Summary
Small heat-shock proteins (sHSPs) are a widely conserved family of molecular chaperones, all containing a conserved α-crystallin domain flanked by variable N- and C-terminal tails. We report that IbpA and IbpB, the sHSPs of Escherichia coli, are substrates for the AAA+ Lon protease. This ATP-fueled enzyme degraded purified IbpA substantially more slowly than purified IbpB, and we demonstrate that this disparity is a consequence of differences in maximal Lon degradation rates and not in substrate affinity. Interestingly, however, IbpB stimulated Lon degradation of IbpA both in vitro and in vivo. Furthermore, although the variable N- and C-terminal tails of the Ibps were dispensable for proteolytic recognition, these tails contain critical determinants that control the maximal rate of Lon degradation. Finally, we show that E. coli Lon degrades variants of human α-crystallin, indicating that Lon recognizes conserved determinants in the folded α-crystallin domain itself. These results suggest a novel mode for Lon substrate recognition and provide a highly suggestive link between the degradation and sHSP branches of the protein quality-control network.
Introduction
Organisms constantly combat environmental insults, which can denature proteins, destroy molecular function and rapidly result in toxic aggregates. Under these circumstances, quality-control networks in the cell attempt to refold damaged proteins (Visick and Clarke, 1995; Feder and Hofmann, 1999). In Escherichia coli, the quality-control network includes IbpA and IbpB, which are members of the small heat-shock protein family (sHSPs), as well as the energy-dependent chaperones GroEL/GroES and DnaK/DnaJ/GrpE, and the ClpB disaggregase (Georgopoulos and Welch, 1993;Parsell and Lindquist, 1993; Lund, 2001). Lon and other AAA+ (ATPases associated with various cellular activities) proteases also play a major role in clearing misfolded and damaged proteins from the cell. Recent work demonstrates that certain branches of the quality-control network can cooperate to refold damaged proteins (Mogk et al., 2003a,b; Doyle et al., 2007). Here we investigate a possible link between the protein degradation branch and the sHSP branch of the protein quality-control network.
The sHSPs are a family of ATP-independent molecular chaperones. Current studies indicate that sHSPs bind and function to protect misfolded proteins from irreversible aggregation (Laskowska et al., 1996; Lee et al., 1997; Narberhaus, 2002). All sHSPs contain a central conserved α-crystallin domain flanked by N- and C-terminal tails of variable length and amino-acid composition (Haslbeck et al., 2005). sHSPs exist as higher-order oligomers, a characteristic that is crucial for client-protein protection (Leroux et al., 1997; van de Klundert et al., 1998).
Escherichia coli has two sHSPs, IbpA and IbpB, which are expressed from the same operon (Allen et al., 1992). The Ibps are important for resistance to heat stress, as well as superoxide and copper-induced oxidative stress (Thomas and Baneyx, 1998;Kitagawa et al., 2000; Matuszewska et al., 2008). Furthermore, IbpA and IbpB interact with each other at elevated temperatures in vitro and cooperate to keep client proteins in a refolding-competent state (Matuszewska et al., 2005). After thermal inactivation, for example, luciferase is reactivated more efficiently by ClpB and DnaK/J/E in vitro, if both IbpA and IbpB are present during the initial thermal inactivation, than if only one of the Ibps is present (Ratajczak et al., 2009). The Ibps also cooperate with ClpB and DnaK to reverse protein aggregation in vivo (Mogk et al., 2003a). Thus, the Ibps interact functionally with the disaggregation and refolding machinery of the cell. However, to date, the Ibps have not been specifically linked to the protein-degradation machinery.
Lon is the principal protease responsible for the degradation of damaged and misfolded proteins in E. coli (Kowit and Goldberg, 1977; Fredriksson et al., 2005). For example, Lon-deficient cells accumulate more aggregated proteins following heat shock than cells missing other AAA+ proteases (Tomoyasu et al., 2001; Rosen et al., 2002). Recent work demonstrates that Lon can directly recognize misfolded substrates by binding to exposed hydrophobic regions, which would normally be buried in properly folded proteins (Gur and Sauer, 2008b), providing insight into one mode by which Lon recognizes substrates.
In this study, we report that IbpA and IbpB are themselves substrates for Lon, suggesting that there is functional cross-talk between the sHSPs and the protein degradation machinery. We further demonstrate that the rates of degradation of the Ibps are controlled by their N- and C-terminal tails. Unexpectedly, however, these tails are not required for Lon recognition. Rather, they influence the maximal speed of Lon proteolysis. Finally, we show that the presence of IbpB accelerates the rate of Lon degradation of IbpA both in vitro and in vivo. Our results therefore provide a new link between the sHSP and the protein-degradation branches of the quality-control network and further suggest a distinct method of substrate recognition by Lon.
Results
The E. coli sHSPs IbpA and IbpB are Lon substrates
IbpA and IbpB were initially identified in a proteomic screen for substrates of E. coli Lon protease (E. Oakes and TAB, pers. comm.), using a strategy similar to previous screens for ClpXP substrates (Flynn et al., 2003; Neher et al., 2006). Briefly, Lon carrying an active-site mutation was used to trap substrates in vivo (Van Melderen and Gottesman, 1999), and the trapped substrates were identified by mass spectrometry. Approximately 200 potential Lon substrates were identified as high-quality Lon-interacting proteins using this method. To determine directly whether IbpA and IbpB are substrates for Lon, we purified 35S-labelled IbpA and IbpB and measured the rate at which Lon proteolysis generated acid-soluble radioactive peptides. Both proteins were degraded by Lon (Fig. 1B). However, despite the fact that IbpA and IbpB are approximately 50% identical at the sequence level (Fig. 1A), Lon degraded IbpB much faster than IbpA (Fig. 1B).
