Summary
Focal cortical dysplasia (FCD) and related malformations of cortical development (MCD) represent an increasingly recognized cause of medically-intractable epilepsy. However, the underlying mechanisms of epileptogenesis are poorly understood, and treatments for epilepsy due to various cortical malformations are often limited or ineffective. Animal models offer a number of advantages for investigating cellular and molecular mechanisms causing epileptogenesis and developing novel, rational therapies for MCD-related epilepsy. This review will highlight specific examples of how animal models have been useful in addressing several clinically-relevant issues about epilepsy due to FCDs and related cortical malformations, including the pathological and clinical features, etiological factors, localization of the epileptogenic zone, neuronal and astrocytic contributions to epileptogenesis, and the development of anti-epileptogenic therapies.
Keywords: mice, rat, epilepsy, epileptogenesis, malformation
Introduction
Epilepsy resulting from focal cortical dysplasia (FCD) and related malformations of cortical development (MCDs) is frequently severe and intractable to available medications (Andermann et al., 2000). Although surgical approaches for epilepsy due to FCD can be successful in some cases (Wyllie et al., 1998; Tassi et al., 2002; Bautista et al., 2003), a significant proportion of these patients are not appropriate surgical candidates or continue to have seizures despite epilepsy surgery. Development of more effective therapies for epilepsy due to FCD and related MCDs depends on obtaining a better understanding of the underlying pathophysiology of epilepsy, or mechanisms of epileptogenesis, in these entities (Wong, 2008). Clinical, radiographic, and pathological studies of people can reveal insights into biological processes causing epilepsy. However, due to limitations of clinical studies involving patients or human tissue, animal models are often utilized to investigate mechanisms of epileptogenesis. In this review, I will attempt to illustrate both the utility and pitfalls of animal models of epilepsy in FCD and related MCDs.
Before discussing animal models of cortical malformations per se, it is worthwhile to consider some broader issues related to the utility of animal models in general. Overall, there are two main applications of animal models for studying human disease. First, animal models allow basic mechanistic studies of pathophysiology of disease involving experimental approaches that are difficult or impossible to perform in people due to practical or ethical reasons. Knowledge obtained from these experimental studies about pathophysiology ideally may be applied to develop novel therapies for disease. Second, animal models are also useful for performing pre-clinical studies testing the efficacy and safety of new treatments. Ultimately, these animal studies can provide important data supporting or refuting the progression to clinical trials in people. Despite the potential utility of model systems, there are clearly limitations of animal studies. The most obvious limitations relate to the unknown applicability of simpler animal systems to humans. Given the intrinsic biological differences between people and lower animals, it is inherently evident that animal models can never exactly reproduce all the features of a human disease. However, given the incredible evolutionary conservation of biological processes, it is possible that certain aspects of human disease can be recapitulated in a lower animal, such as isolated behavioral phenomena, pathological entities, abnormal cell types, or molecular, biochemical, and genetic defects. The important point is to clearly define the specific part of the disease that is being modeled, and the specific question that is being addressed with the model.
With the acknowledged limitations, how well can epilepsy due to FCD and related MCDs be modeled? When identifying specific aspects of the human disease to model, there are two main considerations. First is modeling the pathology of the cortical malformation, or the lesion, including both circuit and cellular abnormalities. Second is, within a given pathology or lesion, modeling the particular cellular and molecular mechanisms that lead to epilepsy, or epileptogenesis. In the ideal model, both of these aspects would be faithfully reproduced, but this is often not the case. This highlights the importance of clearly defining the specific aspect of a disease that is being modeled.
A variety of different animal models have been developed, which mimic different aspects of MCDs and epilepsy. A selective sampling of models of FCD and other related MCDs are listed in Table 1. Analogous to pitfalls in human studies of MCDs, there are often inconsistencies and confusion in the animal model literature about the terminology and classification of various MCDs, such as in the general use of the term “cortical dysplasia”. Table 1 categorizes representative animal models according to the widely-recognized Barkovich classification, which is based on presumed defects in different stages of cortical development: proliferation, migration, and organization (Barkovich et al., 2005). FCD and Tuberous Sclerosis Complex (TSC), which often share identical pathological features, are prototypic examples of MCDs caused primarily by abnormalities in proliferation and differentiation of early neuroglial progenitor cells. Other MCDs with distinct but overlapping features are representative of defects in later stages of cortical development, such as lissencephaly and heterotopias due to abnormal neuronal migration and polymicrogyria due to abnormal cortical organization. Different animal models of MCDs have been generated by introducing a variety of different genetic defects or environmental insults affecting an early stage of cortical development. These various models mimic specific pathological or clinical features of these different MCDs to varying degrees. Although this review focuses primarily on FCD and TSC, animal models of other types of MCDs are also mentioned, because these other models sometimes have more specific insights about mechanisms of epileptogenesis that may also be relevant to FCD. Rather than attempting a comprehensive summary of the literature, this review will highlight selected examples of how animal models have been useful in addressing several clinically-relevant questions about epilepsy due to FCDs (Table 2). Other works have provided more detailed, comprehensive reviews of animal models of different MCDs and more complete listings of relevant references (Chevassus-au-Louis et al., 1999; Schwartzkroin et al., 2004; Najm et al., 2007).
Table 1.
Animal Models of Focal Cortical Dysplasia and Related Malformations of Cortical Development
| Animal Model | Pathological Features | Epilepsy/Hyperexcitability |
|---|---|---|
| Malformations due to abnormal neuronal glial proliferation | ||
| Focal Cortical Dysplasia | ||
| In utero irradiation rat | Abnormal cortical lamination Heterotopic/dysmorphic neurons |
Rare (<20%) spontaneous epilepsy |
| In utero methylazoxymethanol (MAM) rat |
Abnormal cortical lamination Heterotopic neurons |
Rare (<20%) spontaneous epilepsy |
| In utero carmustine (BCNU) rat | Abnormal cortical lamination Dysmorphic neurons |
In vitro hyperexcitability |
| Neonatal focal ibotenate | Abnormal cortical lamination Heterotopic neurons |
In vitro hyperexcitability |
| Prenatal/in utero freeze lesion rat | Abnormal cortical lamination | Interictal spikes/increased kindling |
| Tuberous Sclerosis | ||
| Eker rat (spontaneous Tsc2+/−) | Rare subcortical and Subependymal hamartomas |
None |
| Tsc1-synapsin conditional knock-out mice |
Abnormal cortical lamination Large, dysmorphic neurons |
Rare (<20%) spontaneous epilepsy |
| Tsc1-GFAP conditional knock-out mice |
Glial proliferation | Frequent (~100%) epilepsy |
| Malformations due to abnormal neuronal migration | ||
| Lissencephaly | ||
| Lis1+/− knock-out mice | Abnormal cortical lamination | In vitro hyperexcitability |
| Heterotopia | ||
| Tish spontaneous mutant rat | Subcortical band heterotopias | Spontaneous epilepsy |
| p35 knock-out mice | Heterotopic neurons | Spontaneous epilepsy |
| In utero irradiation or MAM rat | Nodular heterotopias | Rare spontaneous epilepsy |
| Malformations due to abnormal cortical organization | ||
| Polymicrogyria | ||
| Postnatal/neonatal freeze-lesion rat | Microsulcus | In vitro hyperexcitability |
The table summarizes pathological features and epilepsy/hyperexcitability of representative animal models of focal cortical dysplasia and other related malformations of cortical development, categorized according to presumed developmentally-based pathophysiology (based on Barkovich et al. 2005 classification). This table only highlights representative examples from each category and is not intended to be a comprehensive listing of all animal models. See the text for references on selected animal models and more comprehensive reviews with additional references.
