Abstract
Background
Patent foramen ovale (PFO) is associated with cryptogenic stroke (CS), though the pathogenicity of a discovered PFO in the setting of CS is typically unclear. Transesophageal echocardiography (TEE) features such as PFO size, an associated hypermobile septum, and presence of a right-to-left shunt at rest have all been proposed as markers of risk. The association of these TEE features with other markers of pathogenicity has not been examined.
Methods and Results
We used a recently derived score based on clinical and neuroimaging features to stratify patients with PFO and CS by the probability that their stroke is PFO-attributable. We examined whether high risk TEE features are seen more frequently in patients more likely to have had a PFO-attributable stroke (n = 637) compared to those less likely to have a PFO attributable stroke (n = 657). Large physiologic shunt size was not more frequently seen among those with probable PFO-attributable strokes (OR=0.92; p = 0.53). Neither the presence of a hypermobile septum nor a right-to-left shunt at rest were detected more often in those with a probable PFO-attributable stroke (OR=0.80; p = 0.45 and OR=1.15; 0.11 respectively).
Conclusions
We found no evidence that the proposed TEE risk markers of large PFO size, hypermobile septum, and presence of right-to-left shunt at rest are associated with clinical features suggesting that a CS is PFO-attributable. Additional tools to describe PFOs may be useful in helping to determine whether an observed PFO is incidental or pathogenically related to CS.
Keywords: cerebrovascular disease/stroke, echocardiography, cardiovascular imaging, risk factor, congenital heart disease
Patent foramen ovale (PFO) is associated with cryptogenic stroke (CS).1 Despite this association there is continued debate about the causal relationship of discovered PFOs in individual patients with cryptogenic stroke.2, 3 Numerous candidate echocardiographic features have been proposed as ‘high risk’ features that make a PFO more likely to be causally linked to CS, including presence of a hypermobile atrial septum (or atrial septal aneurysm (ASA),4 physiologicshunt size as measured by right-to-left microbubble count,5 and presence of a right-to-left shunt at rest (i.e. without a Valsalva maneuver).6 Investigators have incorporated these potential ‘high risk’ parameters into routine echocardiographic analysis in an attempt to identify high risk PFOs from incidentally discovered ones. Nevertheless, reports raise the possibility that these echocardiographic features are not clearly related either to CS or to the risk of recurrence.3, 7, 8 This uncertainty adds to the debate about causality and raises questions about appropriate therapeutic approaches.9
We report an analysis from the Risk of Paradoxical Embolism (RoPE) Study database,10 a large observational database formed by combining 12 component databases of patients with CS and known PFO status. The rationale for the RoPE Study has been previously described and builds on prior work demonstrating that overall summary trial results may not represent benefits for individual patients and that risk modeling may improve result interpretation.11, 12 These issues are important for trial design and for treating patients since the likelihood that a CS event is attributable to an identified PFO is related to patient-specific factors and PFO closure may not be beneficial for all patients with CS and PFO.13, 14
The RoPE Score is a way to stratify patients with CS with respect to 1) the likelihood that a PFO would be present (prior to TEE evaluation) and 2) the (related) probability that CS is attributable to an observed PFO.15 In brief, among patients with PFO and CS, younger patients, without conventional stroke risk factors or prior stroke and with a visible superficial lesion seen on neuroimaging, appear to be the most likely to have a PFO-attributable CS. Using The RoPE Score we attempt to clarify the clinical significance of the major ‘high risk’ PFO echocardiographic features. Our hypothesis is that ‘high risk’ echocardiographic features will be more prevalent in patients with probable PFO-associated CS and less prevalent in those with probable incidental PFOs.