Figure 1.

E. coli sHSPs IbpA and IbpB are Lon substrates A. Sequence alignment of IbpA and IbpB generated by ClustalW2. Darker letters represent the conserved α-crystallin domain. Identical residues are denoted by (*), residues with the same size and hydropathy are denoted by (:), residues with the same size or hydropathy are denoted by (.). B. Lon (600 nM hexamer) degradation of 5 μM IbpA (0.010 ± 0.001 min−1 Lon6−1) or 5 μM IbpB (0.20 ± 0.02 min−1 Lon6−1). C. Substrate dependence of Lon degradation of IbpA (Vmax = 0.043 ± 0.018 min−1 Lon6−1, KM = 18 ± 16 μM) or IbpB (Vmax = 0.60 ± 0.06 min−1 Lon6−1, KM = 16 ± 2.7 μM). Degradation rates were measured from experiments like the one shown in (B) and were fit to the Michaelis-Menten equation. Error bars (±1 SD) in this and all other figures were calculated from at least three independent experiments. The large error in the KM for IbpA is due to the slow rate of degradation. Inset: The IbpA data are replotted on an expanded scale to show the curvature of the fitted line.
This difference in degradation rates could result from altered Lon recognition, which would be reflected in different KM values for proteolysis. Alternatively (or in addition), Lon processing of IbpA and IbpB might differ at a downstream step, such as substrate unfolding or translocation, leading to differences in Vmax. To distinguish between these possibilities, we determined steady-state rates of degradation of varying concentrations of IbpA and IbpB by Lon and fit these data to the Michaelis-Menten equation (Fig. 1C). Interestingly, the KM values for Lon degradation of IbpA and IbpB were nearly identical (16–18 μM). However, the Vmax for IbpB degradation was almost 15-fold higher than that of IbpA (Fig. 1C; Table 1). Thus, Lon recognizes IbpA and IbpB as substrates equally well but processes IbpB much faster than IbpA.
Table 1. Steady-state kinetic parameters for the degradation of Lon substrates.
| Substrate | KM(μM) | Vmax (substrate degraded min−1 Lon6−1) |
|---|---|---|
| IbpA | 18 ± 16 | 0.043 ± 0.018 |
| IbpB | 16 ± 2.7 | 0.60 ± 0.06 |
| IbpAα | 17 ± 4.7 | 0.47 ± 0.06 |
| IbpBα | 16 ± 2.4 | 0.19 ± 0.01 |
| hαA-crystallin | 50 ± 20 | 0.12 ± 0.05 |
| hαB-crystallin | 58 ± 21 | 0.06 ± 0.02 |
| hαAα-crystallin | 35 ± 8.9 | 2.5 ± 0.3 |
| hαBα-crystallin | 26 ± 12 | 3.8 ± 1.4 |
Ibp tails are not required for Lon recognition
The degradation determinants of AAA+ protease substrates are often (although not always) found near the very N- or C-terminus of the substrate (Tobias et al., 1991;Gottesman et al., 1998; Flynn et al., 2003). Furthermore, the N- and C-terminal tails of sHSPs are known to be important for oligomerization and client-protein protection (Fernando and Heikkila, 2000; Jiao et al., 2005b). To investigate the role of these terminal tails in Lon degradation of IbpA and IbpB, we constructed and purified Ibp variants lacking both tails and containing only the α-crystallin domain (referred to as IbpAα and IbpBα). Intriguingly, at substrate concentrations of 5 μM, Lon degraded IbpBα about a third as fast as IbpB but degraded IbpAα 10 times faster than IbpA (Fig. 2A). The higher rate of degradation of IbpAα than IbpA was not caused by a truncation-induced loss of protein secondary structure, as determined by circular-dichroism spectroscopy (data not shown). Thus, these experiments indicate that the tails of IbpA inhibit Lon degradation, whereas those of IbpB promote degradation.
Figure 2.

Lon degrades the isolated α-crystallin domains of IbpA and IbpB. A. Degradation of 5 μM substrate by 600 nM Lon6. IbpA (filled squares), IbpB (filled circles), IbpAα (open squares) or IbpBα (open circles). B. Michaelis-Menten plot for IbpAα (Vmax = 0.47 ± 0.06 min−1 Lon6−1, KM = 17 ± 4.7 μM). C. Michaelis-Menten plot for IbpBα (Vmax = 0.19 ± 0.01 min−1 Lon6−1, KM = 16 ± 2.4 μM). For comparison, the plots for IbpA and IbpB are shown in grey.
To investigate the contribution of the Ibp tails to Lon recognition, we determined the concentration-dependence of IbpAα and IbpBα degradation (Fig. 2B and C). KM values for Lon degradation of IbpAα and IbpBα were nearly identical and were within error of those measured for IbpA and IbpB (Table 1). Once again, differences in the rates of Lon degradation were completely attributable to differences in Vmax values (Fig. 2B and C; Table 1). These experiments demonstrate that the N- and C-terminal tails of the Ibps are dispensable for Lon recognition. Thus, unlike other specific substrates of Lon, the Ibps are recognized via sequence or structural determinants in the body of the protein, specifically, within the α-crystallin domains of the Ibps.
