Abstract
Intravascular lymphomas are rare and aggressive hematolymphoid tumors. Here we describe a human herpesvirus type-8/Kaposi sarcoma-associated herpesvirus (HHV-8/KSHV) and Epstein-Barr virus (EBV) positive intravascular lymphoma. The patient was a 59 year-old HIV-positive man who presented with diarrhea, abdominal pain, fevers, night sweats, and weight loss. Radiographic studies of the abdomen and pelvis revealed numerous subcentimeter nodules within the subcutaneous fat that lacked connection to the skin. An excisional biopsy demonstrated large atypical cells within vessels in the deep subcutaneous fat, and many of the vessels contained extensive organizing thrombi. The atypical cells lacked strong expression of most B-cell markers but were positive for MUM-1 and showed partial expression of several T-cell markers. An immunohistochemical stain for HHV-8 and an in situ hybridization for EBV were both positive in the neoplastic cells. The disease had a rapidly progressive and fatal course. This lymphoma appears to represent an entirely intravascular form of primary effusion lymphoma, and highlights the propensity for HHV-8 and EBV-positive lymphoid neoplasms to show aberrant expression of T-cell markers, illustrates the utility of skin biopsies for the diagnosis of intravascular lymphoma, and suggests that biopsies to evaluate for intravascular lymphoma should be relatively deep and include subcutaneous fat.
Keywords: intravascular lymphoma, human herpesvirus type-8, Kaposi sarcoma-associated herpesvirus, Epstein-Barr virus, primary effusion lymphoma
BACKGROUND
Several lymphoproliferative disorders (LPDs) are associated with coincident Epstein-Barr virus (EBV) and human herpesvirus-8/Kaposi sarcoma-associated herpesvirus (HHV-8/KSHV) infection, including primary effusion lymphoma (PEL)1–4, extracavitary PEL5–9, germinotropic lymphoproliferative disorder (GLD)10 and some cases of plasmablastic Castleman disease (PCD)11–13 (Table 1). These disorders can be diagnostically challenging because of their relative rarity, characteristic lack of expression of typical B-cell markers, and occasional aberrant expression of T-cell antigens1,4,6,14. They are more common in immunocompromised patients, and usually occur in the setting of human immunodeficiency virus (HIV) infection, but have also been reported in transplant patients15,16 and the elderly8,14. Additionally, there are rare reports of some of EBV and HHV-8 positive LPDs occurring in the absence of immunodeficiency 10,17.
Table 1.
| Description | Presentation | Phenotype | |
|---|---|---|---|
| Primary Effusion Lymphoma |
|
|
|
| Multicentric Castleman Disease and associated plasmablastic lymphoproliferative disorders |
MCD: Angiofollicular hyperplasia and plasma cell infiltration Large B-cell lymphoma arising in HHV8-associated MCD: monoclonal HHV-8-infected lymphoid cells that resemble plasmablasts, arising in the setting of MCD; as disease progresses the plasmablasts may become scattered in the interfollicular area and coalesce to form clusters or sheets termed microlymphomas |
|
|
| Germinotrophic lymphoproliferative disorder | Germinotropic aggregates of plasmablasts |
|
|
Intravascular large B-cell lymphomas (IVLBCL) are also rare. IVLBCL is usually widely disseminated, but characteristically spares lymph nodes. The disease is aggressive, and the tumor cells occlude small vessels and capillaries causing end-organ ischemia. Different organs can be affected, leading to the protean presentation of IVLBCL; however, two major patterns of disease are recognized. A Western form presents with predominantly cutaneous or neurologic symptoms, often in conjunction with B symptoms including fever, weight loss and night sweats. An Asian variant of IVLBCL is associated with multi-organ failure, hepatosplenomegaly, pancytopenia, hemophagocytic syndrome and B symptoms1,18,19.
Here we describe a case of intravascular lymphoma associated with EBV and HHV-8 co-infection, which to our knowledge has not been previously described. The clinical presentation and pathologic features of this unusual lymphoma highlight challenges in the diagnosis both of intravascular lymphomas and of neoplasms with HHV-8 and EBV co-infection.