Table 2.
Clinically-Relevant Questions About Epilepsy and Focal Cortical Dysplasia Addressable by Animal Models
|
How well do animal models mimic the pathological and clinical features of epilepsy due to focal cortical dysplasia?
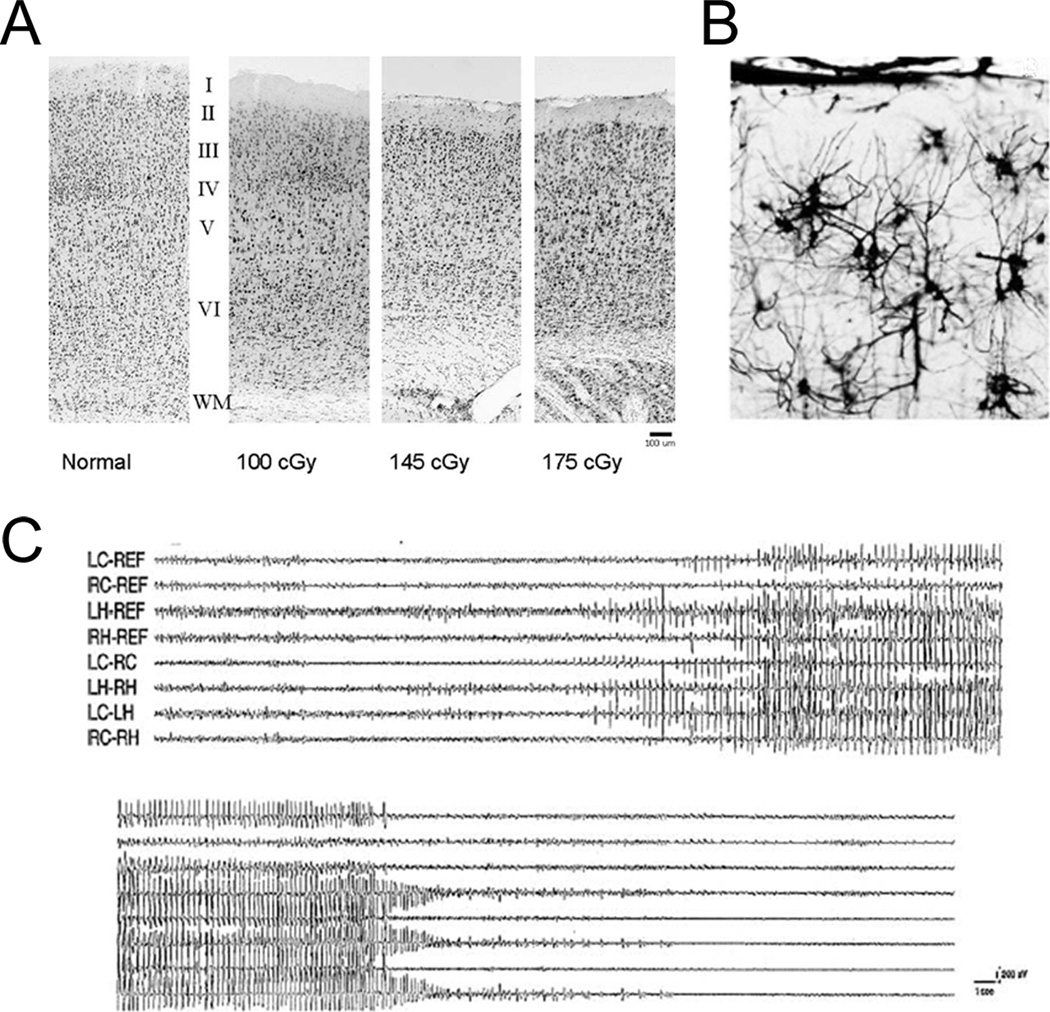
The initial question, relevant to the applicability of animal models, is how well do animal models actually mimic the features of FCDs? In order to serve as a clinically-relevant model, the ideal model would recapitulate both the pathological characteristics of FCDs and the clinical features of epilepsy. The pathological hallmarks of FCD include a focal loss of cortical lamination and the presence of different types of dysmorphic or cytomegalic cells with varying properties. The most characteristic abnormal cell type is the balloon cell, an extremely large cell with both immature neuronal and glial traits. The cellular and pathological characteristics of FCD are consistent with a defect in neuroglial progenitor cells during early cortical development. In terms of modeling the pathological lesion of FCD, there is no perfect animal model that recapitulates all of the cellular and histological features of FCD. However, there are models that reproduce some pathological features of FCD, such as abnormal cortical lamination and dysmorphic cells (Table 1). For example, one of the better-characterized animal models of FCD is the in utero irradiation model, where pregnant rats are exposed to X-rays and the offspring develop cortical malformations. In this model, there is a striking loss of the normal six-layered cortex, with the degree of cortical dyslamination showing a dose-dependence on the initial radiation dose (Fig. 1; Kellinghaus et al., 2004). In terms of cellular abnormalities, although the classic balloon cell has been difficult to recapitulate, varying degrees of cytomegalic or dysmorphic cells have been found in the in utero radiation model and other animal models of FCD (Marin-Padilla et al., 2003). In addition to the in utero irradiation model, prenatal exposure to the teratogen, methylazoxymethanol (MAM), induces similar pathological features that partially mimic human FCD (Sancini et al., 1998; Colacitti et al., 1999). Thus, different animal models can reproduce some of the pathological features of human FCD.
Figure 1.
Animal models can mimic pathological and clinical features of epilepsy due to focal cortical dysplasia. (A, B) In the in utero irradiation model, rats exposed to radiation prenatally develop characteristic pathological abnormalities: a loss of the normal six-layer cortical lamination that is dependent on radiation dose (A) and hypertrophic, dysmorphic neurons with disoriented dendrites (B). (C) A small proportion of rats exposed to radiation prenatally develop spontaneous seizures as adults, as detected by video EEG monitoring. Reproduced with permission from Wiley-Blackwell (for A and C from Kellinghaus et al., 2004) and Wolters Kluwer (for B from Marin-Padilla et al., 2003).
