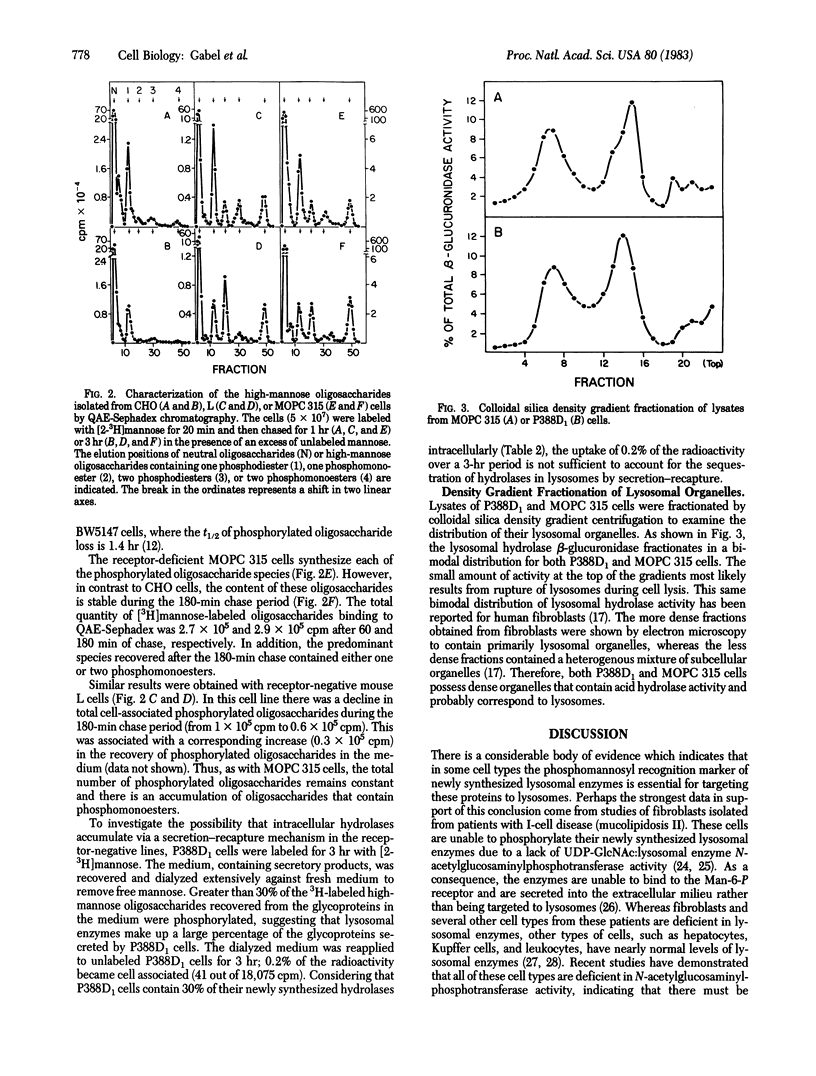
Newly synthesized lysosomal enzymes acquire phosphomannosyl units, which allow binding of the enzymes to the mannose 6-phosphate receptor and subsequent translocation to lysosomes. In some cell types, this sequence of events is necessary for the delivery of these enzymes to lysosomes. Using a slime mold lysosomal hydrolase as a probe, we have identified three murine cell lines that lack the receptor and one line that contains very low (3%) receptor activity. Each of these lines synthesizes the mannose 6-phosphate recognition marker on its lysosomal enzymes, but, unlike cell lines with high levels of receptor, the cells accumulate oligosaccharides containing phosphomonoesters. The receptor-deficient lines possess high levels of intracellular acid hydrolase activity, which is contained in dense granules characteristic of lysosomes. The data suggest that intracellular mechanisms independent of the mannose 6-phosphate receptor must exist in some cells for the delivery of acid hydrolases to lysosomal organelles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies P., Page R. C., Allison A. C. Changes in cellular enzyme levels and extracellular release of lysosomal acid hydrolases in macrophages exposed to group A streptococcal cell wall substance. J Exp Med. 1974 May 1;139(5):1262–1282. doi: 10.1084/jem.139.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimond R. L., Burns R. A., Jordan K. B. Secretion of Lysosomal enzymes in the cellular slime mold, Dictyostelium discoideum. J Biol Chem. 1981 Jul 10;256(13):6565–6572. [PubMed] [Google Scholar]
- Every D., Ashworth J. M. The purification and properties of extracellular glycosidases of the cellular slime mould Dictyostelium discoideum. Biochem J. 1973 May;133(1):37–47. doi: 10.1042/bj1330037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S. Beta-glucuronidase binding to human fibroblast membrane receptors. J Biol Chem. 1980 Jun 10;255(11):5069–5074. [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Freeze H. H., Miller A. L., Kaplan A. Acid hydrolases from Dictyostelium discoideum contain phosphomannosyl recognition markers. J Biol Chem. 1980 Dec 10;255(23):11081–11084. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. A., Goldberg D. E., Kornfeld S. Lysosomal enzyme oligosaccharide phosphorylation in mouse lymphoma cells: specificity and kinetics of binding to the mannose 6-phosphate receptor in vivo. J Cell Biol. 1982 Nov;95(2 Pt 1):536–542. doi: 10.1083/jcb.95.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger P. J., Bessman S. P. Protein determination by Lowry's method in the presence of sulfhydryl reagents. Anal Biochem. 1972 Oct;49(2):467–473. doi: 10.1016/0003-2697(72)90450-2. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Roozen K. J., Brot F. E., Sly W. S. Multiple isoelectric and recognition forms of human beta-glucuronidase activity. Arch Biochem Biophys. 1975 Feb;166(2):536–542. doi: 10.1016/0003-9861(75)90417-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Waheed A., von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981 Feb 12;98(3):761–767. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C. Cytochemical localization of acid phosphatase and trimetaphosphatase activities in exocrine acinar cells. J Histochem Cytochem. 1980 Jan;28(1):78–81. doi: 10.1177/28.1.6243325. [DOI] [PubMed] [Google Scholar]
