Abstract
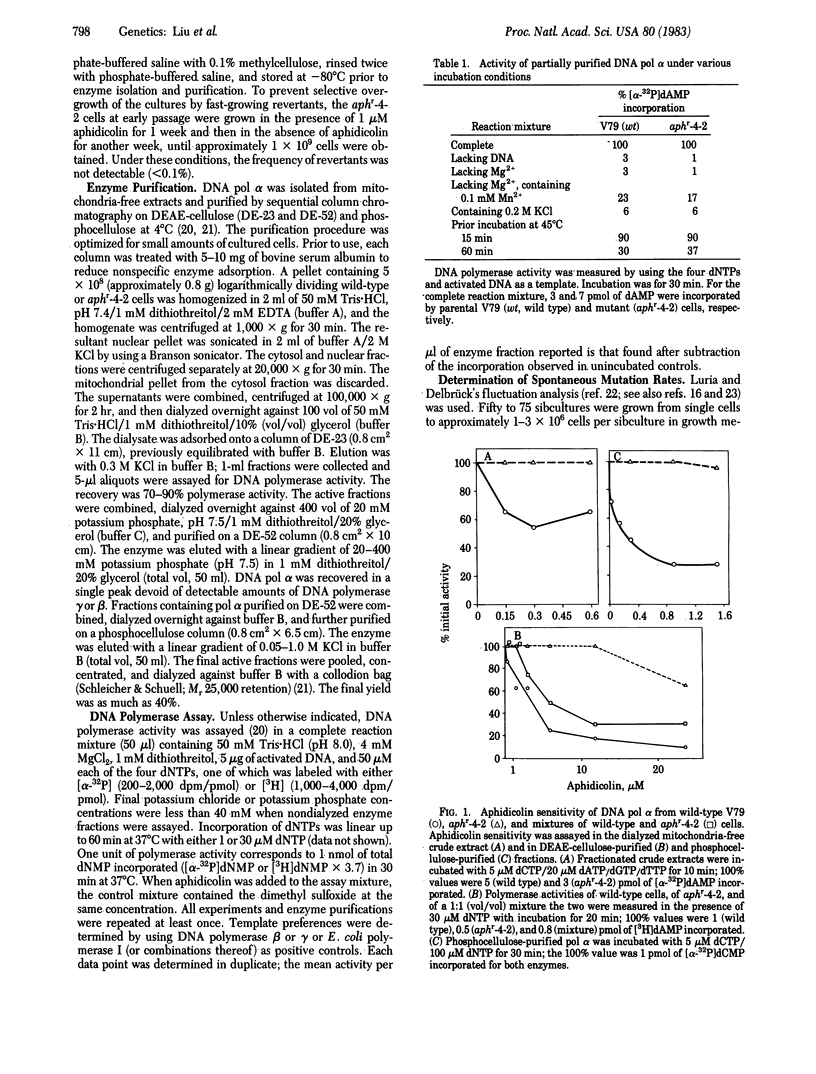
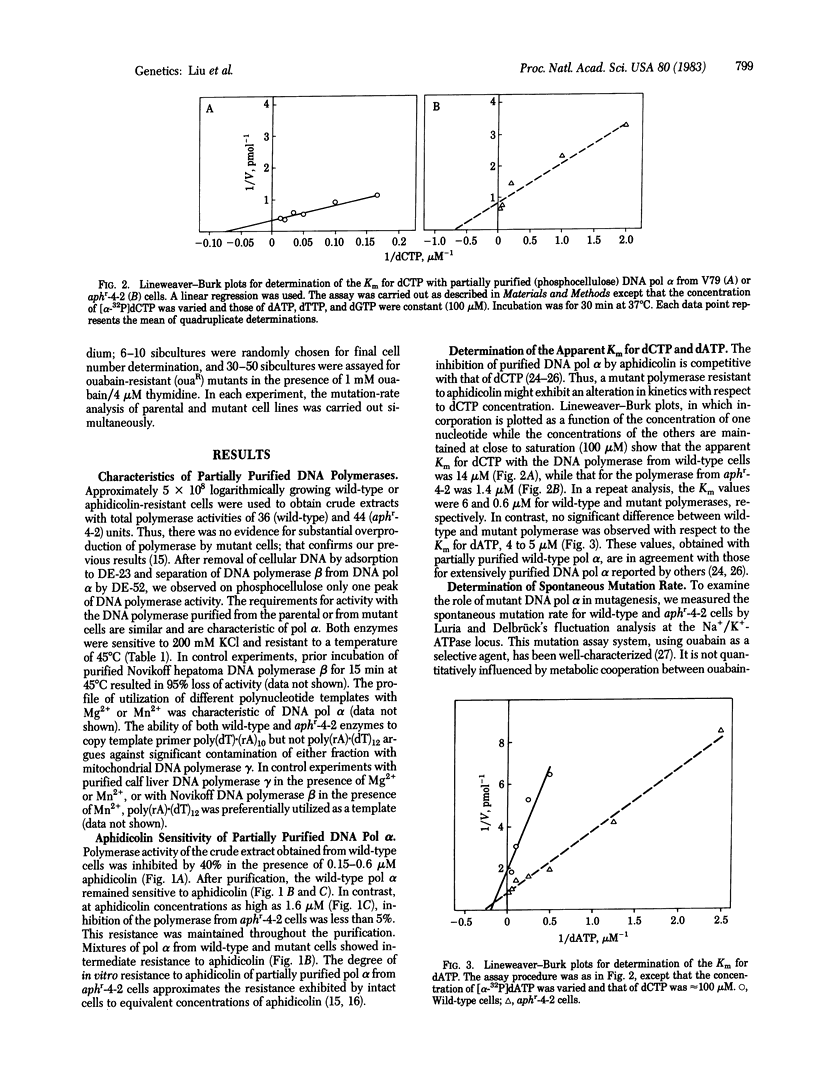
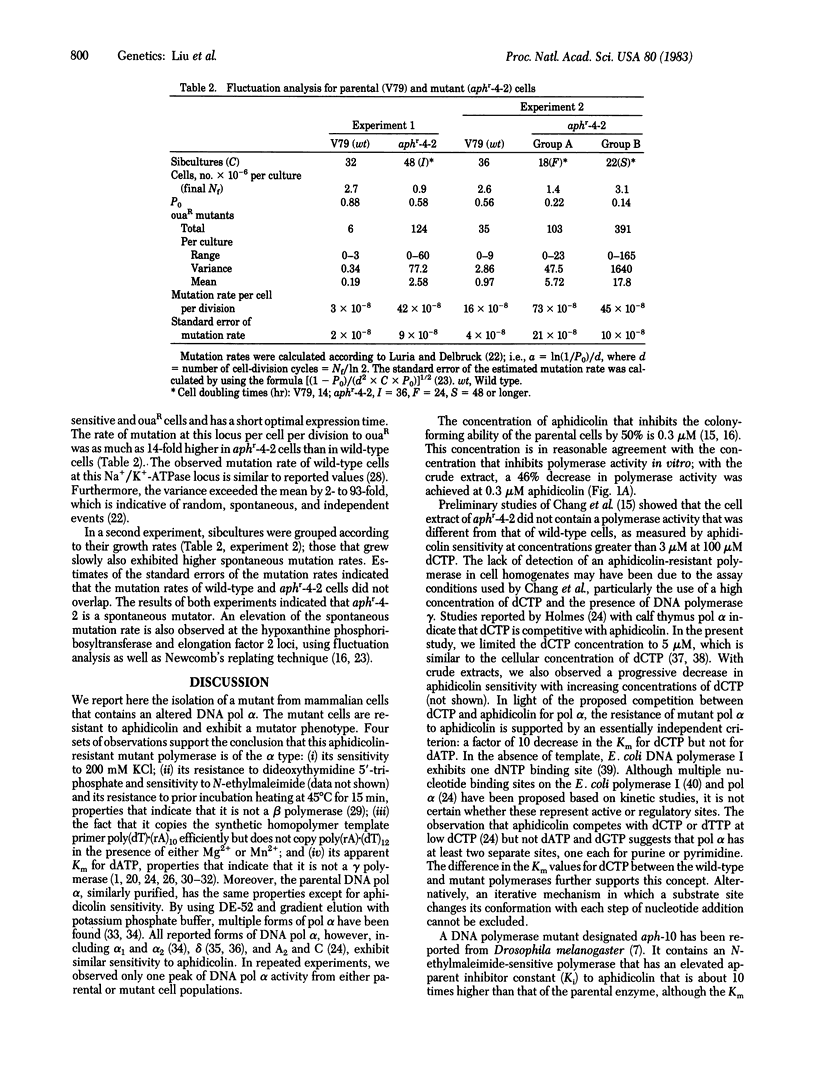
The Chinese hamster V79 cell mutant aphr-4-2, selected for its resistance to aphidicolin, a specific inhibitor of DNA polymerase alpha (DNA nucleotidyltransferase, EC 2.7.7.7), is characterized by slow growth, UV sensitivity, and hypersensitivity to UV-induced mutation. DNA polymerase alpha has been purified from mitochondria-free crude extracts of the mutant and its parental wild-type cells by sequential column chromatography on DEAE-cellulose and phosphocellulose. The major DNA polymerase activity from both cell lines was found to have characteristics of the alpha-type polymerase: sensitivity to 0.2 M KCl, resistance to heat denaturation (45 degrees C for 15 min), an apparent Km of 5 microM for dATP, and an ability to copy poly(dT)X(rA)10 but not poly(rA)X(dT)12. The crude extracts and purified DNA polymerase alpha from the mutant cells are not inhibited by aphidicolin (greater than 0.6 microM). The apparent Km for dCTP with DNA polymerase alpha is 1.0 +/- 0.4 microM (mean +/- SD) for the mutant enzyme. The polymerase from the parental cells, similarly purified, is sensitive to aphidicolin and has an apparent Km for dCTP of 10 +/- 4 microM. The spontaneous mutation rate (per cell per division), determined by fluctuation analysis at the Na+/K+-ATPase (EC 3.6.1.8) locus, is higher for mutant cells (42-73 x 10(-8)) than for parental cells (3-16 x 10(-8)). These data suggest a mechanism for aphidicolin resistance of the mutant--i.e., a decrease in the Km for dCTP. The results also indicate that an altered DNA polymerase alpha may be intrinsically mutagenic during normal semiconservative replicative as well as during UV-induced repair syntheses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger N. A., Kurohara K. K., Petzold S. J., Sikorski G. W. Aphidicolin inhibits eukaryotic DNA replication and repair --- implications for involvement of DNA polymerase alpha in both processes. Biochem Biophys Res Commun. 1979 Jul 12;89(1):218–225. doi: 10.1016/0006-291x(79)90966-5. [DOI] [PubMed] [Google Scholar]