Cortical hamartomas or tubers of TSC often have identical pathological characteristics as human FCD, especially FCD Type IIB according to the Palmini classification (Palmini et al., 2004), including focal loss of cortical lamination, astrogliosis, a spectrum of dysmorphic neurons and glia, and undifferentiated giant cells (analogous to balloon cells of FCD). A number of animal models of TSC have been generated, based on spontaneous or induced mutations of either the Tsc1 or Tsc2 genes, which mimic the pathological features of human TSC to varying degrees. For example, the Eker rat, which carries a spontaneous germline heterozygous mutation of the Tsc2 gene, exhibits hamartomatous lesions, especially in subcortical or subependymal regions (Yeung et al. 1997; Mizuguchi et al., 2000); however, cortical hamartomas have only been reported extremely rarely in the Eker rat (Mizuguchi et al., 2000). Attempts to induce a “second-hit” mutation with early postnatal irradiation of Eker rats results in increased dysmorphic and cytomegalic cells in cortex, but does not stimulate focal tuber formation (Takahashi et al., 2004; Wenzel et al., 2004). Similarly, a number of conditional knock-out mouse models of TSC have been successful in recapitulating selective cytopathological features of TSC, such as disrupted cortical lamination, cytomegalic neurons, and astrogliosis (Uhlmann et al., 2002; Meikle et al., 2007; Way et al., 2009). However, a recognized limitation of these animal models has been the failure to consistently reproduce focal tuber-like lesions. In the future, more targeted gene inactivation techniques, with a higher degree of temporal and spatial specificity aimed at neuroglial progenitor cells, may have a better chance of inducing focal cortical tubers. Nevertheless, as demonstrated in subsequent sections, the existing animal models still provide useful information about specific cellular and functional aspects of TSC related to epilepsy.
While certain pathological aspects of FCD and TSC can be generated in animal models, does this actually lead to epilepsy? There is also some variability in the degree to which animal models of FCD display epilepsy. Most animal models of FCD show some evidence of decreased seizure threshold, or at least increased neuronal excitability, such as with in vitro assays. For example, in the model involving prenatal exposure to MAM, rats exhibit a decreased seizure threshold in response to convulsant drugs and increased spontaneous or evoked epileptiform activity in hippocampal slices (Baraban and Schwartzkroin, 1995, 1996; Chevassus-au-Louis et al., 1998). However, for a long time, there was surprisingly little evidence for spontaneous epilepsy in most animal models of FCD. But recently, with the use of long-term video-EEG monitoring in rodents, spontaneous seizures have been documented in several animal models of FCD, including the irradiation and MAM models (Kondo et al., 2001; Harrington et al, 2007). Even so, the incidence of epilepsy found in these studies is relatively low, with seizure documented in only about 10–20% of animals. There is also variability in animal models of TSC in the incidence of epilepsy. The Eker rat may have a slightly increased susceptibility to convulsants, but has not been documented to have spontaneous seizures (Wenzel et al., 2004). However, some knock-out mouse models of TSC have very frequent, progressive seizures (Uhlmann et al., 2002; Erbayat-Altay et al., 2007). So, there again are some limitations that make animal models less than perfect for reproducing and studying human FCD, but overall animal models do mimic some of the major histological and clinical features of FCD.
What do animal models reveal about the etiologies of focal cortical dysplasia?
While animal models can recapitulate some aspects of the pathological and clinical phenotype of FCD, an important application of these models is to elucidate the underlying pathophysiology and etiology of FCD. In people, it is suspected that a variety of different etiologies, including both environmental injuries and genetic mutations, during early brain development can cause cortical malformations. Animal studies support this idea, as a wide range of different acquired insults or genetic manipulations result in models of cortical dysplasia. A variety of injurious stimuli, such as exposure to radiation, freeze lesions, and various teratogens and other toxins, administered to fetal rodents in utero have been used to produce models of FCD (Table 1; Schwartzkroin et al., 2004). In addition to acquired environmental injuries, genetic animal models suggests that specific genetic mutations may play a role in the pathophysiology of some forms of FCD. Although it is likely that other genes causing isolated FCD will be identified, currently the most relevant models involve mutations affecting the mammalian target of rapamycin (mTOR) signaling pathway, such as PTEN or TSC mutations, and produce FCDs or related pathology in the context of broader syndromes. In general, the wide variety of acquired and genetic manipulations that lead to animal models of FCDs indicate that FCDs are pathophysiologically and etiologically diverse.
Despite the varied and disparate nature of insults producing animal models of FCDs, these models can still identify some common principles about the etiology and pathophysiology of FCD. Although the environmental injuries that have been used to induce malformations in animal models are fairly artificial and do not exactly mimic the kinds of insults to which the human fetus is exposed, shared properties of some of these artificial insults suggest common underlying mechanisms for generating FCD. For example, radiation, MAM, and BCNU, are all alklyating agents that disrupt DNA in proliferating cells. The effectiveness of these agents in animal models suggests that disruption of the proliferating pool of neuroglial progenitors in the developing brain is a key step in causing FCD. Comparisons between animal models also reveal insights about the critical timing of an injury necessary to cause FCD. Freeze lesions, involving direct application of a strongly hypothermic stimulus to cortex, are a popular method for inducing animal models of MCDs. In the classic model, a freeze lesion applied directly to the skull of neonatal rats results in the formation of a cortical microgyrus , which is thought to be a model of polymicrogyria, not FCD (Jacobs et al., 1996; Zilles et al., 1998). However, in a recent adaptation of this model, a similar freeze lesion administered to fetal rats through the walls of the uterus of a pregnant rat causes pathology more closely resembling FCD (Takase et al., 2008). Thus, comparing the effect of prenatal versus postnatal freeze lesions, the timing of the insult during brain development dictates the type of brain malformation generated. Overall, animal models support the idea that FCD is not one disease from an etiological standpoint, but may represent a final common pathway due to a diverse number of environmental or genetic insults at a specific stage of brain development.
What do animal models tell us about the location of the epileptogenic zone?
Identifying the location of the epileptogenic zone in patients with FCD and related MCDs is an issue that is frequently debated and has strong clinical implications. In particular, it’s not entirely clear whether seizures originate directly from within the lesion of the FCD itself or start in normal-appearing areas of cortex surrounding the lesion. This question is important not only for understanding the pathophysiology of epilepsy in FCD, but also has direct clinical implications for the approaches to epilepsy surgery. Is taking out the lesion sufficient or does including a large margin of normal-appearing tissue improve outcome? While epilepsy surgery can be successful in achieving seizure freedom in about 50–75% of patients with medically-intractable epilepsy (Wyllie et al., 1998; Tassi et al., 2002; Bautista et al., 2003), this leaves a substantial proportion of patients that continue to have seizures despite resection of the apparent lesion. In fact, recent electrophysiological studies in some patients with different cortical malformations indicate that seizures may arise from outside the region of the malformation itself (Chassoux et al., 2008; Major et al., 2009). Thus, clinical data suggest that the lesion is necessary for seizure-generation in many cases, but may not account for all aspects of epileptogenesis.