Lon degrades human α-crystallin domains
Our results with the tail-less Ibps clearly indicate that Lon recognizes a region within the α-crystallin domains of the Ibps. We hypothesized that Lon recognizes a feature of the natively folded α-crystallin domain and might therefore degrade other α-crystallin proteins. To test this model, we purified full-length versions of two human α-crystallin proteins, αA-crystallin (hαA) and αB-crystallin (hαB), as well as the tail-less α-crystallin domains of these proteins (hαAα and hαBα), and assayed degradation by Lon in vitro (Fig. 3B and C; Table 1). Strikingly, hαAα and hαBα were both excellent Lon substrates. Indeed, the second-order rate constants (Vmax/KM) for Lon degradation of these α-crystallin domains were twofold to fourfold larger than the value for IbpB, which was the best Lon substrate among the full-length and tail-less Ibp proteins. Furthermore, although the Vmax values for Lon degradation of human αA, αB, αAα and αBα differed substantially (0.06–3.8 min−1 Lon6−1; Table 1), the KM values were similar (26–58 μM). These experiments establish that Lon can degrade α-crystallin domains that are only distantly related to the Ibps (Fig. 3A) and support our hypothesis that Lon may recognize a feature of the folded α-crystallin domain.
Figure 3.
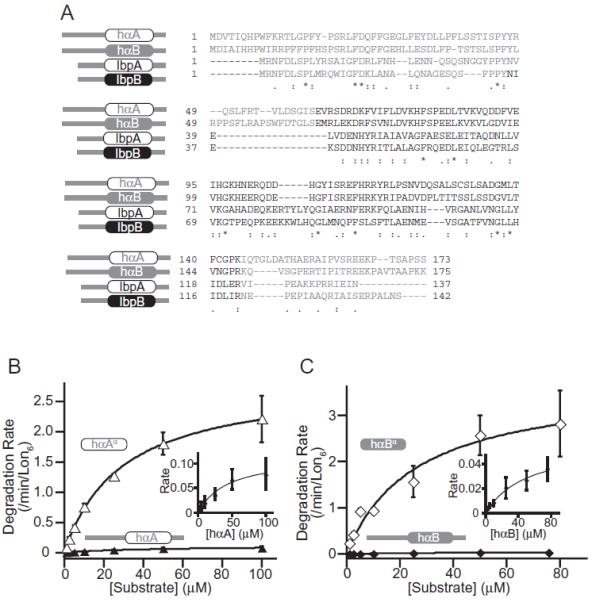
Lon degrades human α-crystallin proteins. A. Sequence alignment of E. coli IbpA and IbpB and human αA-crystallin and αB-crystallin generated by ClustalW2. B. Michaelis-Menten plots for Lon degradation of full-length αA-crystallin (Vmax = 0.12 ± 0.05 min−1 Lon6−1, KM = 50 ± 20 μM) or αAα (Vmax = 2.5 ± 0.3 min−1 Lon6−1, KM = 35 ± 8.9 μM). C. Michaelis-Menten plots for Lon degradation of full-length αB-crystallin (Vmax = 0.06 ± 0.02 min−1 Lon6−1, KM = 58 ± 21 μM) or αBα (Vmax = 3.8 ± 1.4 min−1 Lon6−1, KM = 26 ± 12 μM). Insets: The data for hαA and hαB are replotted on expanded scales.
It is possible that the native and denatured forms of the α-crystallin domain are in dynamic equilibrium and that Lon recognizes a peptide sequence in the unfolded protein. However, given that all of these variants contain the same modular fold, but different amino-acid sequences, and were all degraded with similar KM values, we consider it more likely that Lon recognizes a general characteristic of the folded α-crystallin domain.
The oligomeric-states of full-length and tail-less α-crystallin proteins do not correlate with their rates of degradation
The ability to form higher-order oligomers is thought to be of crucial importance for sHSPs to perform their chaperone functions (Fernando and Heikkila, 2000). Furthermore, oligomerization is disrupted by deletions in the N- and C-terminal tails of sHSPs (Fernando and Heikkila, 2000; Jiao et al., 2005b). To investigate the possible relationship between the oligomeric state of the Ibps/human α-crystallin proteins and their rates of degradation by Lon, we performed gel-filtration analysis on full-length and tail-less IbpB (Fig. 4A) and full-length and tail-less human αB-crystallin (Fig. 4B). Consistent with previous reports, we found that tail-less versions of both IbpB and αB-crystallin eluted as much smaller complexes than the full-length proteins. However, although IbpBα is only degraded one-third as fast as full-length IbpB, αBα is degraded over 60 times faster than full-length αB-crystallin (Figs 2C and 3C; Table 1). Thus, we find that Lon is able to degrade both highly oligomerized versions of α-crystallin proteins (IbpB), as well as smaller oligomeric versions of α-crystallin proteins (αBα) in a robust manner. Therefore, differences in multimeric state do not explain how the tails of the Ibps/α-crystallin proteins control their rate of degradation.
Figure 4.
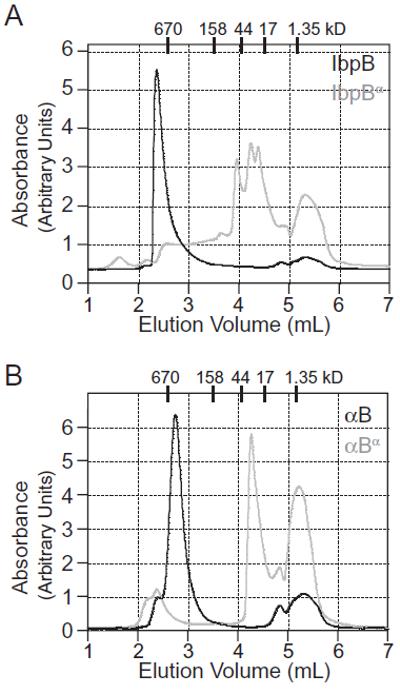
Tail-less E. coli IbpB and human αB-crystallin elute as smaller oligomers than their full-length counterparts. Gel-filtration chromatography of purified proteins monitored by absorbance at 280 nm or 213 nm. A. E. coli IbpB (black line) and IbpBα (grey line). B. Human αB-crystallin (black line) and αBα (grey line). The calculated monomer molecular weights of IbpB, IbpBα, αB and αBα are ~16 kDa, ~10 kDa, ~20 kDa and ~10 kDa respectively. Tick marks at the top of each panel indicate the elution positions of molecular-weight standards.