MATERIALS AND METHODS
Formalin-fixed and paraffin-embedded tissue was subjected to hematoxylin and eosin (H&E) staining as well as immunohistochemical staining and in situ hybridization (ISH) using routine methods. HHV-8 latent nuclear antigen (LANA-1), the product of the viral gene open reading frame 73, was detected using the 13B10 monoclonal antibody (Leica Systems, NCL-HHV8) at a standard dilution of 1:50. EBV was detected using an ISH probe against EBER (Ventana, 760-1209 DNP Probe) and used pre-diluted, as received. Viral interleukin 6 (IL-6) staining was performed using a polyclonal rabbit antiserum raised against viral IL-6 peptides as previously described20. All other immunohistochemical staining was performed using routine methods for clinical diagnosis.
Flow cytometric immunophenotyping was performed on fresh tissue. The material was dissociated by passing it through wire mesh, and the cell suspension was incubated with a combination of 4 monoclonal antibodies specific for CD19, CD3, kappa and lambda (Becton Dickinson, San Jose, CA) that were titrated for optimal staining according to standard protocol.
Analysis for B-cell clonality by immunoglobulin gene heavy chain (IgH) gene rearrangement and T cell clonality by T-cell receptor (TCR) gamma gene rearrangements were performed in the Johns Hopkins molecular pathology facility according to established protocols for clinical use. Slices of formalin-fixed, paraffin-embedded tissue were extracted with xylene and absolute ethanol. DNA was prepared using the QiaAMP DNA Mini kit (Quiagen, Valencia, CA, USA) according to the manufacturer's directions and subjected to PCR using degenerate consensus primers to amplify the hypervariable (VDJ) region of the IgH gene from the 3’ end of the V region to the 3’ end of the J region. The primers used were as follows: FR3A (directed at the 3’ end of the V region) 5’-ACACGGCYSTGTATTACTGTG-3’ and VLJH (directed at the 3’ end of the J region) 5’-GTGACCAGGGTNCCTTGGCCCCAG-3’ where Y=C or T, S=G or C and N=C, T, G, or A. In the presence of a monoclonal population, this amplification results in a distinct 50–150 base pair product. Amplification of the beta-globin gene was used as an internal control to ensure sufficient DNA quality and amplification. PCR amplified products were subjected to gel electrophoresis for analysis of clonal bands. T-cell receptor-gamma gene rearrangement was evaluated using the TCRG Gene Clonality Assay (Invivoscribe Technologies, Inc.) according to the manufacturer’s instructions.
CASE REPORT
A 59 year-old HIV-positive man presented with a three week history of diarrhea, abdominal pain, fevers, night sweats, dizziness, an unintentional 30 pound weight loss and recent cognitive decline. The patient had been diagnosed with HIV 27 years before, and was on highly active anti-retroviral therapy (HAART). The patient was not taking any medications requiring subcutaneous injection for administration and he denied intravenous drug use. Physical exam showed no evidence of skin lesions or lymphadenopathy.
Computed tomography scans of the chest, abdomen and pelvis demonstrated that the spleen was enlarged, but no significant lymphadenopathy or masses were seen. The CT scans were remarkable for numerous subcentimeter nodules scattered through the subcutaneous fat that did not show a connection to the overlying epidermis (Figure 1). These nodules showed mildly increased fluorodeoxyglucose uptake on a positron emission tomography scan.
Figure 1. Radiographic studies of the abdomen and pelvis demonstrate numerous subcutaneous nodules.