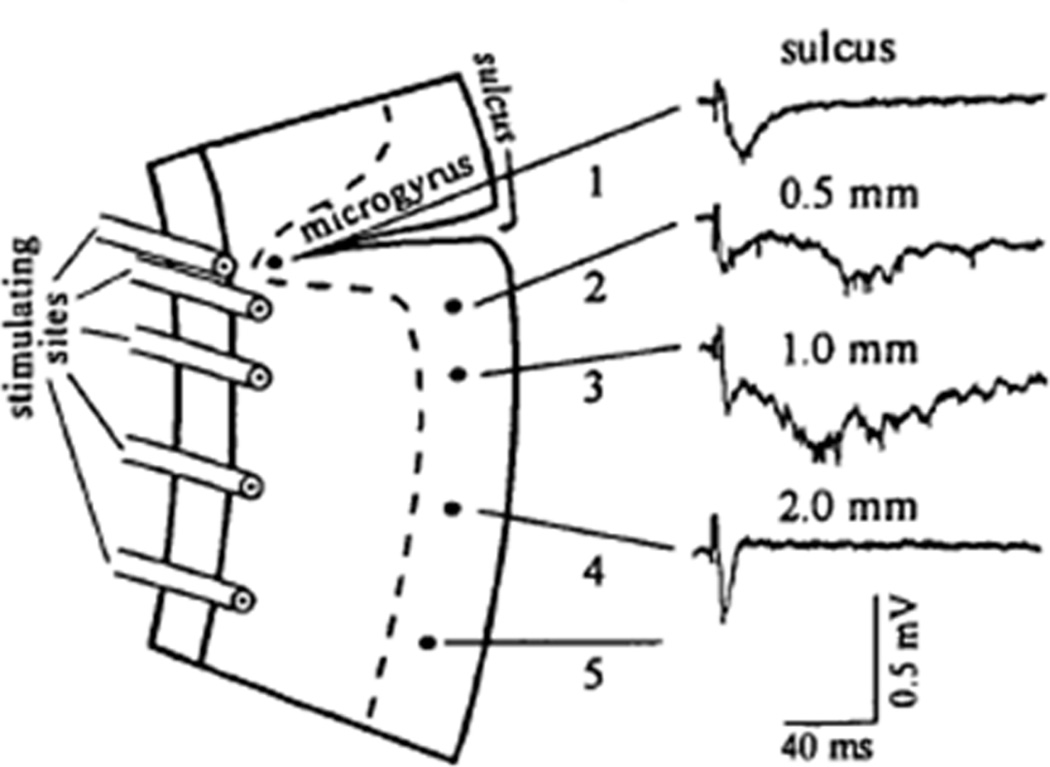
As clinical studies often have practical limitations in characterizing and identifying the precise location of the epileptogenic zone in patients, animal models can offer more direct ways of defining the critical regions and mechanisms causing epileptogenesis and seizure-generation. Although little has been established relevant to this question in standard animal models of FCD per se, some information can be extrapolated from related animal models of other types of cortical malformations. Somewhat surprisingly, much of this data suggests that the peri-lesional region may be more epileptogenic than the lesion itself. For example, in the postnatal freeze-lesion model of a microgyrus, epileptiform activity can be induced in the region adjacent to the microgyrus, but not within the microgyrus itself (Fig. 2; Jacobs et al., 1996). As mentioned earlier, with animal models of TSC, it has been difficult to generate an animal model that has focal tuber-like lesions. Rather, a couple of mouse models of TSC exhibit a diffuse brain enlargement or megencephaly, as well as cellular abnormalities in neurons and glia, but no focal lesions resembling tubers or cortical dysplasia (Uhlmann et al., 2002; Meikle et al., 2007). Despite the absence of focal lesions, these mouse models of TSC do still develop frequent seizures (Uhlmann et al., 2002; Erbayat-Altay et al., 2007). Thus, this indicates that focal lesions are not necessary for epileptogenesis in TSC, and perhaps there are cellular and molecular abnormalities in non-lesional regions of the brain that can cause seizures. If the non-lesional or perilesional regions can similarly generate seizures in people with FCD and related MCDs, this has important implications for optimizing surgical approaches to epilepsy due to focal cortical malformations.
Figure 2.
Animal models implicate the peri-lesional region in epileptogenesis due to cortical malformations. In the postnatal/neonatal freeze lesion model, a microgyrus forms in the cortex of rats. Epileptiform activity can be induced by stimulation in the cortical regions adjacent to the microgyrus, but not within the microgyrus itself. Reproduced with permission from Oxford (from Jacobs et al., 1996).
What do animal models tell us about the cellular and molecular mechanisms of epileptogenesis in focal cortical dysplasia?
Understanding the cellular and molecular mechanisms causing epileptogenesis represents one of the most important goals of epilepsy research, which could lead to more effective, rational therapies for epilepsy. While studies of human brain tissue can reveal important clues to the pathophysiology of epilepsy, this is an area where animal models offer the most advantages, in allowing detailed cellular and molecular studies that may be difficult to perform in human tissue and in offering better experimental controls than can be obtained in clinical studies. Animal studies may discover initial evidence for specific mechanistic abnormalities that could then be tested in human tissue or conversely, may permit more detailed characterization of molecular defects first discovered in human tissue. Although there are numerous examples of animal models identifying cellular and molecular brain abnormalities that may contribute to epilepsy, a couple of representative examples will be highlighted here to demonstrate some general principles.
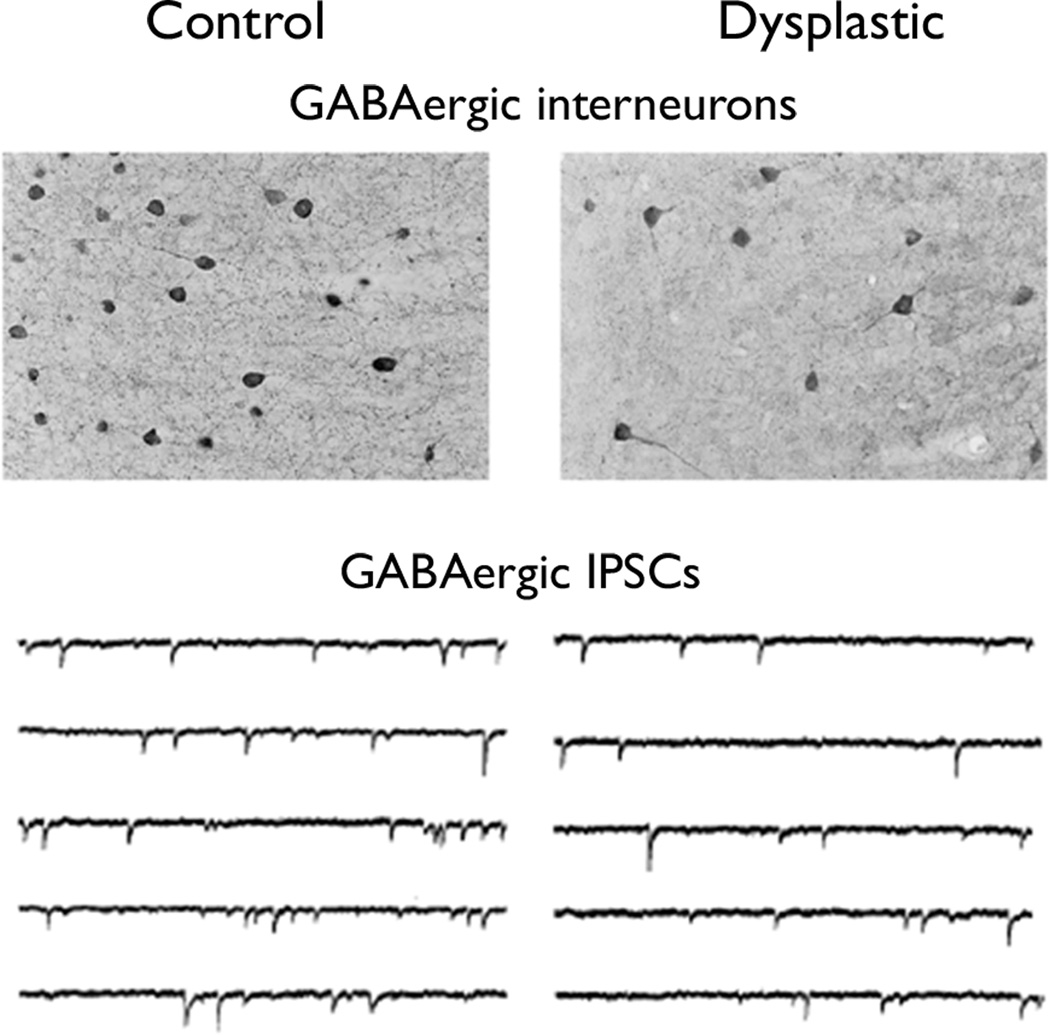