IbpB facilitates Lon degradation of IbpA in vivo and in vitro
IbpA and IbpB interact and cooperate to perform chaperone functions (see Introduction and Matuszewska et al., 2005). To investigate potential synergy in degradation, we assayed Lon degradation of 35S-IbpA in the presence of unlabelled IbpB and vice versa. Importantly, the rate of IbpA degradation increased sevenfold in the presence of equimolar IbpB, indicating that IbpB facilitates Lon degradation of IbpA. In contrast, the rate of IbpB degradation decreased twofold in the presence of equimolar IbpA (Fig. 5A).
Figure 5.

IbpB facilitates IbpA degradation both in vitro and in vivo. A. Degradation of 5 μM 35S-IbpA (grey squares), 5 μM 35S-IbpB (grey circles), 5 μM 35S-IbpA with 5 μM unlabelled IbpB (black squares), and 5 μM 35S-labelled IbpB with 5 μM unlabelled IbpA (black circles) by 600 nM Lon6. Asterisks denote 35S-labelled protein. B. Western blots probed with an IbpA-specific antibody showing the time-course of IbpA degradation in wild-type (top panel, left side), lon− (top panel, right side), ibpB− (bottom panel, left side) or ibpB−lon− (bottom panel, right side) strains after inhibiting translation with spectinomycin. Representative blots from one of three independent experiments are shown. C. Bands from the experiments in panel B were quantified, and relative intensities are plotted.
To determine if IbpB alters Lon degradation of IbpA in vivo, we measured the intracellular turnover of IbpA in the presence or absence of IbpB at 45°C, where both proteins are expressed at reasonably high levels (Laskowska et al., 1996). IbpA was degraded with a half-life of about 15 min in a lon+ strain but was completely stabilized in an otherwise isogenic lon− strain (Fig. 5B, top panel and 5C), supporting the idea that under these conditions, Lon is the predominant protease responsible for IbpA degradation in vivo. Furthermore, in complete agreement with our experiment in vitro (Fig. 5A), IbpA was also stabilized in an ibpB−lon+ strain (Fig. 5B, bottom panel and 5C), indicating that both IbpB and Lon are essential for efficient intracellular degradation of IbpA. Because our IbpB antibody was not as sensitive as the IbpA antibody, we were unable to detect endogenous levels of IbpB, even after heat shock. However, in a strain lacking chromosomal ibpAB, overexpressed IbpB was degraded in a largely Lon-dependent manner (data not shown), consistent with the fact that Lon degrades IbpB in the absence of IbpA in vitro. These degradation results in vivo are also consistent with the finding that IbpA and IbpB were both recovered in substrate-trapping experiments with proteolytically inactive Lon (E. Oakes and TAB, pers. comm.). Thus, we conclude that both IbpA and IbpB are Lon substrates and that IbpB facilitates Lon degradation of IbpA in vitro and in vivo, suggesting that some type of IbpA–IbpB–Lon interaction is important for properly controlled degradation.
Discussion
In this work, we demonstrate that Lon degrades the E. coli sHSPs, IbpA and IbpB. Unlike other specific Lon substrates (non-damaged proteins) whose recognition determinants are at the far N- or C-terminus of the substrate, the Lon-recognition determinants of the Ibps are located within the core α-crystallin domains of the Ibps. However, the N- and/or C-terminal tails of the Ibps play a critical role in controlling their rates of degradation. Interestingly, this control is manifested as a difference in the maximum rate of Ibp degradation and not as a difference in KM. Finally we show that IbpA is degraded more efficiently by Lon when IbpB is present both in vitro and in vivo.
Lon recognition of Ibps
AAA+ proteases typically recognize peptide signals near the N- or C-termini of substrates (Tobias et al., 1991; Gottesman et al., 1998; Flynn et al., 2003), and Lon can also choose substrates by interacting with specific peptide signals (Gonzalez et al., 1998; Ishii and Amano, 2001; Shah and Wolf, 2006; Choy et al., 2007; Gur and Sauer, 2008a,b). However, neither IbpA nor IbpB contain an obvious sequence that resembles well-characterized Lon degradation tags. Instead, we propose that Lon recognizes a portion of the folded α-crystallin domains of IbpA and IbpB (Fig. 6A). This model explains our findings that Lon degrades the full-length Ibps, their tail-less α-crystallin domains, as well as human αA-crystallin and αB-crystallin and their tail-less counterparts. Furthermore, in each case, the KM values for these degradation reactions ranged from 15 to 60 μM (Table 1), suggesting that Lon recognizes these variants with similar affinities. An alternative model is that Lon recognizes a common peptide motif within these α-crystallin domains. However, this mechanism is unlikely given the lack of similarity between the amino-acid sequences of these proteins (Fig. 3A). Moreover, human mitochondrial Lon degrades the folded form of the α-subunit of mitochondrial processing peptidase more efficiently than it degrades the unfolded form of this substrate (Ondrovicováet al., 2005), providing a precedent for Lon recognition of a structural feature of a folded domain.
Figure 6.

