Abstract
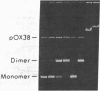
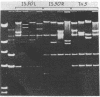
Replicative transposition is signaled by the formation of cointegrates in which donor and target replicons are joined by direct repeats of a transposable element. Elements not generating such cointegrates may move by a conservative breaking and joining process. The IS50 elements forming the terminal repeats of Tn5 [which carries the determinant for kanamycin resistance (Kanr)] contain genes and sites necessary for transposition and mediate the movement of any DNA segment they bracket. To determine if IS50 generates cointegrates, the products of transposition from pBR322::Tn5 plasmids to an F factor in recA-Escherichia coli were examined. With monomeric pBR322::Tn5 plasmids, transposition of Kanr (from Tn5) was generally not accompanied by movement of the determinant for ampicillin resistance (Ampr) (from the pBR322 vector). With dimeric pBR322::Tn5 plasmids, by contrast, half of the transpositions of kanr were accompanied by transposition of ampr. Restriction endonuclease analyses indicated that these F-Kanr Ampr chimeras contained inserts of a single copy of the pBR322 vector sequence bracketed by one Tn5 element and one IS50 element or by a pair of Tn5 elements. None of 79 chimeras tested was a true cointegrate. Because IS50 seems to move only a segment of the donor replicon it is proposed that IS50 transposition is conservative.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur A., Sherratt D. Dissection of the transposition process: a transposon-encoded site-specific recombination system. Mol Gen Genet. 1979 Oct 1;175(3):267–274. doi: 10.1007/BF00397226. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Drummond M. Absence of DNA sequences homologous to transposable element Tn5 (Kan) in the chromosome of Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):419–422. doi: 10.1128/jb.136.1.419-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Egner C., Hirschel B. J., Howard J., Johnsrud L., Jorgensen R. A., Tlsty T. D. Insertion, excision, and inversion of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):115–123. doi: 10.1101/sqb.1981.045.01.020. [DOI] [PubMed] [Google Scholar]
- Berg D. E. Genetic evidence for two types of gene arrangements in new lambdadv plasmid mutants. J Mol Biol. 1974 Jun 15;86(1):59–68. doi: 10.1016/s0022-2836(74)80007-0. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Johnsrud L., McDivitt L., Ramabhadran R., Hirschel B. J. Inverted repeats of Tn5 are transposable elements. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2632–2635. doi: 10.1073/pnas.79.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binding R., Romansky G., Bitner R., Kuempel P. Isolation and properties of Tn10 insertions in the rac locus of Escherichia coli. Mol Gen Genet. 1981;183(2):333–340. doi: 10.1007/BF00270637. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Campbell A., Berg D. E., Botstein D., Lederberg E. M., Novick R. P., Starlinger P., Szybalski W. Nomenclature of transposable elements in prokaryotes. Gene. 1979 Mar;5(3):197–206. doi: 10.1016/0378-1119(79)90078-7. [DOI] [PubMed] [Google Scholar]
- Chaconas G., Harshey R. M., Bukhari A. I. Association of Mu-containing plasmids with the Escherichia coli chromosome upon prophage induction. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1778–1782. doi: 10.1073/pnas.77.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner C., Berg D. E. Excision of transposon Tn5 is dependent on the inverted repeats but not on the transposase function of Tn5. Proc Natl Acad Sci U S A. 1981 Jan;78(1):459–463. doi: 10.1073/pnas.78.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel R. A., James A. A., Kolodner R. recA-independent general genetic recombination of plasmids. Nature. 1981 Nov 12;294(5837):184–186. doi: 10.1038/294184a0. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Chandler M. On the molecular mechanisms of transposition. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4858–4862. doi: 10.1073/pnas.78.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Chandler M. Structure and stability of Tn9-mediated cointegrates. Evidence for two pathways of transposition. J Mol Biol. 1982 Jan 15;154(2):245–272. doi: 10.1016/0022-2836(82)90063-8. [DOI] [PubMed] [Google Scholar]