A general concept to explain epileptogenesis is that epilepsy results from an imbalance between excitatory and inhibitory forces in the brain. This could be due to excessive excitatory influences, such as enhanced glutamatergic synaptic transmission, or a reduction in inhibitory forces, such as deficient GABAergic systems. In some animal models of FCD, there is evidence of abnormalities in either glutamatergic or GABAergic synaptic transmission. For example, in the in utero irradiation model, a decrease in the number of GABAergic interneurons has been documented in dysplastic areas of cortex (Fig. 3; Roper et al., 1999). There is a corresponding reduction in inhibitory synaptic transmission by electrophysiological measures, with a decrease in the frequency and amplitude of inhibitory postsynaptic currents in the dysplastic brains (Zhu and Roper, 2000). In the prenatal, in utero freeze lesion model of focal cortical dysplasia, there is evidence of increased glutamate function with amplified expression of the NR2B subunit of the NMDA glutamate receptor (Takase et al., 2008). Similarly, in the postnatal freeze lesion microsulcus model, there is also increased glutamate receptor binding, as well as decreased GABA receptor expression (Zilles et al., 1998). Thus, a perturbation of the normal balance between the major excitatory and inhibitory neurotransmitter systems may promote epileptogenesis in animal models of FCD and could mimic mechanisms that occur in people. In fact, recent electrophysiological and immunohistochemical studies of human tissue confirm the animal data in demonstrating a number of abnormalities in glutamatergic and GABAergic properties in clinical specimens of FCD, as well as TSC (Crino et al., 2001; Aronica et al., 2003; Andre et al., 2004; Alonso-Nanclares et al., 2005; Calcagnotto et al., 2005; Talos et al., 2008).
Figure 3.
Animal models of focal cortical dysplasia demonstrate cellular defects in GABAergic synaptic inhibition. In the in utero irradiation model, dysplastic areas of cortex exhibit a decrease in the number of GABAergic interneurons (top) and a corresponding reduction in the frequency of inhibitory postsynaptic currents (bottom). Reproduced with permission from Elsevier (for top, Roper et al., 1999) and Society for Neuroscience (for bottom, Zhu and Roper, 2000).
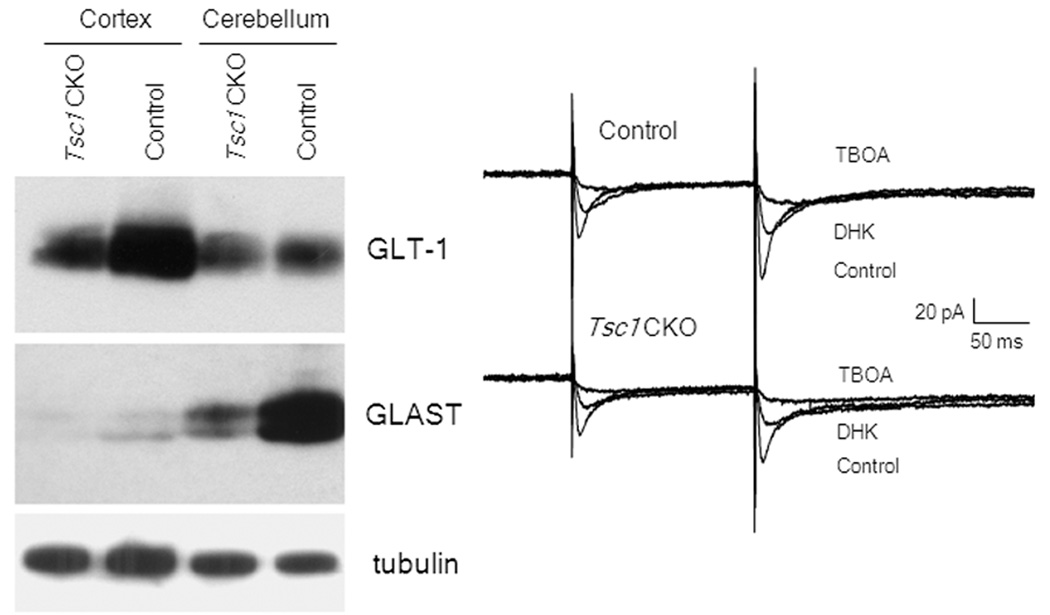
Besides the popular concept of imbalance between excitation and inhibition of neurons causing epilepsy, a recent trend has been recognizing the role of astrocytes in epileptogenesis (Binder and Steinhauser, 2006; Wetherington et al., 2008). Traditionally, glia have simply been viewed as passive, supportive cells in the brain with relatively mundane, housekeeping functions. However, recently astroglia have been recognized to have a much greater diversity of functions that directly affect brain excitability, such as regulation of synaptic neurotransmitter and ion levels and active signaling within networks of both neurons and glia (“gliotransmission”). A defect in these astrocytic mechanisms could also disrupt physiological balance and lead to epileptogenesis and seizure generation. One candidate mechanism involves removal of synaptic glutamate by astrocytes. Astrocytes have specific glutamate transporters, GLT-1 and GLAST, that help take up glutamate from the extracellular space. A deficiency in the astrocyte glutamate transporters could result in elevated extracellular glutamate levels, leading to excessive excitation of neurons or excitotoxic cell death, both of which may promote epileptogenesis. Although there have been limited studies on astrocytes in animal models of FCD per se, there is accumulating evidence in both human specimens and animal models of TSC that astrocyte dysfunction may be key in epileptogenesis. In a knock-out mouse of TSC that involves inactivation of the Tsc1 gene primarily in glia, there is decreased astrocyte glutamate transporter expression and function and an associated impairment of glutamate homeostasis, which likely contributes to epileptogenesis and other neurological deficits in these mice (Fig. 4; Wong et al., 2003; Zeng et al., 2007). Thus, animal models provide evidence for newly-recognized roles of glia in epileptogenesis and suggest novel therapeutic targets for epilepsy aimed specifically at astrocytes.
Figure 4.