- Bray G., Brent T. P. Deoxyribonucleoside 5'-triphosphate pool fluctuations during the mammalian cell cycle. Biochim Biophys Acta. 1972 May 10;269(2):184–191. doi: 10.1016/0005-2787(72)90425-x. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Boezi J. A., Warren S. T., Sabourin C. L., Liu P. K., Glatzer L., Trosko J. E. Isolation and characterization of a UV-sensitive hypermutable aphidicolin-resistant Chinese hamster cell line. Somatic Cell Genet. 1981 Mar;7(2):235–253. doi: 10.1007/BF01567660. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Trosko J. E., Akera T. Characterization of ultraviolet light-induced ouabain-resistant mutations in Chinese hamster cells. Mutat Res. 1978 Jul;51(1):85–98. doi: 10.1016/0027-5107(78)90011-8. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi G., Jose J. G., Linn S. Further characterization of a cell-free system for measuring replicative and repair DNA synthesis with cultured human fibroblasts and evidence for the involvement of DNA polymerase alpha in DNA repair. Nucleic Acids Res. 1979 Nov 10;7(5):1205–1219. doi: 10.1093/nar/7.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Drake J. W. The genetic control of spontaneous and induced mutation rates in bacteriophage T4. Genetics. 1973 Apr;73(Suppl):45–64. [PubMed] [Google Scholar]
- Dresler S. L., Roberts J. D., Lieberman M. W. Characterization of deoxyribonucleic acid repair synthesis in permeable human fibroblasts. Biochemistry. 1982 May 11;21(10):2557–2564. doi: 10.1021/bi00539a040. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Kunkel T. A., Seal G., Loeb L. A. Distinctive properties of mammalian DNA polymerases. Biochim Biophys Acta. 1979 Feb 27;561(2):369–382. doi: 10.1016/0005-2787(79)90145-x. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Travaglini E. C., Loeb L. A. Isolation and characterization of DNA polymerases from eukaryotic cells. Methods Cell Biol. 1978;19:27–42. doi: 10.1016/s0091-679x(08)60007-2. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- Fansler B., Loeb L. A. Sea urchin nuclear DNA polymerase. IV. Reversible association of DNA polymerase with nuclei during the cell cycle. Exp Cell Res. 1972 Dec;75(2):433–441. doi: 10.1016/0014-4827(72)90450-8. [DOI] [PubMed] [Google Scholar]