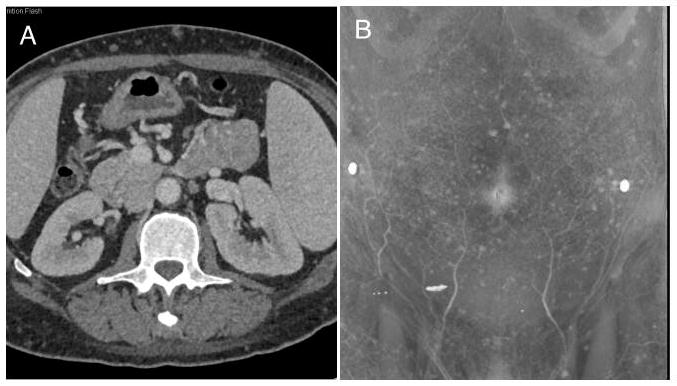
Axial computed tomography scans show multiple nodules in the subcutaneous fat without any enhancement (A). The 3D volume rendered images show the extent of lesion involvement over the abdominal wall (B). These nodules also demonstrated mild FDG uptake (not shown).
Laboratory values on admission were notable for mild anemia (Hb 10.7 g/dL; normal range 13.9–16.3 g/dL) and thrombocytopenia (69 K/cu mm; normal 150–350 K/cu mm) with increased neutrophils (83%; normal 31–76%) and decreased lymphocytes (3%; normal 24–44%). The CD4 count was 526/cubic mm (normal 458–1344/cubic mm), and the HIV-1 viral load was 93 copies/mL. The patient also had an increased lactic dehydrogenase (1329 U/L; normal 118–273 U/L).
A bone marrow biopsy that was performed four days prior to admission at an outside hospital showed hypercellular marrow with trilineage hematopoiesis and increased polyclonal plasma cells with no evidence of lymphoma. Colon biopsies demonstrated severe acute colitis, but were negative for fungi, cytomegalovirus, and viral cytopathic effect. An initial fine needle biopsy of a subcutaneous nodule demonstrated large, atypical cells suggestive of malignancy but the material in the scant specimen was insufficient for a diagnosis.
A subsequent excisional biopsy of one of the subcutaneous nodules in the abdominal wall showed fibroadipose tissue with a patchy mixed inflammatory infiltrate and occasional hemosiderin-laden macrophages. Many of the vessels contained organizing thrombi, and some also contained large atypical cells. The atypical cells had a moderate amount of eosinophilic cytoplasm and round to oval nuclei with one or more prominent nucleoli (Figure 2). There were numerous apoptotic bodies and mitotic figures seen within the vessels.
Figure 2. Thrombosed vessels with a mixed inflammatory infiltrate and prominent atypical intravascular cells.
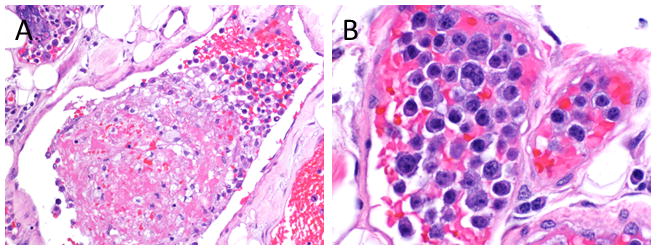
An excisional biopsy of the subcutaneous nodules revealed vessels that contained partially organizing thrombi and a mixed inflammatory infiltrate along with some atypical cells (A, H&E, original magnification 20X). The atypical cells had a moderate amount of eosinophilic cytoplasm and round to oval nuclei with one or more prominent nucleoli (B, H&E, original magnification 50X).
A panel of immunohistochemical stains and in situ hybridization for EBV (EBER) demonstrated that the atypical cells were positive for MUM-1, CD43, HHV-8 (LANA-1),EBV and viral IL-6, with dim expression of CD79a and CD45 (Figure 3). A subset of the atypical cells expressed dim CD4, CD45-RO and cytoplasmic CD3. The atypical cells were restricted to the intravascular compartment, and had a high Ki-67 proliferation index. The B-cell markers CD20, CD19 and Pax-5 as well as the plasma cell markers CD138 and CD38 were negative in the neoplastic cells. Immunostains for IgG, IgM and IgA were difficult to interpret due to high background staining, but many of the atypical cells appeared to express IgG. Immunostains for kappa and lambda light chains were non-contributory due to high background staining. The tumor cells were also negative for CD5, CD7, CD8, TIA-1, CD25, CD30, CD56, CD68, CD163, myeloperoxidase, CD33, CD99, TdT, CD34, CD117, S100, and EMA.