Animal models implicate astrocytes in epileptogenesis due to cortical malformations. In Tsc1-GFAP conditional knock-out mice involving inactivation of the Tsc1 gene primarily in glial cells, astrocytes exhibit a decreased expression of the glutamate transporters, GLT-1 and GLAST (left), and a corresponding reduction in astrocyte glutamate transporter currents as blocked by specific transporter inhibitors, such as TBOA (right). These astrocyte defects cause abnormal glutamate homeostasis and may promote epileptogenesis in these mice. Reproduced with permission from Wiley (Wong et al., 2003).
Can animal models help identify novel treatments for epilepsy due to focal cortical dysplasia?
The ultimate goal of animal models of human disease is to develop and test new treatments that can be translated directly into clinical trials in people. While many treatments for epilepsy have been discovered through a screening or “shot-gun” approach, ideally animal models can be used to identify and test rational, more effective treatments for epilepsy that specifically target and correct mechanistic defects of epileptogenesis. Furthermore, while most current therapies for epilepsy are primarily end-stage, symptomatic treatments that suppress seizures, the holy grail of epilepsy research has been to develop true “anti-epileptogenic” therapies that prevent or reverse the underlying mechanisms causing epileptogenesis and thus improve the long-term course and prognosis of epilepsy. Recently there have been exciting studies in animal models suggesting the possibility of such mechanism-based, anti-epileptogenic therapies for FCD and related MCDs. While alterations in ion channels and neurotransmitter systems represent the final pathways directly causing seizures and are the target of many seizure medications, anti-epileptogenic therapies likely need to be directed at earlier stages of epileptogenesis, such as primary cell signaling pathways that regulate downstream ion channels and neurotransmitter receptors. A very promising candidate for such a signaling mechanism relates to the mTOR pathway. The mTOR pathway, which is directly regulated by insulin and phosphatidylinositol 3-kinase, has been found to be abnormally activated in FCD and TSC, as well as ganglioma, and thus may represent a common signaling mechanism in focal cortical malformations associated with epilepsy (Crino, 2007; Schick et al., 2007). As mTOR is a central protein kinase involved in protein synthesis, stimulating the expression of numerous downstream proteins, it is conceivable that dysregulation of the mTOR pathway could lead to abnormal expression of multiple proteins that promote epileptogenesis.
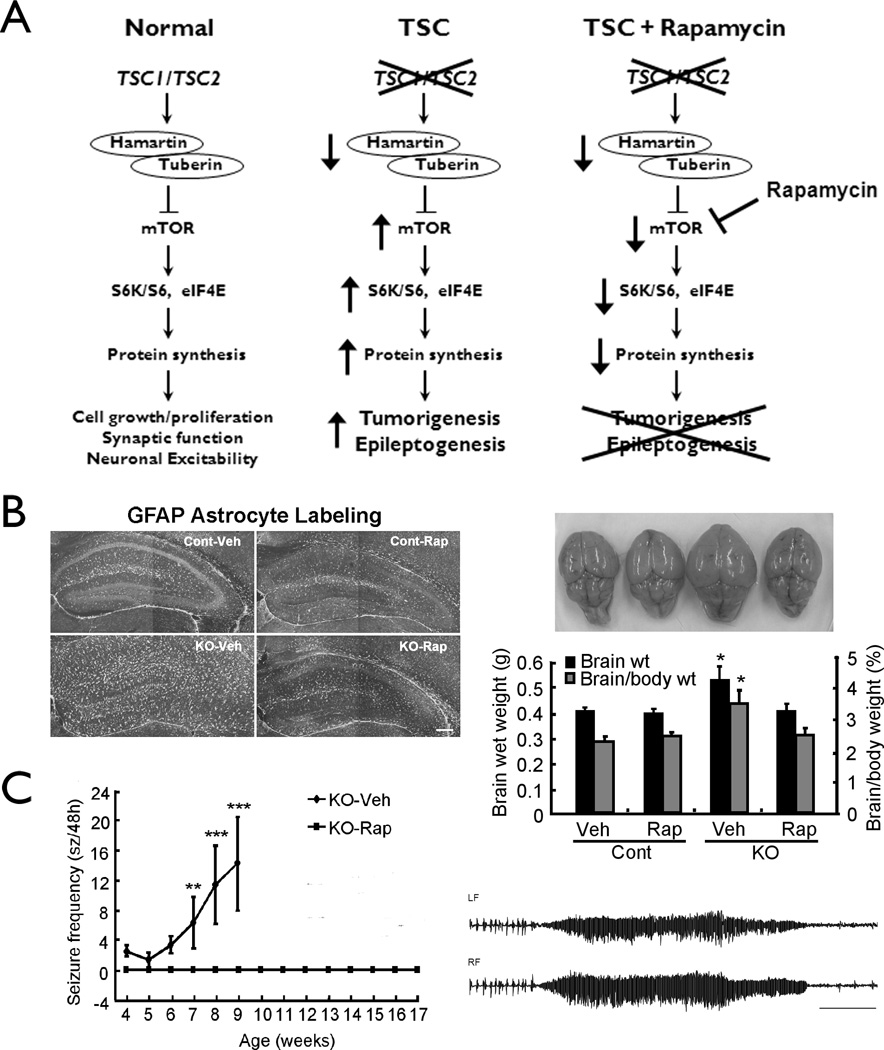
Recent studies demonstrate the critical role of mTOR and the anti-epileptogenic potential of mTOR inhibitors in animal models of TSC. Interestingly, the initial link between mTOR dysregulation and TSC gene mutations was discovered in the fruit fly, Drosophila (Gao et al., 2002), representing a perfect example of basic research in even the simplest animal models having direct clinical relevance to people. Similarly, the mTOR pathway is hyperactivated due to Tsc gene inactivation in neurons or glia in knock-out mouse models of TSC (Uhlmann et al., 2004; Meikle et al., 2007). These mouse models exhibit many pathological features of TSC, including abnormal cortical lamination, dysplastic and ectopic neurons, and astrogliosis, which are reversed by the mTOR inhibitor, rapamycin (Meikle et al., 2008; Zeng et al., 2008). In addition, in the Tsc1-GFAP knock-out mice, other potential molecular mechanisms of epileptogenesis, such as the deficient astrocyte Glt-1 glutamate transporter expression, are prevented by early treatment with rapamycin (Zeng et al., 2008). Most remarkably, early treatment with rapamycin prior to the typical onset of seizures completely prevents development of epilepsy in these mice (Fig. 5), whereas late treatment after the onset of epilepsy decreases progressive seizures. These findings are significant, because rapamycin appears to have true anti-epileptogenic actions, not simply seizure-supressing effects, by correcting an underlying cell signaling defect in TSC. While epilepsy researchers have recognized the need for anti-epileptogenic therapies for years, no such therapies have yet been developed for clinical use. However, rapamycin already shows promising effects on tumor growth in TSC in clinical trials (Franz et al., 2006; Bissler et al., 2008) and preliminary studies are also in progress to test its efficacy for epilepsy in TSC patients. Given the pathological and molecular similarities between TSC and FCD, including involvement of the mTOR pathway (Kwon et al., 2004; Ljungdberg et al. 2006; Crino, 2007; Schick et al., 2007), rapamycin could also be considered as a possible therapy for FCD. Overall, recent work has highlighted the potential importance and role animal models can play in development of new therapies for epilepsy due to cortical malformations.