Methods
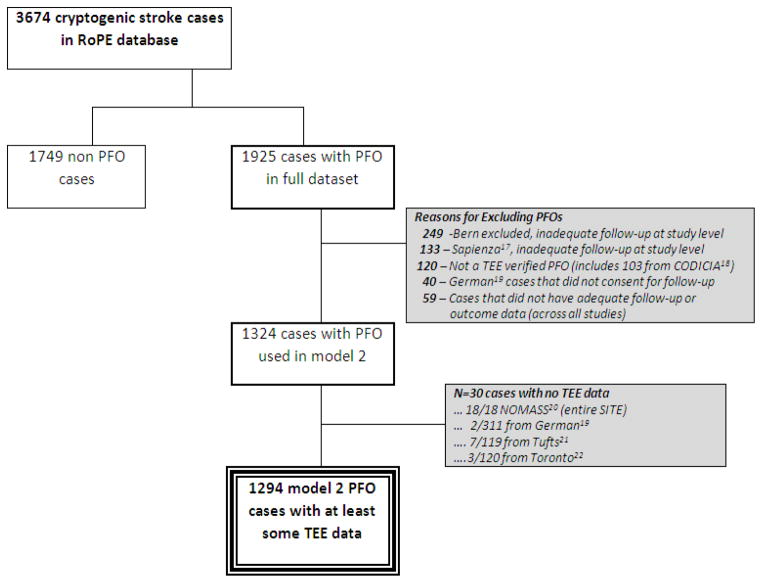
The RoPE database has been described previously.10, 16 Briefly, we combined 12 databases containing clinical, neuroimaging and echocardiographic data for patients with CS who were investigated for PFO. The RoPE Study includes patients with (n=1925) and without (n=1749) PFO (Figure). CS was defined by the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) classification and was diagnosed within component databases.23 As previously described,16 CS definition for this study specifically excluded patients with known stroke mechanisms including large artery atherosclerosis, cardioembolism, small vessel disease, or strokes of other etiology including arterial dissection or hypercoagulable states. “Stroke” was defined as the sudden onset of neurologic deficit in a vascular territory presumed to be due to focal ischemia after a comprehensive workup. If a deficit was present for <24 hours it was considered a TIA if there were no acute MRI or CT changes in appropriate locations. Patients underwent either transesophageal echocardiography (TEE) or transcranial Doppler (TCD) for PFO detection. For this analysis, only patients evaluated with TEE (n = 1324) were included. Component database variables were harmonized and new data were collected when necessary and feasible. This study was approved by the Tufts Medical Center Internal Review Board
Figure.
Cases Included in TEE Analysis
The RoPE score is a prediction tool for determining the probability that an index CS is attributable to PFO (Table 1). For an individual, it is not possible to know with certainty whether or not a PFO is incidental or pathogenic. The 10-point RoPE score allows estimation of the attributable fraction for a PFO in the setting of CS. Attributable fraction is determined by the prevalence of PFO in patients with CS compared to that found in an otherwise similar group of patients without CS. Since the PFO prevalence in CS patients is dependent on other patient characteristics,24 a more patient-specific attributable fraction can be considered by applying a patient-specific PFO prevalence rate.15 Generally, with a decreasing number of conventional stroke risk factors and younger age (resulting in a higher RoPE score and an increasing PFO prevalence), the PFO-attributable fraction (which, assuming causality, can be thought of as the proportion of strokes that would not have occurred if the PFO had been previously eliminated) increases.
Table 1.