Models for Lon recognition and possible roles for Ibp degradation. A. Lon recognizes the α-crystallin domains of IbpA and IbpB and not the N- and C-terminal tails of these proteins. B. Potential roles for Lon degradation of Ibp proteins in vivo. Lon may degrade free Ibps (pathway 1), degrade client proteins and bound Ibps at the same time (pathway 2) or degrade client-bound Ibps to allow refolding of client proteins (pathway 3).
The tails of IbpA and IbpB control the rate of Lon degradation
Interestingly, the Vmax values for Lon degradation of different variants of IbpA, IbpB, αA-crystallin and αB-crystallin varied substantially, in some cases by more than 50-fold (Table 1). Moreover, the tails of IbpA, αA-crystallin and αB-crystallin slow degradation, whereas the tails of IbpB facilitate degradation. Although the tails of sHSPs are known to affect sHSP oligomerization, we found no correlation between the oligomeric state of Ibp/α-crystallin and their rate of degradation (Figs 2C, 3C and 4). This ability of the protein tails to alter the maximal rate of degradation is unusual, as degradation by AAA+ proteases is usually regulated at the level of recognition (for review, see Baker and Sauer, 2006). However, recent studies show that model substrates can programme the speed and efficiency of degradation by Lon (Gur and Sauer, 2009). The Ibps therefore appear to be examples of biological Lon substrates whose degradation can be controlled in this manner.
IbpB activates Lon degradation of IbpA
Our studies show that IbpB facilitates Lon degradation of IbpA both in vitro and in vivo (Fig. 5). This apparent interaction parallels the ability of IbpA and IbpB to cooperate in performing their chaperone activity in vitro (Matuszewska et al., 2005;Ratajczak et al., 2009). However, to our knowledge, cooperation of any kind between endogenous IbpA and IbpB in vivo has not previously been described. It remains to be determined whether IbpB-controlled degradation of IbpA by Lon serves a regulatory role or is related, in some fashion, to chaperone activities in vivo. Furthermore, although the rate of IbpB-stimulated IbpA degradation in vivo is slow compared with the degradation of some of the well-characterized Lon substrates [t1/2-15 min compared with 2 min for SoxS and 1.2 min for SulA (Mizusawa and Gottesman, 1983; Griffith et al., 2004)], there are other examples of biologically important substrates of AAA+ proteases with long half-lives. For example, in minimal media, the half-life of UvrA, the nucleotide excision repair protein and ClpXP substrate, is 70 min (Pruteanu and Baker, 2009).
Despite the relatively slow degradation of the Ibps, the connections between the assembled Ibps and Lon suggest that there may be functional cross-talk between the sHSPs and the protein-degradation machinery. For example, IbpA and IbpB may be degraded by Lon as they cooperate to help Lon recognize and degrade damaged proteins, which cannot be refolded (Fig. 6B). In this model, IbpA and IbpB act analogously to the Bacillus subtilis MecA adaptor protein, which facilitates the degradation of unfolded and aggregated substrates by the protease ClpCP in vitro and is itself degraded in the process (Turgay et al., 1998; Schlothauer et al., 2003). We propose that Lon also degrades free Ibps to keep their concentrations low during non-stress conditions, or during return to normal conditions following heat shock, when Ibp levels rapidly decrease (Mogk et al., 2003a; Jiao et al., 2005a). In this case, Lon degradation of unneeded Ibps might serve to prevent spurious Ibp binding to functional proteins.
Interestingly, we found that IbpA is degraded robustly by Lon under heat stress in vivo (Fig. 5B and C), when the Ibps would be expected to be bound to client proteins (Laskowska et al., 1996). As discussed above, the Ibps might deliver bound client proteins to Lon and be degraded in the process (Fig. 6B, pathway 2). Several reports demonstrate the involvement of eukaryotic sHSPs in delivering specific clients for degradation (den Engelsman et al., 2003; Parcellier et al., 2003; Lin et al., 2006;Ahner et al., 2007), and prokaryotic sHSPs may play a similar role. Alternatively, degradation of client-bound Ibps might be required to remove the Ibps and thus to facilitate client-protein refolding (Fig. 6B, pathway 3). In vitro, ClpB greatly accelerates DnaK/J/E-mediated refolding of sHSP-bound aggregated proteins, probably by disaggregating sHSP-client complexes (Mogk et al., 2003b; Ratajczak et al., 2009). As our results suggest that Lon can degrade client-bound Ibps in vivo (Fig. 5B), Lon may also contribute to enhanced refolding of client proteins by removing bound Ibps. Consistently, preliminary experiments suggest that Lon degrades client-bound Ibps but not Ibp-bound clients in vitro (data not shown). Experiments to more completely investigate this model are underway.
Finally, our results demonstrate that IbpB is required for the efficient degradation of IbpA (Fig. 5). Because IbpA and IbpB are co-transcribed, IbpA is expected to be translated first. As a result, a mechanism could have evolved to prevent Lon degradation of IbpA before it binds to its partner, IbpB. By this model, IbpA degradation is unlikely to simply be a way to clear unassembled subunits but rather is likely to serve an important functional role. Further experiments will be needed to interrogate models for the biological roles of the Ibp-Lon collaboration.