Figure 5.
Animal models of Tuberous Sclerosis Complex demonstrate the potential efficacy of rapamycin as an anti-epileptogenic therapy. (A) The TSC1 and TSC2 genes encode the proteins, hamartin and tuberin, respectively, which bind together to act as a single signaling complex and normally inhibit the mammalian target of rapamycin (mTOR) protein. mTOR functions to activate a cascade of kinases and proteins that stimulates ribosomal biogenesis and protein synthesis, ultimately involved in a variety of functions, such as cell growth and proliferation, synaptic transmission and neuronal excitability. Thus, the TSC genes normally limit excessive cell growth, proliferation, and excitability. In the disease of TSC, mutation of one of the TSC genes results in a decrease in the hamartin-tuberin complex, causing disinhibition or hyperactivation of the mTOR pathway, and resultant tumorigenesis and, most likely, also epileptogenesis. Rapamycin, by inhibiting mTOR, may be able to counteract the effects of TSC gene mutations, thus potentially preventing tumor formation and epileptogenesis. (B) In Tsc1-GFAP conditional knock-out mice, rapamycin treatment prevents abnormal glial proliferation, as reflected by GFAP staining (left), and the corresponding megencephaly that results from glial proliferation (right). (C) Tsc1-GFAP conditional knock-out mice develop seizures (typical ictal EEG trace on right) around 4–5 weeks of age, which become progressively more frequent over the following month, with most mice dying prematurely by 2–3 months of age. Rapamycin treatment initiated at 2 weeks of age completely prevents onset of epilepsy in these mice. Reproduced with permission from Wiley (Zeng et al., 2008).
Conclusions
A number of conclusions can be made about the utility of animal models for epilepsy and FCD with respect to several clinically-relevant issues. First, animal models can recapitulate some, but not all aspects of epilepsy due to FCD, including pathological and clinical features. Second, animal models indicate that FCD can result from multiple etiologies and may represent a final common pathway due to environmental or genetic insults at a specific stage of brain development. Third, the peri-lesional region may be as important as the lesion in causing epileptogenesis. Fourth, animal models can be useful for studying different cellular and molecular mechanisms of epileptogenesis in both neurons and glia in FCD. And finally and most importantly from a clinical standpoint, animal models can be used to develop and test novel anti-epileptogenic therapies based on rational, mechanistic hypotheses. In particular, the recent work in animal models of TSC elucidating the role of the mTOR pathway and demonstrating the potential clinical benefits of rapamycin exemplify the direct translational relevance of findings from animal models to human disease. Although there are definite limitations to animal models, future work will undoubtedly continue to add examples of clinical applications of animal models and lead to new therapies for epilepsy in FCD.
Acknowledgements
The author receives funding from the National Institutes of Health (K02 NS045583, R01 NS056872), Tuberous Sclerosis Alliance, and Citizens United for Research in Epilepsy. The author has read the Journal's position on issues involved in ethical publication and affirms that this report is consistent with those guidelines.
Footnotes
Disclosure of Conflicts of Interest
The author declares no conflicts of interest.
References
- Alonso-Nanclares L, Garbelli R, Sola RG, Pastor J, Tassi L, Spreafico R, DeFelipe J. Microanatomy of the dysplastic neocortex from epileptic patients. Brain. 2005;128:158–173. doi: 10.1093/brain/awh331. [DOI] [PubMed] [Google Scholar]
- Andermann F. Cortical dysplasias and epilepsy: a review of the architectonic, clinical, and seizure patterns. Adv Neurol. 2000;84:479–496. [PubMed] [Google Scholar]
- Andre VM, Flores-Hernandez J, Cepeda C, Starling AJ, Nguyen S, Lobo MK, Vinters HV, Levine MS, Mathern GW. NMDA receptor alterations in neurons from pediatric cortical dysplasia tissue. Cerebral Cortex. 2004;14:634–646. doi: 10.1093/cercor/bhh024. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Jansen GH, van Veelen CW, van Rijen PC, Ramkema M, Troost D. Expression and cell distribution of group I and group II metabotropic glutamate receptor subtypes in taylor-type focal cortical dysplasia. Epilepsia. 2003;44:785–795. doi: 10.1046/j.1528-1157.2003.54802.x. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Schwartzkroin PA. Electrophysiology of CA1 pyramidal neurons in an animal model of neuronal migration disorders: prenatal methylazoxymethanol treatment. Epilepsy Res. 1995;22:145–156. doi: 10.1016/0920-1211(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Schwartzkroin PA. Flurothyl seizure susceptibility in rats following prenatal methylazoxymethanol treatment. Epilepsy Res. 1996;23:189–194. doi: 10.1016/0920-1211(95)00094-1. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–1887. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- Bautista JF, Foldvary-Schaefer N, Bingaman WE, Luders HO. Focal cortical dysplasia and intractable epilepsy in adults: clinical, EEG, imaging, and surgical features. Epilepsy Res. 2003;55:131–136. doi: 10.1016/s0920-1211(03)00118-9. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schimithorst VJ, Laor T, Broady AS, Bean J, Salisbury S, Franz DN. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnotto ME, Paredes MF, Tihan T, Barbaro NM, Baraban SC. Dysfunction of synaptic inhibition in epilepsy associated with focal cortical dysplasia. J Neurosci. 2005;25:9469–9657. doi: 10.1523/JNEUROSCI.2687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassoux F, Landre E, Rodrigo S, Beuvon F, Turak B, Semah F, Devaux B. Intralesional recordings and epileptogenic zone in focal polymicrogyria. Epilepsia. 2008;49:51–64. doi: 10.1111/j.1528-1167.2007.01267.x. [DOI] [PubMed] [Google Scholar]
- Chesvassus-au-Louis N, Ben-Ari Y, Vergnes M. Decreased seizure threshold and more rapid kindling in rats with prenatal treatment with methylzoxymethanol. Brain Res. 1998;812:422–425. doi: 10.1016/s0006-8993(98)00932-9. [DOI] [PubMed] [Google Scholar]
- Chevassus-au-Louis N, Baraban SC, Gaiarsa JL, Ben-Ari Y. Cortical malformations and epilepsy: new insights from animal models. Epilepsia. 1999;40:811–821. doi: 10.1111/j.1528-1157.1999.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Colacitti C, Sancini G, DeBiasi S, Franceschetti S, Caputi A, Frassoni C, Cattabeni F, Avanzini G, Spreafico R, DiLuca M, Battaglia G. Prenatal methylazoxymethanol treatment in rats produces brain abnormalities with morphological similarities to human developmental brain dysgeneses. J Neuropathol Exp Neurol. 1999;58:92–106. doi: 10.1097/00005072-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Crino PB. Focal brain malformations: a spectrum of disorders along the mTOR cascade. Novartis Found Symp. 2007;288:260–272. doi: 10.1002/9780470994030.ch18. [DOI] [PubMed] [Google Scholar]