RoPE Score Calculator
| Characteristic | Points | RoPE SCORE |
|---|---|---|
| No history of hypertension | 1 | |
| No history of diabetes | 1 | |
| No history of stroke or TIA | 1 | |
| Non-smoker | 1 | |
| Cortical infarct on imaging | 1 | |
| Age | ||
| 18 to 29 years | 5 | |
| 30 to 39 years | 4 | |
| 40 to 49 years | 3 | |
| 50 to 59 years | 2 | |
| 60 to 69 years | 1 | |
| ≥ 70 years | 0 | |
| Total score (sum of individual points) = | ||
| Maximum score (a patient less than 30 years with no hypertension, no diabetes, no history of stroke or TIA, non-smoker, and cortical infarct) | 10 | |
| Minimum score (a patient ≥ 70 years with hypertension, diabetes, priorstroke, current smoker, and no cortical infarct) | 0 | |
For this analysis, individual RoPE scores were calculated for each of the 1324 cases with PFO investigated by TEE. Echocardiographic variables were harmonized values based on clinical rationale and primary data from the component databases (published and unpublished). RoPE Study investigators, through e-mail, telephone, teleconference, and face-to-face meetings, came to consensus regarding how to harmonize the echocardiographic parameters.16 Hypermobility of the interatrial septum (yes/no) was defined as maximum septal excursion from the midline into the right or left atrium (Bern published25, PICSS8, German19, Lausanne, Tufts21, APRIS26, French PFO/ASA27), total excursion between right and left atria (CODICIA18). The consensus definition of hypermobility in our database refers to ≥ 10 mm of excursion from midline and is approximately equivalent to ‘atrial septal aneurysm’ used in the literature. Shunting across the PFO at rest (yes/no) was considered present if right-to-left shunting of bubbles was observed even in the absence of a Valsalva maneuver. All centers were likely to inject microbubbles from the upper extremity. Physiologic shunt size (large/small) was based on counting bubbles in the left atrium ≤ 3 cardiac cycles after right atrial opacification. Microbubbles observed after 3 cardiac cycles were not used to assess shunt severity. Large shunt size was defined differently in component databases: > 10 bubbles (APRIS26, Bern published25, CODICIA18, Lausanne and PICSS8), ≥ 10 bubbles (French PFO/ASA27, German19), and > 15 bubbles (Tufts21). The consensus definition of large shunt size was defined in our database as > 10 bubbles in the left atrium ≤ 3 cardiac cycles after right atrial opacification.16 Our hypothesis is that these echocardiographic features will be more frequently observed in RoPE score strata with a higher probability of PFO-attributable stroke.
Statistical Analysis
For our primary analysis, we divided the population into those with a RoPE score that was above or below the median. i.e. those with scores >6 (higher probability of PFO-attributable stroke) and ≤ 6 (lower probability of PFO-attributable stroke). Significance was determined using t-test and chi-square analyses with significance set at p = 0.05. We used a generalized linear mixed model that included a random-effect term representing each component database when determining the significance of the various echocardiographic findings across RoPE score categories. For these analyses the independent variable was the RoPE score, the dependent variable was presence or absence of putative ‘high risk’ TEE features. Our secondary analysis consisted of a test of linear trend over 7 ordered RoPE Score categories, again using Generalized Mixed models where the study site was again included as a random effect. We also performed extensive sensitivity analyses that evaluated association between RoPE score categories and composite PFO ‘risk’ profiles a) Large size and hypermobile septum, b) not large and not hypermobile, c) large size or hypermobile. We evaluated the interreader reliability (Kappa) for the proposed ‘high risk’ TEE features by having 3 blinded readers re-read a sampling of the studies from the French PFO/ASA and PICSS studies. Additionally we explored for association by re-defining ‘large shunt size’ using a higher “uncountable” number of bubbles.
Results
The characteristics of the subjects included in this analysis are shown in (Table 2). There were 1294 subjects with CS and PFO and TEE data (Figure). The mean age was 50 years, 59% of patients were Caucasian. There were 637 subjects with RoPE scores > 6 and 657 with RoPE scores ≤ 6. This stratification produced subgroups with very different characteristics: those in the low RoPE score group were 10-fold more likely to have diabetes, 5-fold more likely to have coronary artery disease, and about 8-fold more likely to have hypertension (p <0.0001 for age, the presence of diabetes, coronary artery disease, hypertension, hypercholesterolemia, and history of stroke or TIA, p=0.03 for current smoking).
Table 2. Patient characteristics across RoPE Score strata.
Patient level data were extracted from the individual databases. Age represents the age at the time of index event.