Experimental procedures
Protein purification
All Ibp and human α-crystallin proteins were purified using a method modified from Malakhov et al. (2004). The human α-crystallin constructs were a gift from Ligia Acosta-Sampson and Jonathan King, MIT. His-SUMO fusions of each gene were cloned downstream of the T7 promoter and expressed in BL21 δibp::kan. Cultures of each strain (1 l) were grown in Luria-Bertani (LB) broth at 37°C, induced at OD600 0.8 with 0.5 mM IPTG, grown for an additional 3 h at 30°C, harvested and stored frozen at −80°C until purification. Cells were thawed, resuspended in 3 ml of 50 mM HEPES-KOH (pH 8.0), and 0.5 ml of the nuclease Benzonase (Novagen) was added. The cells were lysed in a cell disruptor (Constant Systems) at 25 MPa, 10 ml of lysate dilution buffer (50 mM HEPES-KOH [pH 8.0], 4.5 M urea, 1.5 mM β-mercaptoethanol [BME], 600 mM potassium glutamate) was added, the lysate was cleared by centrifugation at 30000 × g for 20 min, and the supernatant was incubated with 2 ml Ni-NTA beads (Qiagen) for 30 min at 4°C. The beads were packed in a column, rinsed with 250 ml LG-5 buffer (50 mM HEPES-KOH [pH 8.0], 4 M urea, 1 mM BME, 400 mM potassium glutamate, 5 mM imidazole) followed by 250 ml LG-20 buffer (identical to LG-5 buffer except with 20 mM imidazole). The protein was eluted with 10 ml elution buffer (50 mM HEPES-KOH [pH 8.0], 3 M urea, 1 mM BME, 400 mM potassium glutamate, 500 mM imidazole, 10% glycerol). Peak fractions were pooled and dialysed overnight against 2 l of dialysis buffer 1 (50 mM HEPES-KOH [pH 8.0], 2 M urea, 10% glycerol, 200 mM potassium glutamate, 1 mM BME). The His-SUMO domain was cleaved off by incubating the dialysed protein with 100 μl of ULP protease (purified as in Malakov et al. (2004)) for 6 h at 37°C. The protein was then flowed over 2 ml of packed Ni-NTA beads, and the flow-through fraction was collected and dialysed against 2 l of dialysis buffer 2 (50 mM HEPES-KOH [pH 8.0], 800 mM potassium glutamate, 20% sucrose, 1 mM BME). The molecular weight of each protein was confirmed by MALDI mass spectrometry.
35S-labelled versions of substrates were expressed and purified as follows. Cultures of 100 ml were grown in LB broth at 37°C to an OD600 of 1.0. Cells were harvested and resuspended in 100 ml of M9 media supplemented with MAM and grown for an additional 15 min at 37°C. Cultures were induced with 0.5 mM IPTG and grown for an additional 20 min. 35S-methionine (3.5 mCi) was added, the culture was grown for 3.5 h at 37°C, and the cells were harvested and subsequently resuspended in 3 ml of 50 mM HEPES-KOH (pH 8.0). Lysozyme was then added to a final concentration of 0.3 mg ml−1, the cells were incubated on ice for 20 min, and then frozen at −80°C until purification. 35S-labelled proteins were purified essentially as described above. Lon was purified using the procedure of Gur and Sauer (2008b).
The isolated α-crystallin domains of IbpA, IbpB, αA-crystallin and αB-crystallin consisted of the following residues: IbpA (40–123), IbpB (36–121), αA-crystallin (63–145), αB-crystallin (67–149). A methionine was added to the N-terminus of each of these constructs to facilitate labelling with 35S-methionine.
Degradation in vitro
Degradation of substrates in vitro was performed using 600 nM Lon6 in 50 mM Tris (pH 8.0), 15 mM MgCl2, 5 mM KCl, 1 mM DTT, 2% DMSO, 25 mM HEPES-KOH (pH 8.0), 400 mM potassium glutamate, 10% sucrose and 0.5 mM BME. Lon and substrate were incubated at 37°C for 2 min before the addition of the ATP-regeneration mix (32 mM ATP, 400 mg ml−1 creatine kinase, 40 mM creatine phosphate). Time points were taken every 30 s for 2.5 min, or every 5 min for 30 min. Reactions were quenched in 10% trichloroacetic acid (TCA). The TCA-insoluble material was removed by centrifugation and 35S radioactivity in the supernatant was measured in a Tri-Carb liquid scintillation counter (Perkin Elmer). The fraction of substrate that had been degraded at each time point was calculated by dividing the TCA-soluble counts by the total counts in an equal volume of each reaction.
Determination of oligomeric-state by gel-filtration chromatography
Samples of IbpB, IbpBα, αB-crystallin or αBα (70 μM in 50 μl) were loaded onto a Tosoh Super SW 3000 HPLC column (pre-equilibrated with a buffer containing 50 mM HEPES-KOH [pH 8.0], 300 mM potassium glutamate) and eluted at a flow rate of 0.3 ml min−1. Peaks were detected by absorbance at 280 nm or 213 nm. A mix of molecular-weight standards (Bio-Rad), which included thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa) and vitamin B12 (1.35 kDa), was chromatographed under the same conditions.
Antibody production
Polyclonal antibodies specific to IbpA and IbpB were generated by inoculating rabbits with a peptide specific to IbpA (RVIPEAKKPRRIEIN) or IbpB (IDLIRNEPEPIAAQR). Antibodies were produced by Covance Research Products.
Degradation in vivo
For degradation assays in vivo, a 60 ml culture in LB broth was grown at 37°C in a shaking water bath to OD600 0.2. The culture was then subjected to 45°C heat shock for 30 min before a 900 μl aliquot of the culture was added to 100 μl 100% TCA for the t = 0 sample. Spectinomycin was then added (to 400 μg ml−1), and samples were taken at 15 min intervals for 60 min. For each sample, OD600 was determined, and then TCA was added to a final concentration of 10%. The cultures were maintained at 45°C during the time-course. Samples were recovered by centrifugation and the pellets were rinsed with acetone and resuspended in enough 2X Tris-tricine sample buffer so that each sample contained the same cell density. An equal volume of each sample was run on a 16.5% Tris-tricine polyacrylamide gel. IbpA levels were detected using the anti-IbpA antibody described above and were quantified using ImageQuant software (GE Health Sciences).