Figure 3. Immunostains and in situ hybridization reveal an HHV-8+, EBV+ B-cell lymphoma with aberrant T-cell marker expression.
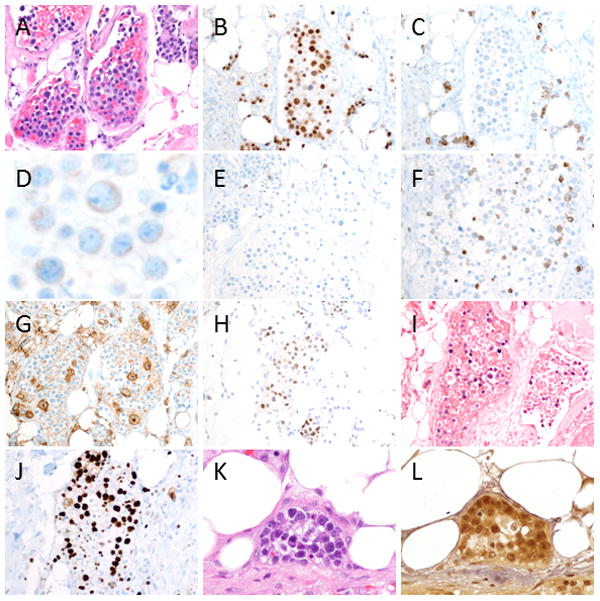
The atypical cells (A, H&E 20x) were positive for MUM-1 (B) and had dim expression of CD79a (C and D), but were negative for CD20 (E), CD19, Pax-5 and CD138 (not shown). The tumor cells also expressed dim cytoplasmic CD3 (F), dim CD4 (G), and CD45-RO (not shown), but were negative for CD5, CD7, CD8, CD56 and TIA-1 (not shown). An immunostain for HHV8 (LANA-1) (H) and an in situ hybridization for EBV (EBER) (I) were positive in the neoplastic cells, which were restricted to the intravascular compartment. The tumor cells had a high Ki-67 proliferation index (J). The neoplastic cells (K, H&E 50x) also showed expression of viral IL-6 (L).
Flow cytometry studies were performed on a portion of the excisional biopsy, but showed predominantly debris with no evidence of a monoclonal B-cell population. A PCR assay for immunoglobulin heavy chain (IgH) gene rearrangements showed an oligoclonal pattern, and a PCR assay for T-cell receptor gamma gene rearrangements was notable for poor amplification although there was a significant polyclonal component.
The patient’s mental and respiratory status waxed and waned over the course of his hospital stay. Shortly after diagnostic tissue was obtained, the patient’s condition abruptly declined and he was transitioned to comfort care. He died approximately 2 months from the reported onset of symptoms.
DISCUSSION
Here we report an intravascular lymphoma that is associated with both EBV and HHV-8 viral infection, which joins a small but growing family of LPDs driven by EBV and HHV-8 co-infection. EBV and HHV-8-positive lymphomas are often clinically aggressive in immunocompromised individuals, and include PEL presenting as an effusion2–4 or mass lesion5,6, germinotrophic lymphoproliferative disorder10, rare cases of multicentric Castleman-associated plasmablastic lymphoma or microlymphoma12,13, a cutaneous plasmablastic lymphoma arising in a transplant patient16, and rare large B-cell lymphomas17. Lymphomas in this family tend to have large, atypical cells with an unusual phenotype4,7,9. The tumor cells typically lack pan B-cell markers such as CD20, CD19 and CD79a, but do show expression of one or more activation or plasma cell markers including MUM-1, CD138 or CD381,10,12,17. This case of EBV and HHV-8-positive intravascular lymphoma also demonstrated aberrant expression of T-cell markers, which can occasionally be seen in EBV/HHV-8-positive lymphomas 6,8,14. The expression of T-cell antigens on tumor cells initially raised the possibility that the biopsy findings represented a primary T-cell intravascular lymphoma.