- Fridlender B., Fry M., Bolden A., Weissbach A. A new synthetic RNA-dependent DNA polymerase from human tissue culture cells (HeLa-fibroblast-synthetic oligonucleotides-template-purified enzymes). Proc Natl Acad Sci U S A. 1972 Feb;69(2):452–455. doi: 10.1073/pnas.69.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulotto E., Mondello C. Aphidicolin does not inhibit the repair synthesis of mitotic chromosomes. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1287–1294. doi: 10.1016/0006-291x(81)90759-2. [DOI] [PubMed] [Google Scholar]
- Goscin L. P., Byrnes J. J. DNA polymerase delta: one polypeptide, two activities. Biochemistry. 1982 May 11;21(10):2513–2518. doi: 10.1021/bi00539a034. [DOI] [PubMed] [Google Scholar]
- Holmer A. M., Hesslewood I. P., Johnston I. R. The occurrence of multiple activities in the high-molecular-weight DNA polymerase fraction of mammalian tissues. A preliminary study of some of their properties. Eur J Biochem. 1974 Apr 16;43(3):487–499. doi: 10.1111/j.1432-1033.1974.tb03436.x. [DOI] [PubMed] [Google Scholar]
- Holmes A. M. Studies on the inhibition of highly purified calf thymus 8S and 7.3S DNA polymerase alpha by aphidicolin. Nucleic Acids Res. 1981 Jan 10;9(1):161–168. doi: 10.1093/nar/9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Fidelity of fractionated deoxyribonucleic acid polymerases from human placenta. Biochemistry. 1980 Jan 8;19(1):220–228. doi: 10.1021/bi00542a033. [DOI] [PubMed] [Google Scholar]
- Krokan H., Wist E., Krokan R. H. Aphidicolin inhibits DNA synthesis by DNA polymerase alpha and isolated nuclei by a similar mechanism. Nucleic Acids Res. 1981 Sep 25;9(18):4709–4719. doi: 10.1093/nar/9.18.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Eckstein F., Mildvan A. S., Koplitz R. M., Loeb L. A. Deoxynucleoside [1-thio]triphosphates prevent proofreading during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6734–6738. doi: 10.1073/pnas.78.11.