Acknowledgements
We thank Elizabeth Oakes for sharing unpublished data and for helpful discussions, Eyal Gur for sharing reagents and helpful discussions, and Peter Chien, Jennifer Hou, Anne Meyer and Giselle Román-Hernández for comments on the manuscript. We also thank Ligia Acosta-Sampson and Jonathan King for reagents. This work was supported by NIH grants GM49224 and AI16892. T. A. B. is an employee of the Howard Hughes Medical Institute.
References
- Ahner A, Nakatsukasa K, Zhang H, Frizzell RA, Brodsky JL. Small heat-shock proteins select deltaF508-CFTR for endoplasmic reticulum-associated degradation. Mol Biol Cell. 2007;18:806–814. doi: 10.1091/mbc.E06-05-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SP, Polazzi JO, Gierse JK, Easton AM. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J Bacteriol. 1992;174:6938–6947. doi: 10.1128/jb.174.21.6938-6947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TA, Sauer RT. ATP-dependent proteases of bacteria: recognition logic and operating principles. Trends Biochem Sci. 2006;31:647–653. doi: 10.1016/j.tibs.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy JS, Aung LL, Karzai AW. Lon protease degrades transfer-messenger RNA-tagged proteins. J Bacteriol. 2007;189:6564–6571. doi: 10.1128/JB.00860-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SM, Hoskins JR, Wickner S. Collaboration between the ClpB AAA+ remodeling protein and the DnaK chaperone system. Proc Natl Acad Sci USA. 2007;104:11138–11144. doi: 10.1073/pnas.0703980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Engelsman J, Keijsers V, De Jong WW, Boelens WC. The small heat-shock protein alpha B-crystallin promotes FBX4-dependent ubiquitination. J Biol Chem. 2003;278:4699–4704. doi: 10.1074/jbc.M211403200. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fernando P, Heikkila JJ. Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity. Cell Stress Chaperones. 2000;5:148–159. doi: 10.1379/1466-1268(2000)005<0148:fcoxsh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol Cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Ballesteros M, Dukan S, Nyström T. Defense against protein carbonylation by DnaK/DnaJ and proteases of the heat shock regulon. J Bacteriol. 2005;187:4207–4213. doi: 10.1128/JB.187.12.4207-4213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Frank EG, Levine AS, Woodgate R. Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Roche E, Zhou Y, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith KL, Shah IM, Wolf RE. Proteolytic degradation of Escherichia coli transcription activators SoxS and MarA as the mechanism for reversing the induction of the superoxide (SoxRS) and multiple antibiotic resistance (Mar) regulons. Mol Microbiol. 2004;51:1801–1816. doi: 10.1046/j.1365-2958.2003.03952.x. [DOI] [PubMed] [Google Scholar]
- Gur E, Sauer RT. Evolution of the ssrA degradation tag in Mycoplasma: specificity switch to a different protease. Proc Natl Acad Sci USA. 2008a;105:16113–16118. doi: 10.1073/pnas.0808802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur E, Sauer RT. Recognition of misfolded proteins by Lon, a AAA+ protease. Genes Dev. 2008b;22:2267–2277. doi: 10.1101/gad.1670908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur E, Sauer RT. Degrons in protein substrates program the speed and operating efficiency of the AAA+ Lon proteolytic machine. Proc Natl Acad Sci USA. 2009;106:18503–18508. doi: 10.1073/pnas.0910392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Amano F. Regulation of SulA cleavage by Lon protease by the C-terminal amino acid of SulA, histidine. Biochem J. 2001;358:473–480. doi: 10.1042/0264-6021:3580473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao W, Li P, Zhang J, Zhang H, Chang Z. Small heat-shock proteins function in the insoluble protein complex. Biochem Biophys Res Commun. 2005a;335:227–231. doi: 10.1016/j.bbrc.2005.07.065. [DOI] [PubMed] [Google Scholar]
- Jiao W, Qian M, Li P, Zhao L, Chang Z. The essential role of the flexible termini in the temperature-responsiveness of the oligomeric state and chaperone-like activity for the polydisperse small heat shock protein IbpB from Escherichia coli. J Mol Biol. 2005b;347:871–884. doi: 10.1016/j.jmb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Matsumura Y, Tsuchido T. Small heat shock proteins, IbpA and IbpB, are involved in resistances to heat and superoxide stresses in Escherichia coli. FEMS Microbiol Lett. 2000;184:165–171. doi: 10.1111/j.1574-6968.2000.tb09009.x. [DOI] [PubMed] [Google Scholar]
- Van De Klundert FA, Smulders RH, Gijsen ML, Lindner RA, Jaenicke R, Carver JA, De Jong WW. The mammalian small heat-shock protein Hsp20 forms dimers and is a poor chaperone. Eur J Biochem. 1998;258:1014–1021. doi: 10.1046/j.1432-1327.1998.2581014.x. [DOI] [PubMed] [Google Scholar]
- Kowit JD, Goldberg AL. Intermediate steps in the degradation of a specific abnormal protein in Escherichia coli. J Biol Chem. 1977;252:8350–8357. [PubMed] [Google Scholar]
