Abstract
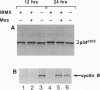
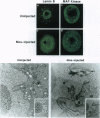
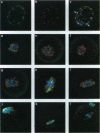
Mitogen-activated protein kinase (MAPK) is selectively activated by injecting either mos or MAPK kinase (mek) RNA into immature mouse oocytes maintained in the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX). IBMX arrests oocyte maturation, but Mos (or MEK) overexpression overrides this block. Under these conditions, meiosis I is significantly prolonged, and MAPK becomes fully activated in the absence of p34cdc2 kinase or maturation-promoting factor. In these oocytes, large openings form in the germinal vesicle adjacent to condensing chromatin, and microtubule arrays, which stain for both MAPK and centrosomal proteins, nucleate from these regions. Maturation-promoting factor activation occurs later, concomitant with germinal vesicle breakdown, the contraction of the microtubule arrays into a precursor of the spindle, and the redistribution of the centrosomal proteins into the newly forming spindle poles. These studies define important new functions for the Mos/MAPK cascade in mouse oocyte maturation and, under these conditions, reveal novel detail of the early stages of oocyte meiosis I.
Full text
PDF


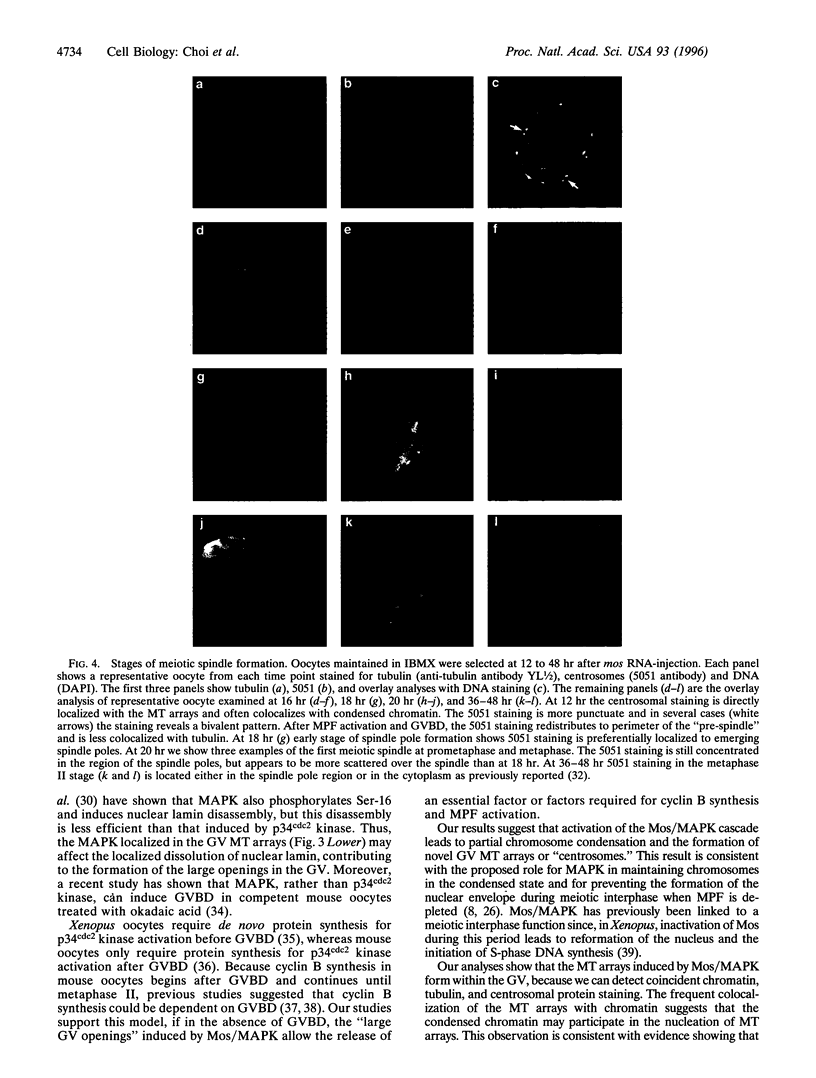

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calarco-Gillam P. D., Siebert M. C., Hubble R., Mitchison T., Kirschner M. Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell. 1983 Dec;35(3 Pt 2):621–629. doi: 10.1016/0092-8674(83)90094-6. [DOI] [PubMed] [Google Scholar]
- Chesnel F., Eppig J. J. Induction of precocious germinal vesicle breakdown (GVB) by GVB-incompetent mouse oocytes: possible role of mitogen-activated protein kinases rather than p34cdc2 kinase. Biol Reprod. 1995 Apr;52(4):895–902. doi: 10.1095/biolreprod52.4.895. [DOI] [PubMed] [Google Scholar]
- Choi T., Aoki F., Mori M., Yamashita M., Nagahama Y., Kohmoto K. Activation of p34cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development. 1991 Nov;113(3):789–795. doi: 10.1242/dev.113.3.789. [DOI] [PubMed] [Google Scholar]
- Colledge W. H., Carlton M. B., Udy G. B., Evans M. J. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994 Jul 7;370(6484):65–68. doi: 10.1038/370065a0. [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P., Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994 Jun 17;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Dessev G., Iovcheva-Dessev C., Bischoff J. R., Beach D., Goldman R. A complex containing p34cdc2 and cyclin B phosphorylates the nuclear lamin and disassembles nuclei of clam oocytes in vitro. J Cell Biol. 1991 Feb;112(4):523–533. doi: 10.1083/jcb.112.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S. J., Stein P., Evans L., Calarco P. D., Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994 Feb 25;76(4):639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K., Murakami M. S., Blair D. G., Kuriyama R., Hunt T., Fischinger P., Vande Woude G. F. Similarities between somatic cells overexpressing the mos oncogene and oocytes during meiotic interphase. Cell Growth Differ. 1994 Oct;5(10):1093–1103. [PubMed] [Google Scholar]
- Furuno N., Nishizawa M., Okazaki K., Tanaka H., Iwashita J., Nakajo N., Ogawa Y., Sagata N. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994 May 15;13(10):2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Moriyama K., Matsuda S., Okumura E., Kishimoto T., Kawasaki H., Suzuki K., Yahara I., Sakai H., Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991 Sep;10(9):2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Haccard O., Jessus C., Cayla X., Goris J., Merlevede W., Ozon R. In vivo activation of a microtubule-associated protein kinase during meiotic maturation of the Xenopus oocyte. Eur J Biochem. 1990 Sep 24;192(3):633–642. doi: 10.1111/j.1432-1033.1990.tb19270.x. [DOI] [PubMed] [Google Scholar]
