Abstract
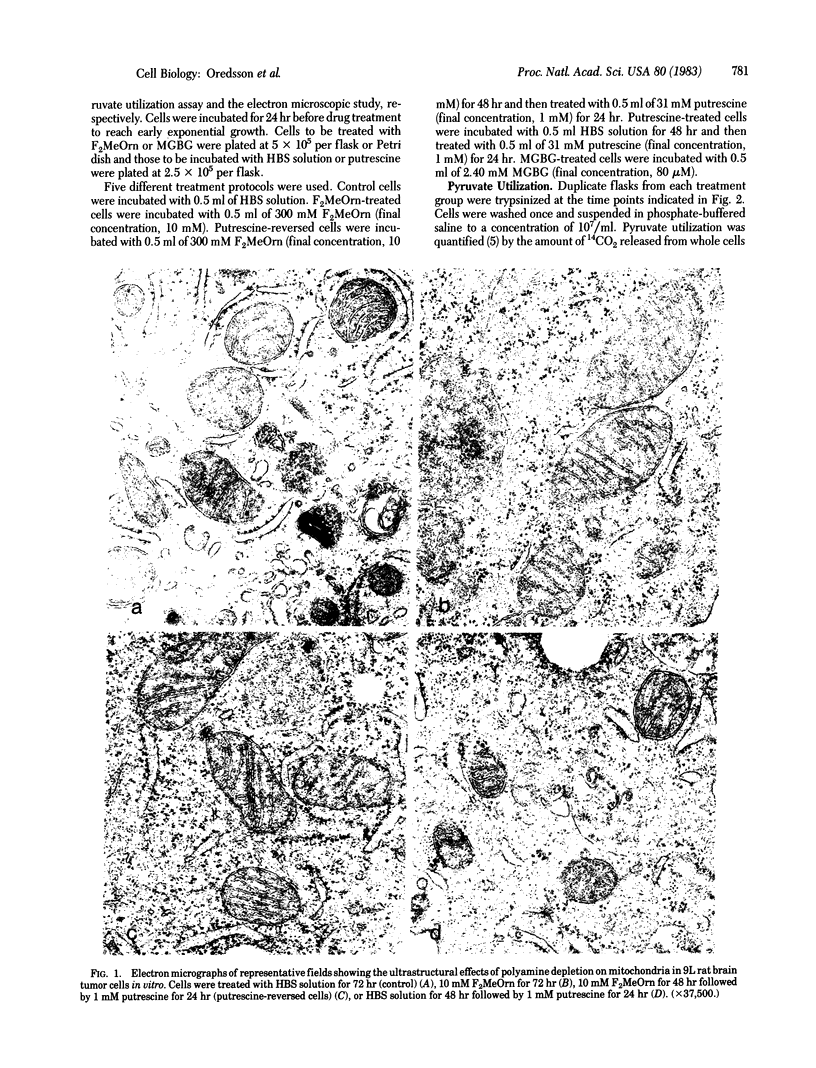
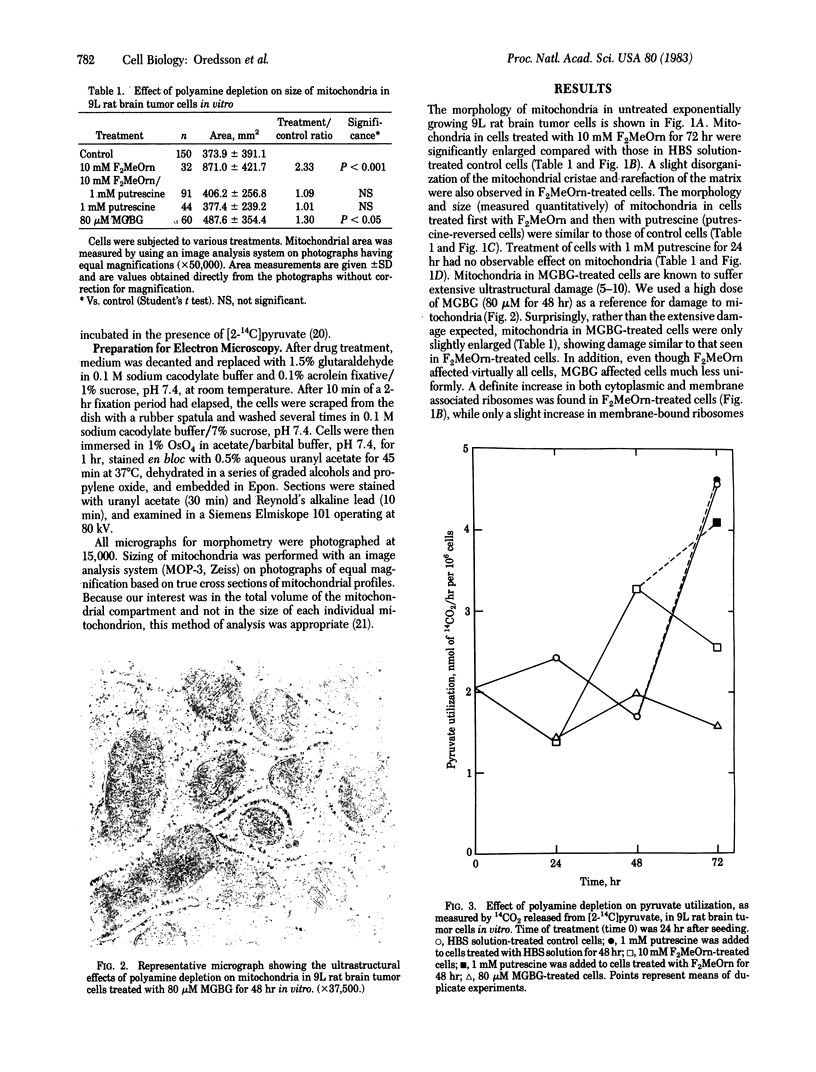
Mitochondrial structure and function were studied in 9L rat brain tumor cells depleted of polyamines by α-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase. Cells treated with methylglyoxal bis(guanylhydrazone), a reversible inhibitor of S-adenosylmethionine decarboxylase, were used for comparison because this polyamine biosynthesis inhibitor is known to cause structural and functional disruption of mitochondria. A significant increase in mitochondrial size, measured quantitatively, was found in α-difluoromethylornithine-treated cells (10 mM for 72 hr) compared with untreated cells (P < 0.001). This increase in mitochondrial size was reversed when putrescine was added to the cultures for 24 hr after α-difluoromethylornithine treatment. Putrescine alone had no effect on the size of mitochondria. Treatment of cells with methylglyoxal bis(guanylhydrazone) (80 μM for 48 hr) caused only a slight increase in mitochondrial size compared with mitochondria in untreated cells (P < 0.05) and failed to produce the dramatic ultrastructural changes reported in other cell lines. Ultrastructural examination revealed an increase in cytoplasmic and membrane-associated ribosomes in α-difluoromethylornithine-treated cells, an increase in cytoplasmic ribosomes in methylglyoxal bis(guanylhydrazone)-treated cells, and an increase in membrane-bound ribosomes in putrescine-treated cells. In cells treated first with α-difluoromethylornithine and then with putrescine, the distribution of ribosomes was normal. The distributions of ribosomes were not quantitatively assessed. Pyruvate utilization, a measure of mitochondrial function, was decreased in cells treated with 10 mM α-difluoromethylornithine for 72 hr, compared with untreated cells. Restoration of intracellular polyamine levels by the addition of putrescine 24 hr before analysis reversed this phenomenon. Putrescine treatment alone did not affect pyruvate utilization. Pyruvate utilization in methylglyoxal bis(guanylhydrazone)-treated cells was depressed to a greater extent than that in α-difluoromethylornithine-treated cells.
Keywords: electron microscopy, pyruvate utilization, methylglyoxal bis(guanylhydrazone), ribosomes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breen G. A., Scheffler I. E. Respiration-deficient Chinese hamster cell mutants: biochemical characterization. Somatic Cell Genet. 1979 Jul;5(4):441–451. doi: 10.1007/BF01538879. [DOI] [PubMed] [Google Scholar]