- Guarente L. P., Isberg R. R., Syvanen M., Silhavy T. J. Conferral of transposable properties to a chromosomal gene in Escherichia coli. J Mol Biol. 1980 Aug 15;141(3):235–248. doi: 10.1016/0022-2836(80)90179-5. [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- Harshey R. M., Bukhari A. I. A mechanism of DNA transposition. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1090–1094. doi: 10.1073/pnas.78.2.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshey R. M., McKay R., Bukhari A. I. DNA intermediates in transposition of phage Mu. Cell. 1982 Jun;29(2):561–571. doi: 10.1016/0092-8674(82)90172-6. [DOI] [PubMed] [Google Scholar]
- Hirschel B. J., Berg D. E. A derivative of Tn5 with direct terminal repeats can transpose. J Mol Biol. 1982 Feb 25;155(2):105–120. doi: 10.1016/0022-2836(82)90439-9. [DOI] [PubMed] [Google Scholar]
- Hirschel B. J., Galas D. J., Berg D. E., Chandler M. Structure and stability of transposon 5-mediated cointegrates. J Mol Biol. 1982 Aug 25;159(4):557–580. doi: 10.1016/0022-2836(82)90101-2. [DOI] [PubMed] [Google Scholar]
- Hirschel B. J., Galas D. J., Chandler M. Cointegrate formation by Tn5, but not transposition, is dependent on recA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4530–4534. doi: 10.1073/pnas.79.15.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Aoki K., Naito A. Illegitimate recombination mediated in vitro by DNA gyrase of Escherichia coli: structure of recombinant DNA molecules. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3724–3728. doi: 10.1073/pnas.79.12.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Syvanen M. Replicon fusions promoted by the inverted repeats of Tn5. The right repeat is an insertion sequence. J Mol Biol. 1981 Jul 25;150(1):15–32. doi: 10.1016/0022-2836(81)90322-3. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Kostriken R., Morita C., Heffron F. Transposon Tn3 encodes a site-specific recombination system: identification of essential sequences, genes, and actual site of recombination. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4041–4045. doi: 10.1073/pnas.78.7.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebart J. C., Ghelardini P., Paolozzi L. Conservative integration of bacteriophage Mu DNA into pBR322 plasmid. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4362–4366. doi: 10.1073/pnas.79.14.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster C. J., Shapiro J. A. Recombination involving transposable elements: on replicon fusion. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):239–242. doi: 10.1101/sqb.1981.045.01.036. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile K. L., DasSarma S., King S. R., Jaskunas S. R. Recombination between bacteriophage lambda and plasmid pBR322 in Escherichia coli. J Bacteriol. 1980 Jun;142(3):992–1003. doi: 10.1128/jb.142.3.992-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. R. Resolution of cointegrates between transposons gamma delta and Tn3 defines the recombination site. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3428–3432. doi: 10.1073/pnas.78.6.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Berg D. E. IS50-mediated inverse transposition. Discrimination between the two ends of an IS element. J Mol Biol. 1982 Aug 5;159(2):257–271. doi: 10.1016/0022-2836(82)90495-8. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Lowe J. B., McDivitt L., Berg D. E. Control of transposon Tn5 transposition in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7450–7454. doi: 10.1073/pnas.79.23.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Uno Y., Yoshikawa M. The requirement for both DNA polymerase and 5' to 3' exonuclease activities of DNA polymerase I during Tn5 transposition. Mol Gen Genet. 1981;182(1):19–24. doi: 10.1007/BF00422761. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starlinger P. IS elements and transposons. Plasmid. 1980 May;3(3):241–259. doi: 10.1016/0147-619x(80)90039-6. [DOI] [PubMed] [Google Scholar]
- Syvanen M., Hopkins J. D., Clements M. A new class of mutants in DNA polymerase I that affects gene transposition. J Mol Biol. 1982 Jun 25;158(2):203–212. doi: 10.1016/0022-2836(82)90429-6. [DOI] [PubMed] [Google Scholar]