While most intravascular lymphomas are large B-cell lymphomas, T-cell and NK/T-cell intravascular lymphomas have also been reported. NK/T-cell intravascular lymphomas are the most common of the intravascular T-cell lymphomas, and are characterized by frequent skin involvement and an aggressive clinical course. These express cytotoxic markers including CD8, TIA-1 and CD56, and most are EBV-positive 21–23. The lack of cytotoxic T-cell marker expression and clonal rearrangement of the TCR-gamma gene argues against a diagnosis of NK/T-cell intravascular T-cell lymphoma in this case. There have also been rare reports of intravascular anaplastic large cell lymphomas (ALCL) 24–26. These lymphomas express CD4 and CD30 but are ALK and EBV-negative and typically form a discrete cutaneous mass with tumor cells localized to the lymphatics. Intravascular ALCLs are reported to have a relatively indolent clinical course. Finally, there are rare reports of skin lesions that contain a proliferation of atypical CD30-positive cells within small vessels, and these cases are thought to represent a reactive process27. CD30, which is not a lineage-associated antigen, is not expressed in this case, making a diagnosis of intravascular ALCL or a reactive proliferation unlikely28.
Although the immunophenotype of the tumor cells is consistent with PEL, we are unable to definitively assign a lineage to this intravascular lymphoma. The neoplastic cells are largely negative for B-cell specific antigens, there is no evidence of a B-cell clone on flow cytometric analysis, and IgH gene rearrangement studies did not reveal a clone. Additionally, while the tumor cells expressed dim CD3 and CD4, T-cell receptor (TCR) gene rearrangement studies showed no definitive evidence of a clonal T-cell population. Molecular studies were hampered by the sparse distribution of tumor cells and admixed inflammatory cells, which also precluded microdissection. Despite our inability to demonstrate a B or T-cell clone the immunophenotype is typical of PEL, which is currently considered a mature B-cell lymphoma despite the fact that it characteristically lacks expression of B-cell antigens as well as surface and cytoplasmic immunoglobulin1,2,29. Of note, PEL can also show aberrant expression of T-cell antigens, and may even demonstrate rearrangement of both TCR and immunoglobulin genes or TCR genes alone30,31,32.
Another feature of the tumor in this patient was the finding of extensive organizing thrombi within the small vessels, which surrounded and obscured the neoplastic cells. While fibrin thrombi associated with hemorrhage and necrosis have occasionally been observed in both IVLBCL1,33 and intravascular cytotoxic T-cell lymphoma34, the thrombi were particularly pronounced in this HHV-8 and EBV-positive lymphoma. Thrombosis in the setting of intravascular lymphoma could further accelerate end-organ ischemic damage, leading to a more rapid and aggressive disease course. The mechanism underlying the formation of thrombi in lymphoma is unclear but may relate to production of cytokines by the tumor cells, which could also be potentiated by viral infection. In particular, IL-6, an analogue of which is produced by HHV-8 viral infection, is known to promote coagulation without affecting fibrinolysis35. In one study of the risk factors for developing thrombotic complications in HIV-infected individuals, malignancy in the form of Kaposi sarcoma was one of the most frequently identified risk factors36. While the exact mechanism of thrombosis in this case is uncertain, it is intriguing to speculate that the production of viral cytokines by HHV-8 infected cells contributed to the formation of fibrin thrombi, and the fact that viral IL-6 is expressed by the tumor cells provides support for this hypothesis.
The diagnosis of intravascular lymphoma in this patient required sampling of deep subcutaneous adipose tissue. The excisional biopsy was prompted by the radiographic findings and insufficient sampling on fine needle aspiration. Even when no overt skin findings are present, blind skin biopsies have been shown to be a relatively sensitive way to diagnose intravascular lymphoma37–41. In one case series, 10 of 12 consecutive patients with a known diagnosis of intravascular lymphoma had evidence of lymphoma on skin biopsies, both in lesions and in areas with healthy appearing skin38. Several studies have shown that IVLBCL tends to involve the vessels of the subcutaneous fat but not the dermis37,39. These studies and the findings in this case illustrate that a superficial shave or punch skin biopsy may not be sufficient to diagnose intravascular lymphoma.