- Haccard O., Sarcevic B., Lewellyn A., Hartley R., Roy L., Izumi T., Erikson E., Maller J. L. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993 Nov 19;262(5137):1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Hampl A., Eppig J. J. Translational regulation of the gradual increase in histone H1 kinase activity in maturing mouse oocytes. Mol Reprod Dev. 1995 Jan;40(1):9–15. doi: 10.1002/mrd.1080400103. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol. 1988 Apr;126(2):242–252. doi: 10.1016/0012-1606(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Watanabe N., Furuta Y., Tamemoto H., Sagata N., Yokoyama M., Okazaki K., Nagayoshi M., Takeda N., Ikawa Y. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994 Jul 7;370(6484):68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Theurkauf W. E. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 1993 Sep;9(9):310–317. doi: 10.1016/0168-9525(93)90249-h. [DOI] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kosako H., Gotoh Y., Matsuda S., Ishikawa M., Nishida E. Xenopus MAP kinase activator is a serine/threonine/tyrosine kinase activated by threonine phosphorylation. EMBO J. 1992 Aug;11(8):2903–2908. doi: 10.1002/j.1460-2075.1992.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., Eppenberger H. M., Fakan S., Nigg E. A. Nuclear substructure antigens. Monoclonal antibodies against components of nuclear matrix preparations. Exp Cell Res. 1986 Jan;162(1):205–219. doi: 10.1016/0014-4827(86)90439-8. [DOI] [PubMed] [Google Scholar]
- Mansour S. J., Matten W. T., Hermann A. S., Candia J. M., Rong S., Fukasawa K., Vande Woude G. F., Ahn N. G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994 Aug 12;265(5174):966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Maro B., Johnson M. H., Webb M., Flach G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J Embryol Exp Morphol. 1986 Mar;92:11–32. [PubMed] [Google Scholar]
- Matten W., Daar I., Vande Woude G. F. Protein kinase A acts at multiple points to inhibit Xenopus oocyte maturation. Mol Cell Biol. 1994 Jul;14(7):4419–4426. doi: 10.1128/mcb.14.7.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger S. M., Albertini D. F. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci. 1991 Oct;100(Pt 2):289–298. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
- Minshull J., Sun H., Tonks N. K., Murray A. W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994 Nov 4;79(3):475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Nebreda A. R., Hunt T. The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993 May;12(5):1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. MAP kinases: charting the regulatory pathways. Science. 1992 Sep 4;257(5075):1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Peter M., Sanghera J. S., Pelech S. L., Nigg E. A. Mitogen-activated protein kinases phosphorylate nuclear lamins and display sequence specificity overlapping that of mitotic protein kinase p34cdc2. Eur J Biochem. 1992 Apr 1;205(1):287–294. doi: 10.1111/j.1432-1033.1992.tb16779.x. [DOI] [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Posada J., Yew N., Ahn N. G., Vande Woude G. F., Cooper J. A. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993 Apr;13(4):2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Montgomery R. R., Belanoff J. R. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983 Jun;97(2):264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Shibuya E. K., Boulton T. G., Cobb M. H., Ruderman J. V. Activation of p42 MAP kinase and the release of oocytes from cell cycle arrest. EMBO J. 1992 Nov;11(11):3963–3975. doi: 10.1002/j.1460-2075.1992.tb05490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Hawley R. S. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J Cell Biol. 1992 Mar;116(5):1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarfaty I., Resau J. H., Rulong S., Keydar I., Faletto D. L., Vande Woude G. F. The met proto-oncogene receptor and lumen formation. Science. 1992 Aug 28;257(5074):1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- Verlhac M. H., Kubiak J. Z., Clarke H. J., Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994 Apr;120(4):1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Verlhac M. H., de Pennart H., Maro B., Cobb M. H., Clarke H. J. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol. 1993 Aug;158(2):330–340. doi: 10.1006/dbio.1993.1192. [DOI] [PubMed] [Google Scholar]
- Yew N., Mellini M. L., Vande Woude G. F. Meiotic initiation by the mos protein in Xenopus. Nature. 1992 Feb 13;355(6361):649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]
- Yew N., Oskarsson M., Daar I., Blair D. G., Vande Woude G. F. mos gene transforming efficiencies correlate with oocyte maturation and cytostatic factor activities. Mol Cell Biol. 1991 Feb;11(2):604–610. doi: 10.1128/mcb.11.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]