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., McGOVERN A. P., MARGESON K. B. Changes in the distribution of polymerase activity during DNA synthesis in mouse fibroblasts. Proc Natl Acad Sci U S A. 1963 Jan 15;49:102–107. doi: 10.1073/pnas.49.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Structural and functional properties of calf thymus DNA polymerase delta. Prog Nucleic Acid Res Mol Biol. 1981;26:83–96. doi: 10.1016/s0079-6603(08)60396-7. [DOI] [PubMed] [Google Scholar]
- Lewis B. J., Abrell J. W., Smith R. G., Gallo R. C. Human DNA polymerase 3 (R-DNA polymerase): distinction from DNA polymerase I and reverse transcriptase. Science. 1974 Mar 1;183(4127):867–869. doi: 10.1126/science.183.4127.867. [DOI] [PubMed] [Google Scholar]
- Liu P. K., Chang C. C., Trosko J. E. Association of mutator activity with UV sensitivity in an aphidicolin-resistant mutant of Chinese hamster V79 cells. Mutat Res. 1982 Dec;106(2):317–332. doi: 10.1016/0027-5107(82)90113-0. [DOI] [PubMed] [Google Scholar]
- Liu P. K., Trosko J. E., Chang C. C. Hypermutability of a UV-sensitive aphidicolin-resistant mutant of Chinese hamster fibroblasts. Mutat Res. 1982 Dec;106(2):333–345. doi: 10.1016/0027-5107(82)90114-2. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Agarwal S. S., Woodside A. M. Induction of DNA polymerase in human lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Nov;61(3):827–834. doi: 10.1073/pnas.61.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A. Purification and properties of deoxyribonucleic acid polymerase from nuclei of sea urchin embryos. J Biol Chem. 1969 Apr 10;244(7):1672–1681. [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R., Chinault D. N. Evidence that DNA polymerases alpha and beta participate differentially in DNA repair synthesis induced by different agents. J Biol Chem. 1982 Jan 10;257(1):46–49. [PubMed] [Google Scholar]
- Oguro M., Suzuki-Hori C., Nagano H., Mano Y., Ikegami S. The mode of inhibitory action by aphidicolin on eukaryotic DNA polymerase alpha. Eur J Biochem. 1979 Jul;97(2):603–607. doi: 10.1111/j.1432-1033.1979.tb13149.x. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Aphidicolin allows a rapid and simple evaluation of DNA-repair synthesis in damaged human cells. Mutat Res. 1980 May;70(3):389–394. doi: 10.1016/0027-5107(80)90029-9. [DOI] [PubMed] [Google Scholar]