The relative risk of developing a B-cell lymphoma is increased by a factor of at least 80-fold in patients with HIV as compared to the general population 1. The incidence of some types of viral-driven neoplasms in HIV-positive individuals has decreased dramatically with the introduction of HAART, including primary CNS lymphoma, which is nearly uniformly EBV-positive42–44. However, the incidence of other types of lymphoma has been relatively unaffected, including Hodgkin and Burkitt lymphoma11,42,44. Reductions have also been seen in the frequency of neoplasms, such as Kaposi sarcoma, that arise in the presence of very low CD4 counts44. Interestingly, lymphoproliferative disorders with HHV-8 and EBV co-infection may arise in patients with well controlled HIV viral loads and CD4 counts greater than 500, as occurred in this case3. Thus, as patients live longer with HIV infection and fewer deaths are attributable to opportunistic infections or other AIDS-defining illnesses, HHV-8 and EBV driven lymphomas may become increasingly prominent.
In summary, EBV and HHV-8 are recognized as important contributors toward lymphomagenesis, but only rare LPDs involving co-infection by these viruses have been described. The findings in this case suggest that inhibition of IL-6 signaling could provide a potential strategy for the amelioration of symptoms in some patients with HHV-8 lymphomas45, and also demonstrate that HHV-8 and EBV-positive lymphomas can present not only as effusions or solid tumor masses but also as intravascular lymphomas.
Acknowledgments
This work was supported in part by grant P30CA006973 from the National Cancer Institute (R.F.A.). We thank Dr. Christopher Gocke and Dr. Guoli Chen for technical advice regarding the molecular assays performed in this study.
References
- 1.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. p. 439. [Google Scholar]
- 2.Nador RG, Cesarman E, Chadburn A, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645–56. [PubMed] [Google Scholar]
- 3.Boulanger E, Gérard L, Gabarre J, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:4372–80. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. The New England journal of medicine. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 5.Deloose STP, Smit LA, Pals FT, et al. High incidence of Kaposi sarcoma-associated herpesvirus infection in HIV-related solid immunoblastic/plasmablastic diffuse large B-cell lymphoma. Leukemia. 2005;19:851–5. doi: 10.1038/sj.leu.2403709. [DOI] [PubMed] [Google Scholar]
- 6.Dong HY, Wang W, Uldrick TS, et al. Human herpesvirus 8- and Epstein-Barr virus-associated solitary B cell lymphoma with a T cell immunophenotype. Leukemia & lymphoma. 2013 doi: 10.3109/10428194.2012.747680. [DOI] [PubMed] [Google Scholar]
- 7.DePond W, Said JW, Tasaka T, et al. Kaposi’s sarcoma-associated herpesvirus and human herpesvirus 8 (KSHV/HHV8)-associated lymphoma of the bowel. Report of two cases in HIV-positive men with secondary effusion lymphomas. The American journal of surgical pathology. 1997;21:719–24. doi: 10.1097/00000478-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: a tissue-based variant of primary effusion lymphoma. The Journal of molecular diagnostics: JMD. 2005;7:17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadburn A, Hyjek E, Mathew S, et al. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. The American journal of surgical pathology. 2004;28:1401–16. doi: 10.1097/01.pas.0000138177.10829.5c. [DOI] [PubMed] [Google Scholar]
- 10.Du MQ, Diss TC, Liu H, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–8. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 11.Dong HY, Scadden DT, de Leval L, et al. Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. The American journal of surgical pathology. 2005;29:1633–41. doi: 10.1097/01.pas.0000173023.02724.1f. [DOI] [PubMed] [Google Scholar]