| All PFO patients with at least some TEE data (n=1294) | RoPE Score > 6 (n=637) | RoPE Score ≤ 6 (n=657) | p-value * | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age in yrs, mean ± stdev | 49.6±14.6 (1294) | 38.9 ± 9.2 (637) | 60.0±10.8 (657) | |
| Age over 65, % | 17.9% (232/1294) | 0.0% (0/637) | 35.3% (232/657) | |
| Male gender, % | 59.4% (768/1294) | 54.6% (348/637) | 63.9% (420/657) | 0.0007 |
| White race, % | 86.6% (453/523) | 93.4% (283/303) | 77.3% (170/220) | <.0001 |
| Incident event was TIA, % | 14.0% (181/1294) | 9.7% (62/637) | 18.1% (119/657) | <.0001 |
| Medical history, % yes | ||||
| Diabetes | 8.7% (112/1289) | 1.3% (8/636) | 15.9% (104/653) | |
| Coronary artery disease | 7.1% (63/885) | 2.0% (7/343) | 10.3% (56/542) | <.0001 |
| Hypertension | 29.2% (377/1290) | 6.0% (38/637) | 51.9% (339/653) | |
| Hypercholesterolemia | 24.2% (242/998) | 12.1% (62/512) | 37.0% (180/486) | <.0001 |
| Current smoker | 29.6% (380/1282) | 26.9% (171/636) | 32.4% (209/646) | |
| History of stroke or TIA | 11.9% (154/1290) | 5.0% (32/635) | 18.6% (122/655) |
The proposed high-risk PFO characteristics seen on TEE were commonly seen in the RoPE database (Table 3). A sampling of TEE studies was re-read by 3 readers to establish inter-reader reliability within the RoPE database. For 29 TEE studies from the French PFO/ASA study: hypermobile septum, Kappa 0.57; large PFO, Kappa 0.42; shunt at rest, Kappa 0.75, for 31 TEE studies from the PICSS study: hypermobile septum, Kappa 0.33; large PFO, Kappa 0.14; shunt at rest, Kappa 0.33. Inter-reader agreement for these variables was consistent with previously published standards.28 Overall 25.3% had a hypermobile septum, 64.4% had a large shunt, and 69.6% had a shunt at rest. However, there was no difference in the frequency of these echocardiographic PFO features between the high RoPE score (higher probability of PFO-related index stroke) and low RoPE score (lower probability of PFO-related index stroke) cohorts (OR=0.92; p = 0.53 for large number of bubbles, OR=1.15; p = 0.45 for right-to-left shunt at rest, and OR=0.80; p = 0.11 for presence of a hypermobile septum). Extensive exploratory analyses of echocardiographic features across RoPE score strata demonstrated no trend towards increased frequency as RoPE score increased after correcting for site effect. The prevalence of these TEE features varied across different centers for any given RoPE Score stratum. (Supplement I). Furthermore, we saw no trends after exploring different definitions of ‘large shunt size’ and no association between RoPE score category and composite PFO risk profiles (Supplement II).
Table 3.
Putative ‘high risk TEE features’ across high and low RoPE Score strata.
| TEE findings | All PFO patients with at least some TEE data (n=1294) | RoPE Score > 6 (n=637) | RoPE Score ≤ 6 (n=657) | p-value ** |
|---|---|---|---|---|
|
| ||||
| Large # bubbles vs. not large | 64.4% (695/1079) | 67.4% (347/515) | 61.7% (348/564) | 0.5286 |
| Shunt at rest vs. no shunt | 69.6% (484/695) | 67.6% (238/352) | 71.7% (246/343) | 0.4474 |
| Hypermobile septum vs. not | 25.3% (320/1265) | 23.0% (144/626) | 27.5% (176/639) | 0.1063 |
p-values from Generalized Mixed models (TEE Variables ONLY) adjusting for random site effect.
Discussion
Since treatment decisions may rely on whether or not a discovered PFO is believed to be pathogenically related to the index stroke, it is critically important to identify reliable ways to stratify the likelihood that an identified PFO is associated with stroke. While some proposed ‘high risk’ TEE features have been reported in prior case control studies to be associated with CS, these previous studies each report on a small number of patients and are often not statistically significant.8, 29–37 So too recognized potential high risk features, including a persistent Eustachian Valve, are not consistently reported throughout the literature.38 This analysis from the RoPE Study demonstrates that previously proposed ‘high risk’ TEE findings of septal hypermobility, shunt at rest, and a physiologically large shunt do not appear to be found more frequently in patients whose clinical and neuroimaging features (i.e. superficially located lesions) are highly suggestive of a PFO-attributable index stroke.