- Chaffee R. R., Arine R. M., Rochelle R. H. The possible role of intracellular polyamines in mitochondrial metabolic regulation. Biochem Biophys Res Commun. 1979 Jan 30;86(2):293–299. doi: 10.1016/0006-291x(79)90865-9. [DOI] [PubMed] [Google Scholar]
- Diala E. S., Evans I. H., Wilkie D. Primary antimitochondrial activity of the cancer drug methylglyoxal bis(guanylhydrazone) in yeast cells. J Gen Microbiol. 1980 Jul;119(1):35–40. doi: 10.1099/00221287-119-1-35. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. STABILIZATION OF STREPTOCOCCUS FAECALIS PROTOPLASTS BY SPERMINE. J Bacteriol. 1964 Nov;88:1416–1420. doi: 10.1128/jb.88.5.1416-1420.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J. J., Porter C. W., Morris D. R. Induction of polyamine limitation in Chinese hamster ovary cells by alpha-methylornithine. J Cell Physiol. 1981 Jun;107(3):413–426. doi: 10.1002/jcp.1041070313. [DOI] [PubMed] [Google Scholar]
- Hecker D., Rose H. Histochemische und ultrastrukturelle Untersuchungen über den Einfluss von Methylglyoxal (bis)guanylhydrazon (Methyl-GAG) auf Organkulturen von malignen Tumoren. Arch Geschwulstforsch. 1977;47(1):37–44. [PubMed] [Google Scholar]
- Hung D. T., Deen D. F., Seidenfeld J., Marton L. J. Sensitization of 9L rat brain gliosarcoma cells to 1,3-bis(2-chloroethyl)-1-nitrosourea by alpha-difluoromethylornithine, an ornithine decarboxylase inhibitor. Cancer Res. 1981 Jul;41(7):2783–2785. [PubMed] [Google Scholar]
- Hölttä E., Hannonen P., Pispa J., Jänne J. Effect of methylglyoxal bis(guanylhydrazone) on polyamine metabolism in normal and regenerating rat liver and rat thymus. Biochem J. 1973 Nov;136(3):669–676. doi: 10.1042/bj1360669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja J. A. Interaction of ribosomes and ribosomal subparticles with endoplasmic reticulum membranes in vitro: effect of spermine and magnesium. Biochim Biophys Acta. 1971 Nov 29;254(1):117–128. doi: 10.1016/0005-2787(71)90118-3. [DOI] [PubMed] [Google Scholar]
- Khawaja J. A., Raina A. Effect of spermine and magnesium on the attachment of free ribosomes to endoplasmic reticulum membranes. Biochem Biophys Res Commun. 1970 Oct 23;41(2):512–518. doi: 10.1016/0006-291x(70)90536-x. [DOI] [PubMed] [Google Scholar]