- Owada M., Neufeld E. F. Is there a mechanism for introducing acid hydrolases into liver lysosomes that is independent of mannose 6-phosphate recognition? Evidence from I-cell disease. Biochem Biophys Res Commun. 1982 Apr 14;105(3):814–820. doi: 10.1016/0006-291x(82)91042-7. [DOI] [PubMed] [Google Scholar]
- Reitman M. L., Kornfeld S. UDP-N-acetylglucosamine:glycoprotein N-acetylglucosamine-1-phosphotransferase. Proposed enzyme for the phosphorylation of the high mannose oligosaccharide units of lysosomal enzymes. J Biol Chem. 1981 May 10;256(9):4275–4281. [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R. The mannose 6-phosphate receptor of Chinese hamster ovary cells. Compartmentalization of acid hydrolases in mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10623–10627. [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R., Youle R. J., Murray G. J., Neville D. M., Jr The mannose 6-phosphate receptor of Chinese Hamster ovary cells. Isolation of mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10618–10622. [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Sahagian G. G., Distler J., Jourdian G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4289–4293. doi: 10.1073/pnas.78.7.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Steiner A. W., Rome L. H. Assay and purification of a solubilized membrane receptor that binds the lysosomal enzyme alpha-L-iduronidase. Arch Biochem Biophys. 1982 Apr 1;214(2):681–687. doi: 10.1016/0003-9861(82)90074-1. [DOI] [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Identification of a rat liver alpha-N-acetylglucosaminyl phosphodiesterase capable of removing "blocking" alpha-N-acetylglucosamine residues from phosphorylated high mannose oligosaccharides of lysosomal enzymes. J Biol Chem. 1980 Sep 25;255(18):8398–8401. [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. UDP-N-acetylglucosamine:lysosomal enzyme precursor N-acetylglucosamine-1-phosphotransferase. Partial purification and characterization of the rat liver Golgi enzyme. J Biol Chem. 1982 Oct 25;257(20):12322–12331. [PubMed] [Google Scholar]
- Waheed A., Pohlmann R., Hasilik A., von Figura K. Subcellular location of two enzymes involved in the synthesis of phosphorylated recognition markers in lysosomal enzymes. J Biol Chem. 1981 May 10;256(9):4150–4152. [PubMed] [Google Scholar]
- Waheed A., Pohlmann R., Hasilik A., von Figura K., van Elsen A., Leroy J. G. Deficiency of UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase in organs of I-cell patients. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1052–1058. doi: 10.1016/0006-291x(82)91076-2. [DOI] [PubMed] [Google Scholar]