- 12.Seliem RM, Griffith RC, Harris NL, et al. HHV-8+, EBV+ multicentric plasmablastic microlymphoma in an HIV+ Man: the spectrum of HHV-8+ lymphoproliferative disorders expands. The American journal of surgical pathology. 2007;31:1439–45. doi: 10.1097/PAS.0b013e31804d43d8. [DOI] [PubMed] [Google Scholar]
- 13.Peker D, Alkan S, Zhang L, et al. HIV-associated plasmablastic multicentric Castleman disease with microlymphoma coinfected with HHV8 and EBV. Journal of Hematopathology. 2013;6:109–114. [Google Scholar]
- 14.Boulanger E, Hermine O, Fermand JP, et al. Human herpesvirus 8 (HHV-8)-associated peritoneal primary effusion lymphoma (PEL) in two HIV-negative elderly patients. American journal of hematology. 2004;76:88–91. doi: 10.1002/ajh.20048. [DOI] [PubMed] [Google Scholar]
- 15.Boulanger E, Afonso PV, Yahiaoui Y, et al. Human herpesvirus-8 (HHV-8)-associated primary effusion lymphoma in two renal transplant recipients receiving rapamycin. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:707–10. doi: 10.1111/j.1600-6143.2007.02110.x. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, Nuovo GJ, Porcu P, et al. Epstein-Barr virus- and human herpesvirus 8-associated primary cutaneous plasmablastic lymphoma in the setting of renal transplantation. Journal of cutaneous pathology. 2005;32:35–9. doi: 10.1111/j.0303-6987.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferry JA, Sohani AR, Longtine JA, et al. HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma and HHV8-positive intravascular large B-cell lymphoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22:618–26. doi: 10.1038/modpathol.2009.36. [DOI] [PubMed] [Google Scholar]
- 18.Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109:478–85. doi: 10.1182/blood-2006-01-021253. [DOI] [PubMed] [Google Scholar]
- 19.Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. The lancet oncology. 2009;10:895–902. doi: 10.1016/S1470-2045(09)70140-8. [DOI] [PubMed] [Google Scholar]
- 20.Cannon JS, Nicholas J, Orenstein JM, et al. Heterogeneity of viral IL-6 expression in HHV-8-associated diseases. The Journal of infectious diseases. 1999;180:824–8. doi: 10.1086/314956. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita Y, Tsuzuki T, Nakayama A, et al. A case of natural killer/T cell lymphoma of the subcutis resembling subcutaneous panniculitis-like T cell lymphoma. Pathology international. 1999;49:241–6. doi: 10.1046/j.1440-1827.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Said JW, Ames ED, et al. First reported cases of intravascular large cell lymphoma of the NK cell type: clinical, histologic, immunophenotypic, and molecular features. American journal of clinical pathology. 2005;123:603–11. doi: 10.1309/X597-G3QM-XAFB-CM5V. [DOI] [PubMed] [Google Scholar]
- 23.Cerroni L, Massone C, Kutzner H, et al. Intravascular large T-cell or NK-cell lymphoma: a rare variant of intravascular large cell lymphoma with frequent cytotoxic phenotype and association with Epstein-Barr virus infection. The American journal of surgical pathology. 2008;32:891–8. doi: 10.1097/PAS.0b013e31815d29c9. [DOI] [PubMed] [Google Scholar]
- 24.Deetz CO, Gilbertson KG, Anadkat MJ, et al. A rare case of intravascular large T-cell lymphoma with an unusual T helper phenotype. The American Journal of dermatopathology. 2011;33:e99–102. doi: 10.1097/DAD.0b013e318221bc25. [DOI] [PubMed] [Google Scholar]
- 25.Rieger KE, Polidore T, Warnke R, et al. ALK-negative systemic intravascular anaplastic large cell lymphoma presenting in the skin. Journal of cutaneous pathology. 2011;38:216–20. doi: 10.1111/j.1600-0560.2010.01528.x. [DOI] [PubMed] [Google Scholar]
- 26.Metcalf RA, Bashey S, Wysong A, et al. Intravascular ALK-negative anaplastic large cell lymphoma with localized cutaneous involvement and an indolent clinical course: toward recognition of a distinct clinicopathologic entity. The American journal of surgical pathology. 2013;37:617–23. doi: 10.1097/PAS.0b013e318280aa9c. [DOI] [PubMed] [Google Scholar]