- MAGER J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959 Dec;36:529–531. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Danzin C., Wagner J., Siat M., Joder-Ohlenbusch A. M., Claverie N. Accumulation of decarboxylated S-adenosyl-L-methionine in mammalian cells as a consequence of the inhibition of putrescine biosynthesis. Eur J Biochem. 1982 Apr;123(3):499–504. doi: 10.1111/j.1432-1033.1982.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Mikles-Robertson F., Feuerstein B., Dave C., Porter C. W. The generality of methylglyoxal bis(guanylhydrazone)-induced mitochondrial damage and the dependence of this effect on cell proliferation. Cancer Res. 1979 Jun;39(6 Pt 1):1919–1926. [PubMed] [Google Scholar]
- Nag D., Ghosh J. J. Imipramine-induced changes of brain adenosine triphosphatase activity. The role of spermine in counteracting the disorganizing effect of the drug on membrane ATPase. J Neurochem. 1973 Apr;20(4):1021–1027. doi: 10.1111/j.1471-4159.1973.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Oredsson S. M., Deen D. F., Marton L. J. Decreased cytotoxicity of cis-diamminedichloroplatinum(II) by alpha-difluoromethylornithine depletion of polyamines in 9L rat brain tumor cells in vitro. Cancer Res. 1982 Apr;42(4):1296–1299. [PubMed] [Google Scholar]
- Pegg A. E., Pösö H., Shuttleworth K., Bennett R. A. Effect of inhibition of polyamine synthesis on the content of decarboxylated S-adenosylmethionine. Biochem J. 1982 Feb 15;202(2):519–526. doi: 10.1042/bj2020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H. W., Ahlers J., Günther T. The dependence of polyamine and phospholipid contents in maring bacteria on the osmotic strength of the medium. Int J Biochem. 1978;9(5):313–316. doi: 10.1016/0020-711x(78)90103-9. [DOI] [PubMed] [Google Scholar]
- Pleshkewych A., Kramer D. L., Kelly E., Porter C. W. Independence of drug action on mitochondria and polyamines in L1210 leukemia cells treated with methylglyoxal-bis(guanylhydrazone). Cancer Res. 1980 Dec;40(12):4533–4540. [PubMed] [Google Scholar]
- Porter C. W., Mikles-Robertson F., Kramer D., Dave C. Correlation of ultrastructural and functional damage to mitochondria of ascites L1210 cells treated in vivo with methylglyoxal-bis(guanylhydrazone) or ethidium bromide. Cancer Res. 1979 Jul;39(7 Pt 1):2414–2421. [PubMed] [Google Scholar]
- Quarfoth G., Ahmed K., Foster D. Effects of polyamines on partial reactions of membrane (Na+ + K+)-ATPase. Biochim Biophys Acta. 1978 Oct 12;526(2):580–590. doi: 10.1016/0005-2744(78)90148-1. [DOI] [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenfeld J., Gray J. W., Marton L. J. Depletion of 9L rat brain tumor cell polyamine content by treatment with D,L-alpha-difluoromethylornithine inhibits proliferation and the G1 to S transition. Exp Cell Res. 1981 Jan;131(1):209–216. doi: 10.1016/0014-4827(81)90420-1. [DOI] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]