- Crino PB, Duhaime AC, Baltuch G, White R. Differential expression of glutamate and GABA-A receptor subunit mRNA in cortical dysplasia. Neurology. 2001;56:906–913. doi: 10.1212/wnl.56.7.906. [DOI] [PubMed] [Google Scholar]
- Erbayat-Altay E, Zeng LH, Xu L, Gutmann D, Wong M. The natural history and treatment of epilepsy in a murine model of tuberous sclerosis. Epilepsia. 2007;48:1470–1476. doi: 10.1111/j.1528-1167.2007.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dimopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- Harrington EP, Moddel G, Najm IM, Baraban SC. Altered glutamate receptor-transporter expression and spontaneous seizures in rats exposed to methylazoxymethanol in utero. Epilepsia. 2007;48:158–168. doi: 10.1111/j.1528-1167.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Gutnick MJ, Prince DA. Hyperexcitability in a model of cortical maldevelopment. Cerebral Cortex. 1996;6:514–523. doi: 10.1093/cercor/6.3.514. [DOI] [PubMed] [Google Scholar]
- Kellinghaus C, Kunieda T, Ying Z, Pan A, Luders HO, Najm IM. Severity of histopathologic abnormalities and in vivo epileptogenicity in the in utero radiation model of rats is dose dependent. Epilepsia. 2004;45:583–591. doi: 10.1111/j.0013-9580.2004.41103.x. [DOI] [PubMed] [Google Scholar]
- Kondo S, Najm I, Kunieda T, Perryman S, Yacubova K, Luders HO. Electroencephalographic characterization of an adult rat model of radiation-induced cortical dysplasia. Epilepsia. 2001;42:1221–1227. doi: 10.1046/j.1528-1157.2001.38300.x. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. mTOR is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci USA. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Bhattacharjee MB, Lu Y, Armstrong DL, Yoshor D, Swann JW, Sheldon M, D’Arcangelo G. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol. 2006;60:420–429. doi: 10.1002/ana.20949. [DOI] [PubMed] [Google Scholar]
- Major P, Rakowski S, Simon MV, Cheng ML, Eskandar E, Baron J, Leeman BA, Frosch MP, Theile EA. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009;50:147–154. doi: 10.1111/j.1528-1167.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M, Tsai RJ, King MA, Roper SN. Altered corticogenesis and neuronal morphology in irradiation-induced cortical dysplasia: a Golgi-Cox study. J Neuropathol Exp Neurol. 2003;62:1129–1143. doi: 10.1093/jnen/62.11.1129. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J. Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi M, Takashima S, Yamanouchi H, Nakazato Y, Mitani H, Hino O. Novel cerebral lesions in the Eker rat model of tuberous sclerosis: cortical tuber and anaplastic ganglioglioma. J Neuropath Exp Neurol. 2000;59:188–196. doi: 10.1093/jnen/59.3.188. [DOI] [PubMed] [Google Scholar]
- Najm IM, Tilelli CQ, Oghlakian R. Pathophysiological mechanisms of focal cortical dysplasia: a critical review of human tissue studies and animal models. Epilepsia. 2007;48(Suppl 2):21–32. doi: 10.1111/j.1528-1167.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, Vinters HV. Terminology and classification of cortical dysplasias. Neurology. 2004;62(Suppl 3):S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- Roper SN, Eisenschenk S, King MA. Reduced density of parvalbumin- and calbindin D28k-immunoreactive neurons in experimental cortical dysplasia. Epilepsy Res. 1999;26:442–449. doi: 10.1016/s0920-1211(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Sancini G, Franceschetti S, Battaglia G, Colacitti C, Di Luca M, Spreafico R, Avanzini G. Dysplastic neocortex and subcortical heterotopias in methylazoxymethanol-treated rats: an intracellular study of identified pyramidal neurones. Neurosci Lett. 1998;246:181–185. doi: 10.1016/s0304-3940(98)00258-4. [DOI] [PubMed] [Google Scholar]
- Schick V, Majores M, Koch A, Elger CE, Schramm J, Urbach H, Becker AJ. Alterations of phosphatidylinositol 3-kinase pathway components in epilepsy-associated glioneuronal lesions. Epilepsia. 2007;48(Suppl 5):65–73. doi: 10.1111/j.1528-1167.2007.01291.x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Roper SN, Wenzel HJ. Cortical dysplasia and epilepsy: animal models. Adv Exp Med Biol. 2004;548:145–174. doi: 10.1007/978-1-4757-6376-8_12. [DOI] [PubMed] [Google Scholar]
- Takahashi DK, Dinday MT, Barbaro NM, Baraban SC. Abnormal cortical cells and astrocytomas in the Eker rat model of tuberous sclerosis complex. Epilepsia. 2004;45:1525–1530. doi: 10.1111/j.0013-9580.2004.23004.x. [DOI] [PubMed] [Google Scholar]
- Takase K, Shigeto H, Suzuki SO, Kikuchi H, Ohyagi Y, Kira J. Prenatal freeze lesioning produces epileptogenic focal cortical dysplasia. Epilepsia. 2008;49:997–1010. doi: 10.1111/j.1528-1167.2008.01558.x. [DOI] [PubMed] [Google Scholar]
- Talos DM, Kwiatkowski DJ, Cordero K, Black PM, Jensen FE. Cell-specific alterations of glutamate receptor expression in tuberous sclerosis complex cortical tubers. Ann Neurol. 2008;63:454–465. doi: 10.1002/ana.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, Cardinale F, Cossu M, Ferrario A, Galli C, Bramerio M, Citterio A, Spreafico R. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–1732. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada KA, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Li W, Scheidenhelm DK, Gau CL, Tamanoi F, Gutmann DH. Loss of tuberous sclerosis complex 1 (Tsc1) expression results in increased Rheb/S6K pathway signalling important for astrocyte cell size regulation. Glia. 2004;47:180–188. doi: 10.1002/glia.20036. [DOI] [PubMed] [Google Scholar]
- Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Patel LS, Robbins CA, Emmi A, Yeung RS, Schwartkzroin PA. Morphology of cerebral lesions in the Eker rat model of tuberous sclerosis. Acta Neuropathol. 2004;108:97–108. doi: 10.1007/s00401-004-0865-8. [DOI] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Ess KE, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired astrocyte glutamate transport in a mouse epilepsy model of tuberous sclerosis complex. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740–748. doi: 10.1002/ana.410440507. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Katsetos CD, Klein-Szanto A. Subependymal astrocytic hamartomas in the Eker rat model of Tuberous Sclerosis. Am J Pathol. 1997;151:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Ouyang Y, Gazit V, Cirrito JR, Jansen LA, Ess LA, Ess KC, Yamada KA, Wozniak DF, Holtzman DM, Gutmann DH, Wong M. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2007;28:184–196. doi: 10.1016/j.nbd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Roper SN. Reduced inhibition in an animal model of cortical dysplasia. J Neurosci. 2000;20:8925–8931. doi: 10.1523/JNEUROSCI.20-23-08925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Qu M, Schleicher A, Luhmann HJ. Characterization of neuronal migration disorders in neocortical structures: quantitative receptor autoradiography of ionotrophic glutamate, GABA(A) and GABA(B) receptors. Eur J Neurosci. 1998;10:3095–3106. doi: 10.1046/j.1460-9568.1998.00322.x. [DOI] [PubMed] [Google Scholar]