- Laskowska E, Wawrzynów A, Taylor A. IbpA and IbpB, the new heat-shock proteins, bind to endogenous Escherichia coli proteins aggregated intracellularly by heat shock. Biochimie. 1996;78:117–122. doi: 10.1016/0300-9084(96)82643-5. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux MR, Melki R, Gordon B, Batelier G, Candido EP. Structure-function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides. J Biol Chem. 1997;272:24646–24656. doi: 10.1074/jbc.272.39.24646. [DOI] [PubMed] [Google Scholar]
- Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund PA. Microbial molecular chaperones. Adv Microb Physiol. 2001;44:93–140. doi: 10.1016/s0065-2911(01)44012-4. [DOI] [PubMed] [Google Scholar]
- Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics. 2004;5:75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- Matuszewska E, Kwiatkowska J, Kuczynska-Wisnik D, Laskowska E. Escherichia coli heat-shock proteins IbpA/B are involved in resistance to oxidative stress induced by copper. Microbiology. 2008;154:1739–1747. doi: 10.1099/mic.0.2007/014696-0. [DOI] [PubMed] [Google Scholar]
- Matuszewska M, Kuczynska-Wisnik D, Laskowska E, Liberek K. The small heat shock protein IbpA of Escherichia coli cooperates with IbpB in stabilization of thermally aggregated proteins in a disaggregation competent state. J Biol Chem. 2005;280:12292–12298. doi: 10.1074/jbc.M412706200. [DOI] [PubMed] [Google Scholar]
- Mizusawa S, Gottesman S. Protein degradation in Escherichia coli: the lon gene controls the stability of sulA protein. Proc Natl Acad Sci USA. 1983;80:358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Deuerling E, Vorderwülbecke S, Vierling E, Bukau B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol. 2003a;50:585–595. doi: 10.1046/j.1365-2958.2003.03710.x. [DOI] [PubMed] [Google Scholar]
- Mogk A, Schlieker C, Friedrich KL, Schönfeld HJ, Vierling E, Bukau B. Refolding of substrates bound to small Hsps relies on a disaggregation reaction mediated most efficiently by ClpB/DnaK. J Biol Chem. 2003b;278:31033–31042. doi: 10.1074/jbc.M303587200. [DOI] [PubMed] [Google Scholar]
- Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher SB, Villén J, Oakes EC, Bakalarski CE, Sauer RT, Gygi SP, Baker TA. Proteomic profiling of ClpXP substrates after DNA damage reveals extensive instability within SOS regulon. Mol Cell. 2006;22:193–204. doi: 10.1016/j.molcel.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Ondrovicová G, Liu T, Singh K, Tian B, Li H, Gakh O, et al. Cleavage site selection within a folded substrate by the ATP-dependent lon protease. J Biol Chem. 2005;280:25103–25110. doi: 10.1074/jbc.M502796200. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantôme A, et al. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pruteanu M, Baker TA. Controlled degradation by ClpXP protease tunes the levels of the excision repair protein UvrA to the extent of DNA damage. Mol Microbiol. 2009;71:912–924. doi: 10.1111/j.1365-2958.2008.06574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak E, Zietkiewicz S, Liberek K. Distinct activities of Escherichia coli small heat shock proteins IbpA and IbpB promote efficient protein disaggregation. J Mol Biol. 2009;386:178–189. doi: 10.1016/j.jmb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Rosen R, Biran D, Gur E, Becher D, Hecker M, Ron EZ. Protein aggregation in Escherichia coli: role of proteases. FEMS Microbiol Lett. 2002;207:9–12. doi: 10.1111/j.1574-6968.2002.tb11020.x. [DOI] [PubMed] [Google Scholar]
- Schlothauer T, Mogk A, Dougan DA, Bukau B, Turgay K. MecA, an adaptor protein necessary for ClpC chaperone activity. Proc Natl Acad Sci USA. 2003;100:2306–2311. doi: 10.1073/pnas.0535717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah IM, Wolf RE. Sequence requirements for Lon-dependent degradation of the Escherichia coli transcription activator SoxS: identification of the SoxS residues critical to proteolysis and specific inhibition of in vitro degradation by a peptide comprised of the N-terminal 21 amino acid residues. J Mol Biol. 2006;357:718–731. doi: 10.1016/j.jmb.2005.12.088. [DOI] [PubMed] [Google Scholar]
- Thomas JG, Baneyx F. Roles of the Escherichia coli small heat shock proteins IbpA and IbpB in thermal stress management: comparison with ClpA, ClpB, and HtpG In vivo. J Bacteriol. 1998;180:5165–5172. doi: 10.1128/jb.180.19.5165-5172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias JW, Shrader TE, Rocap G, Varshavsky A. The N-end rule in bacteria. Science. 1991;254:1374–1377. doi: 10.1126/science.1962196. [DOI] [PubMed] [Google Scholar]
- Tomoyasu T, Mogk A, Langen H, Goloubinoff P, Bukau B. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol Microbiol. 2001;40:397–413. doi: 10.1046/j.1365-2958.2001.02383.x. [DOI] [PubMed] [Google Scholar]
- Turgay K, Hahn J, Burghoorn J, Dubnau D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998;17:6730–6738. doi: 10.1093/emboj/17.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Melderen L, Gottesman S. Substrate sequestration by a proteolytically inactive Lon mutant. Proc Natl Acad Sci USA. 1999;96:6064–6071. doi: 10.1073/pnas.96.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick JE, Clarke S. Repair, refold, recycle: how bacteria can deal with spontaneous and environmental damage to proteins. Mol Microbiol. 1995;16:835–845. doi: 10.1111/j.1365-2958.1995.tb02311.x. [DOI] [PubMed] [Google Scholar]