The null results from our study can be interpreted in several ways. First, so-called ‘high risk’ PFO features may play no etiologic role in stroke; while the association between PFO and CS is presumed to be due to paradoxical embolism, there may be other important mechanisms unrelated to shunting or septal hypermobility. Second, while the features may in fact lead to higher risk of stroke (through paradoxical embolism or other mechanisms), their ascertainment by TEE is imperfect and highly variable. Even when examining the same studies, inter-reader agreement can be surprisingly low and (as discussed below) the studies are highly dependent on technique and on patient factors that vary over time. Extensive exploratory analyses failed to identify trends when evaluating data from individual sites with uniform protocols (Supplement I). Third, in this study, TEE variables were collected at multiple sites under independent research protocols. These “pragmatic” conditions may have increased measurement error further. Fourth, while the relationship between the RoPE score and the presence of PFO was robust in this database, it is not possible to segregate perfectly those patients for whom PFO is and is not causally related to their stroke. Finally, it is possible that the PFO association is mediated by different mechanisms some of which depend on a large shunt, e.g. paradoxical embolism, and others that depend on a small shunt, e.g. in situ thrombus formation. As previously published, assuming a control PFO prevalence rate of 25%, the PFO-attributable fraction for patients with CS ranged from 0% (95% CI 0% – 4%) for patients with RoPE score 0–3 to 88% (95% CI 83% to 91%) for patients with RoPE score 9–10.15 Since patients with and without a true association between CS and PFO were of course included in both comparison groups the potential effect of the high risk features may be underestimated.
While it is likely that each of these sources contributed to our null findings, the inability of TEE robustly to identify ‘high risk’ PFOs is a concern since TEE remains the gold standard by which anatomic characteristics of PFOs are characterized. Specific TEE protocols are not standardized across institutions, instantaneous loading conditions may fluctuate, and anatomic and functional features are variably reported in the literature.39
ASA is characterized by a saccular formation of the interatrial septum that may protrude into either atrium. The term itself represents a spectrum of atrial septal morphologic changes.40 It is variably defined in the literature as septal movement of > 10 mm or > 15 mm.8, 41 The causal relationship between ASA and CS has not been firmly established though hypotheses include embolization of thrombi formed within the ASA, thrombus formation secondary to subclinical atrial arrhythmias, and alterations of septal movement that promote right-left shunting.27 Recently reported exploratory analyses suggest that those with ASA present benefit from device closure when compared to those without this septal anatomy, although this was not seen consistently14, 42 Interestingly, as reported in the literature there is significant interobserver and intraobserver variability in detecting this abnormality (even in research settings), likely limiting the discriminatory ability of this finding.28 Morphologic heterogeneity, varying definitions, and inconsistent detection may all contribute to the explanation as to why our analysis showed no clear relationship with RoPE strata in the RoPE database.
Our analysis evaluated microbubble count, one of the most commonly used tools for semi-quantitative characterization of shunt size. The difficulty and inconsistency of fine gradations of microbubble count likely result because these counts are made based on a single frame in a single imaging plane and thus may not represent the actual amount of shunting.43 The number of microbubbles, moreover, does not correlate well with the anatomic size of a patent foramen ovale.44 Quantification of PFO size can be determined by the separation between septum primum and septum secundum in the bicaval view both at rest and during Valsalva maneuver.45 This view, which is possible for most medium and large sized PFOs, was not consistently performed across the component RoPE databases. A further limitation of microbubble count as determined in this study is that important variation in shunt size may occur well beyond our cut-off of 10. While we also saw no effect using higher cut-offs, technical limitations of TEE prevent measuring shunt size when the bubbles are “uncountable”, although variation in this range may be clinically significant. Newer methods of shunt detection may offer an increased ability to quantify the shunt objectively and over a wider range.46 The measured shunt size may also differ depending on whether microbubbles are injected from the upper extremity (as is standard) or the lower extremities (perhaps better reflecting the presumed mechanism due to lower extremity or pelvic vein thrombi). In part because shunting from the lower extremity may depend on the presence and characteristics of a Eustachian Valve (unmeasured across most RoPE component studies), measures of shunting from these two sources may be poorly correlated.47.45 A minority of included databases (Bern and PICSS) systematically ascertained information on the Eustachian valve.47 As a result this feature was not included in our analysis. These limitations, in association with significant inter-reader variability in bubble count, create noise that may contribute to the null result for this variable.27, 28
Similarly, the presence of a right-to-left shunt at rest is highly variable and dependent on technique and loading conditions. Physiologic pressure differences between the right and left atria usually push the septum primum against the septum secundum. Momentary changes in pressures can result in a transient elevation of right atrial pressure so that it is greater than left atrial pressure. The free edge of the septum primum may move resulting in enlargement of the PFO orifice.9 Transient shunting and directional shift can be seen in the setting of changes in volume status or body positioning. An effective Valsalva maneuver, defined by complete bowing of the interatrial septum toward the left atrium, may be difficult when sedation is too heavy.39 Moreover, this hemodynamic state represents one of many circumstances where right atrial pressure may rise above that seen in the left atrium. Since the presence of right-to-left shunting is critically dependent on instantaneous interatrial pressure differences, it is perhaps not surprising that this short term observation as documented in the component RoPE database studies is an unreliable marker of long-term paradoxical embolism risk.