- RADDING C. M., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XIII. Kinetics of primed and de novo synthesis of deoxynucleotide polymers. J Biol Chem. 1962 Sep;237:2877–2882. [PubMed] [Google Scholar]
- Robert-Guroff M., Schrecker A. W., Brinkman B. J., Gallo R. C. DNA polymerase gamma of human lymphoblasts. Biochemistry. 1977 Jun 28;16(13):2866–2873. doi: 10.1021/bi00632a010. [DOI] [PubMed] [Google Scholar]
- Seal G., Shearman C. W., Loeb L. A. On the fidelity of DNA replication. Studies with human placenta DNA polymerases. J Biol Chem. 1979 Jun 25;254(12):5229–5237. [PubMed] [Google Scholar]
- Seki S., Ohashi M., Ogura H., Oda T. Possible involvement of DNA polymerases alpha and beta in bleomycin-induced unscheduled DNA synthesis in permeable HeLa cells. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1502–1508. doi: 10.1016/0006-291x(82)91421-8. [DOI] [PubMed] [Google Scholar]
- Spadari S., Weissbach A. The interrelation between DNA synthesis and various DNA polymerase activities in synchronized HeLa cells. J Mol Biol. 1974 Jun 15;86(1):11–20. doi: 10.1016/s0022-2836(74)80003-3. [DOI] [PubMed] [Google Scholar]
- Sugino A., Nakayama K. DNA polymerase alpha mutants from a Drosophila melanogaster cell line. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7049–7053. doi: 10.1073/pnas.77.12.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglini E. C., Mildvan A. S., Loeb L. A. Kinetic analysis of Escherichia coli deoxyribonucleic acid polymerase I. J Biol Chem. 1975 Nov 25;250(22):8647–8656. [PubMed] [Google Scholar]
- Walters R. A., Tobey R. A., Ratliff R. L. Cell-cycle-dependent variations of deoxyribonucleoside triphosphate pools in Chinese hamster cells. Biochim Biophys Acta. 1973 Sep 7;319(3):336–347. doi: 10.1016/0005-2787(73)90173-1. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Baltimore D., Bollum F., Gallo R., Korn D. Nomenclature of eukaryotic DNA polymerases. Eur J Biochem. 1975 Nov 1;59(1):1–2. doi: 10.1111/j.1432-1033.1975.tb02416.x. [DOI] [PubMed] [Google Scholar]