- 27.Riveiro-Falkenbach E, Fernández-Figueras MT, Rodríguez-Peralto JL. Benign atypical intravascular CD30(+) T-cell proliferation: a reactive condition mimicking intravascular lymphoma. The American Journal of dermatopathology. 2013;35:143–50. doi: 10.1097/DAD.0b013e3182323119. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt S, Ashlock BM, Natkunam Y, et al. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood. 2013;122:1233–42. doi: 10.1182/blood-2013-01-481713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792–9. [PubMed] [Google Scholar]
- 30.Hollingsworth HC, Stetler-Stevenson M, Gagneten D, et al. Immunodeficiency-associated malignant lymphoma. Three cases showing genotypic evidence of both T- and B-cell lineages. The American journal of surgical pathology. 1994;18:1092–101. [PubMed] [Google Scholar]
- 31.Said JW, Shintaku IP, Asou H, et al. Herpesvirus 8 inclusions in primary effusion lymphoma: report of a unique case with T-cell phenotype. Archives of pathology & laboratory medicine. 1999;123:257–60. doi: 10.5858/1999-123-0257-HIIPEL. [DOI] [PubMed] [Google Scholar]
- 32.Coupland SE, Charlotte F, Mansour G, et al. HHV-8-associated T-cell lymphoma in a lymph node with concurrent peritoneal effusion in an HIV-positive man. The American journal of surgical pathology. 2005;29:647–52. doi: 10.1097/01.pas.0000157937.01624.1d. [DOI] [PubMed] [Google Scholar]
- 33.Vercambre-Darras S, Mortier L, Lambert M, et al. Intravascular lymphoma revealed by generalized arborescent telangiectasia and repeated venous thrombosis. Annales de dermatologie et de vénéréologie. 2008;135:397–401. doi: 10.1016/j.annder.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Gleason BC, Brinster NK, Granter SR, et al. Intravascular cytotoxic T-cell lymphoma: A case report and review of the literature. Journal of the American Academy of Dermatology. 2008;58:290–4. doi: 10.1016/j.jaad.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. British journal of haematology. 2001;115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson MC, Dezube BJ, Aboulafia DM. Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;39:1214–22. doi: 10.1086/424664. [DOI] [PubMed] [Google Scholar]
- 37.Higashi Y, Kawai K, Yonekura K, et al. Indication for Random Skin Biopsy for the Diagnosis of Intravascular Large B Cell Lymphoma. Dermatology Basel Switzerland. 2012;8520:1–5. doi: 10.1159/000336885. [DOI] [PubMed] [Google Scholar]
- 38.Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Annals of hematology. 2011;90:417–21. doi: 10.1007/s00277-010-1101-3. [DOI] [PubMed] [Google Scholar]
- 39.Asada N, Odawara J, Kimura S, et al. Use of random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Mayo Clinic proceedings. Mayo Clinic. 2007;82:1525–7. doi: 10.1016/s0025-6196(11)61097-5. [DOI] [PubMed] [Google Scholar]
- 40.Gill S, Melosky B, Haley L, et al. Use of random skin biopsy to diagnose intravascular lymphoma presenting as fever of unknown origin. The American journal of medicine. 2003;114:56–8. doi: 10.1016/s0002-9343(02)01378-5. [DOI] [PubMed] [Google Scholar]
- 41.Le EN, Gerstenblith MR, Gelber AC, et al. The use of blind skin biopsy in the diagnosis of intravascular B-cell lymphoma. Journal of the American Academy of Dermatology. 2008;59:148–51. doi: 10.1016/j.jaad.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Kirk O, Pedersen C, Cozzi-Lepri A, et al. Non-Hodgkin lymphoma in HIV-infected patients in the era of highly active antiretroviral therapy. Blood. 2001;98:3406–12. doi: 10.1182/blood.v98.12.3406. [DOI] [PubMed] [Google Scholar]
- 43.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS (London, England) 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. Journal of the National Cancer Institute. 2000;92:1823–30. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 45.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–32. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 46.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–80. [PubMed] [Google Scholar]