In summary, we found no evidence that subjects with CS and clinical features suggestive of PFO-attributable strokes are more likely to have putative ‘high risk’ TEE features than those whose clinical features suggest CS unrelated to their PFO. Due to numerous technical limitations, TEE may be unreliable in risk stratifying PFO on the basis of physiologic and anatomic features. While some of the limitations discussed above relate to limitations in how TEE was applied in the component RoPE studies, and may be partially addressable through more rigorous standardization of imaging procedures, our results can also be seen as reflecting the limitations of TEE measurements as they are usually performed in routine clinical practice. Further development of technologies that might better and more consistently characterize PFO features is needed.
Supplementary Material
Acknowledgments
Sources of Funding
This study was partially funded by grants UL1 RR025752, R01 NS062153, and R21 NS079826, all from the National Institutes of Health.
Footnotes
Disclosures
Both David M. Kent and David E Thaler have consulted for WL Gore Associates. David E. Thaler is a consultant to AGA Medical Corporation. There are no other conflicts of interest to disclose.
References
- 1.Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 2.Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005;112:1063–1072. doi: 10.1161/CIRCULATIONAHA.104.524371. [DOI] [PubMed] [Google Scholar]
- 3.Kent DM, Thaler DE. Is patent foramen ovale a modifiable risk factor for stroke recurrence? Stroke. 2010;41:S26–S30. doi: 10.1161/STROKEAHA.110.595140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agmon Y, Khandheria BK, Meissner I, Gentile F, Whisnant JP, Sicks JD, O’Fallon WM, Covalt JL, Wiebers DO, Seward JB. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation. 1999;99:1942–1944. doi: 10.1161/01.cir.99.15.1942. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Song JK, Song JM, Kang DH, Yun SC, Kang DW, Kwon SU, Kim JS. Association between anatomic features of atrial septal abnormalities obtained by omni-plane transesophageal echocardiography and stroke recurrence in cryptogenic stroke patients with patent foramen ovale. Am J Cardiol. 2010;106:129–134. doi: 10.1016/j.amjcard.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Rigatelli G, Dell’Avvocata F, Cardaioli P, Giordan M, Braggion G, Aggio S, Chinaglia M, Mandapaka S, Kuruvilla J, Chen JP, Nanjundappa A. Permanent right-to-left shunt is the key factor in managing patent foramen ovale. J Am Coll Cardiol. 2011;58:2257–2261. doi: 10.1016/j.jacc.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 7.Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol. 2007;49:797–802. doi: 10.1016/j.jacc.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 8.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 9.Kutty S, Sengupta PP, Khandheria BK. Patent foramen ovale: the known and the to be known. J Am Coll Cardiol. 2012;59:1665–1671. doi: 10.1016/j.jacc.2011.09.085. [DOI] [PubMed] [Google Scholar]
- 10.Kent DM, Thaler DE. The Risk of Paradoxical Embolism (RoPE) Study: Developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials. 2011;12:185. doi: 10.1186/1745-6215-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 12.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40:2349–2355. doi: 10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 15.Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, Di AE, Di Tullio MR, Lutz JS, Elkind MS, Griffith J, Jaigobin C, Mattle HP, Michel P, Mono ML, Nedeltchev K, Papetti F, Thaler DE. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–625. doi: 10.1212/WNL.0b013e3182a08d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaler DE, Di Angelantonio E, Di Tullio MR, Donovan JS, Griffith J, Homma S, Jaigobin C, Mas JL, Mattle HP, Michel P, Mono ML, Nedeltchev K, Papetti F, Ruthazer R, Serena J, Weimar C, Elkind MS, Kent DM. The Risk of Paradoxical Embolism (RoPE) Study: initial description of the completed database. Int J Stroke. 2012 doi: 10.1111/j.1747-4949.2012.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Castro S, Papetti F, Di AE, Razmovska B, Truscelli G, Tuderti U, Puca E, Correnti A, Fiorelli M, Prencipe M, Toni D. Feasibility and clinical utility of transesophageal echocardiography in the acute phase of cerebral ischemia. Am J Cardiol. 2010;106:1339–1344. doi: 10.1016/j.amjcard.2010.06.066. [DOI] [PubMed] [Google Scholar]
- 18.Serena J, Marti-Fabregas J, Santamarina E, Rodriguez JJ, Perez-Ayuso MJ, Masjuan J, Segura T, Gallego J, Davalos A. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke. 2008;39:3131–3136. doi: 10.1161/STROKEAHA.108.521427. [DOI] [PubMed] [Google Scholar]
- 19.Weimar C, Holle DN, Benemann J, Schmid E, Schminke U, Haberl RL, Diener HC, Goertler M. Current management and risk of recurrent stroke in cerebrovascular patients with right-to-left cardiac shunt. Cerebrovasc Dis. 2009;28:349–356. doi: 10.1159/000229553. [DOI] [PubMed] [Google Scholar]
- 20.Steiner MM, Di Tullio MR, Rundek T, Gan R, Chen X, Liguori C, Brainin M, Homma S, Sacco RL. Patent foramen ovale size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;29:944–948. doi: 10.1161/01.str.29.5.944. [DOI] [PubMed] [Google Scholar]
- 21.Kitsios GD, Lasker A, Singh J, Thaler DE. Recurrent stroke on imaging and presumed paradoxical embolism: A cross-sectional analysis. Neurology. 2012;78:993–997. doi: 10.1212/WNL.0b013e31824d58bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casaubon L, McLaughlin P, Webb G, Yeo E, Merker D, Jaigobin C. Recurrent stroke/TIA in cryptogenic stroke patients with patent foramen ovale. Can J Neurol Sci. 2007;34:74–80. doi: 10.1017/s0317167100005825. [DOI] [PubMed] [Google Scholar]
- 23.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422–431. doi: 10.1161/STROKEAHA.111.631648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedeltchev K, Arnold M, Wahl A, Sturzenegger M, Vella EE, Windecker S, Meier B, Mattle HP. Outcome of patients with cryptogenic stroke and patent foramen ovale. J Neurol Neurosurg Psychiatry. 2002;72:347–350. doi: 10.1136/jnnp.72.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Tullio MR, Homma S, Jin Z, Sacco RL. Aortic atherosclerosis, hypercoagulability, and stroke the APRIS (Aortic Plaque and Risk of Ischemic Stroke) study. J Am Coll Cardiol. 2008;52:855–861. doi: 10.1016/j.jacc.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–1746. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 28.Cabanes L, Coste J, Derumeaux G, Jeanrenaud X, Lamy C, Zuber M, Mas JL. Interobserver and intraobserver variability in detection of patent foramen ovale and atrial septal aneurysm with transesophageal echocardiography. J Am Soc Echocardiogr. 2002;15:441–446. doi: 10.1067/mje.2002.116718. [DOI] [PubMed] [Google Scholar]
- 29.Hanna JP, Sun JP, Furlan AJ, Stewart WJ, Sila CA, Tan M. Patent foramen ovale and brain infarct. Echocardiographic predictors, recurrence, and prevention. Stroke. 1994;25:782–786. doi: 10.1161/01.str.25.4.782. [DOI] [PubMed] [Google Scholar]
- 30.Natanzon A, Goldman ME. Patent foramen ovale: anatomy versus pathophysiology--which determines stroke risk? J Am Soc Echocardiogr. 2003;16:71–76. doi: 10.1067/mje.2003.34. [DOI] [PubMed] [Google Scholar]
- 31.Force M, Massabuau P, Larrue V. Prevalence of atrial septal abnormalities in older patients with cryptogenic ischemic stroke or transient ischemic attack. Clin Neurol Neurosurg. 2008;110:779–783. doi: 10.1016/j.clineuro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Goel SS, Tuzcu EM, Shishehbor MH, de Oliveira EI, Borek PP, Krasuski RA, Rodriguez LL, Kapadia SR. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am J Cardiol. 2009;103:124–129. doi: 10.1016/j.amjcard.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 33.Mesa D, Ruiz M, Delgado M, Suarez de LJ, Pan M, Tejero I, Garcia D, Crespin M, Leon C, Toledano F, Mazuelos F, Ochoa JJ, Bescansa E. Prevalence of patent foramen ovale determined by transesophageal echocardiography in patients with cryptogenic stroke aged 55 years or older. Same as younger patients? Rev Esp Cardiol. 2010;63:315–322. doi: 10.1016/s1885-5857(10)70064-5. [DOI] [PubMed] [Google Scholar]
- 34.Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, Chedru F, Guerin F, Bousser MG, de RJ. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–1873. doi: 10.1161/01.str.24.12.1865. [DOI] [PubMed] [Google Scholar]
- 35.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–2268. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 36.Job FP, Ringelstein EB, Grafen Y, Flachskampf FA, Doherty C, Stockmanns A, Hanrath P. Comparison of transcranial contrast Doppler sonography and transesophageal contrast echocardiography for the detection of patent foramen ovale in young stroke patients. Am J Cardiol. 1994;74:381–384. doi: 10.1016/0002-9149(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 37.Petty GW, Khandheria BK, Chu CP, Sicks JD, Whisnant JP. Patent foramen ovale in patients with cerebral infarction. A transesophageal echocardiographic study. Arch Neurol. 1997;54:819–822. doi: 10.1001/archneur.1997.00550190013008. [DOI] [PubMed] [Google Scholar]
- 38.Schuchlenz HW, Saurer G, Weihs W, Rehak P. Persisting eustachian valve in adults: relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr. 2004;17:231–233. doi: 10.1016/j.echo.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Di Tullio MR. Patent foramen ovale: echocardiographic detection and clinical relevance in stroke. J Am Soc Echocardiogr. 2010;23:144–155. doi: 10.1016/j.echo.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Olivares-Reyes A, Chan S, Lazar EJ, Bandlamudi K, Narla V, Ong K. Atrial septal aneurysm: a new classification in two hundred five adults. J Am Soc Echocardiogr. 1997;10:644–656. doi: 10.1016/s0894-7317(97)70027-0. [DOI] [PubMed] [Google Scholar]
- 41.Hara H, Virmani R, Ladich E, key-Bojack S, Titus J, Reisman M, Gray W, Nakamura M, Mooney M, Poulose A, Schwartz RS. Patent foramen ovale: current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 2005;46:1768–1776. doi: 10.1016/j.jacc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Juni P. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 43.De CS, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, Beccia M, Colonnese C, Fedele F, Fieschi C, Pandian NG. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–2413. doi: 10.1161/01.str.31.10.2407. [DOI] [PubMed] [Google Scholar]
- 44.Schuchlenz HW, Weihs W, Horner S, Quehenberger F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med. 2000;109:456–462. doi: 10.1016/s0002-9343(00)00530-1. [DOI] [PubMed] [Google Scholar]
- 45.Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Li MG, Mohr JP. Characteristics of patent foramen ovale associated with cryptogenic stroke. A biplane transesophageal echocardiographic study. Stroke. 1994;25:582–586. doi: 10.1161/01.str.25.3.582. [DOI] [PubMed] [Google Scholar]
- 46.Sommer R, Brandwein R, Dobson D, Eggers E, Eggers A. Novel System for Detection of Cardiac Right to Left Shunts. Journal of American College of Cardiology. 2012:60. doi: 10.1016/j.jacc.2012.08.819. [DOI] [Google Scholar]
- 47.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Atrial anatomy in non-cardioembolic stroke patients: effect of medical therapy. J Am Coll Cardiol. 2003;42:1066–1072. doi: 10.1016/s0735-1097(03)00907-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.