Abstract
AbstractThe ubiquitin proteasome system is required for the rapid and precise control of protein abundance that is essential for synaptic function. USP14 is a proteasome-bound deubiquitinating enzyme that recycles ubiquitin and regulates synaptic short-term synaptic plasticity. We previously reported that loss of USP14 in axJ mice causes a deficit in paired pulse facilitation (PPF) at hippocampal synapses. Here we report that USP14 regulates synaptic function through a novel, deubiquitination-independent mechanism. Although PPF is usually inversely related to release probability, USP14 deficiency impairs PPF without altering basal release probability. Instead, the loss of USP14 causes a large reduction in the number of synaptic vesicles. Over-expression of a catalytically inactive form of USP14 rescues the PPF deficit and restores synaptic vesicle number, indicating that USP14 regulates presynaptic structure and function independently of its role in deubiquitination. Finally, the PPF deficit caused by loss of USP14 can be rescued by pharmacological inhibition of proteasome activity, suggesting that inappropriate protein degradation underlies the PPF impairment. Overall, we demonstrate a novel, deubiquitination-independent function for USP14 in influencing synaptic architecture and plasticity.
Introduction
Regulated protein degradation by the ubiquitin proteasome system controls many specialized cellular processes in neurons such as synaptic remodelling (Yi & Ehlers, 2007), as well as supporting a multitude of general cellular functions (Glickman & Ciechanover, 2002). The ubiquitin proteasome system is important for regulation of synaptic protein abundance (Ehlers, 2003; DiAntonio & Hicke, 2004), presynaptic structure (Willeumier et al. 2006; Yao et al. 2007), short-term plasticity (Wilson et al. 2002) and learning and memory (Bingol & Sheng, 2011; Fioravante & Byrne, 2011; Kaang & Choi, 2012). Furthermore, ubiquitin proteasome system dysfunction has been implicated in neurodegenerative diseases (Paul, 2008; Singhal et al. 2008). However, little is known about the regulatory mechanisms that modulate protein turnover by the proteasome. Because of the importance of the proteasome to synaptic function, it is essential to understand how the regulatory proteins that bind the proteasome control protein degradation.
Ubiquitin-specific protease 14 (USP14) is a deubiquitinating enzyme that binds to the proteasome (Hu et al. 2005) and removes ubiquitin from substrates prior to their destruction (Lee et al. 2010), limiting proteasomal degradation of ubiquitin (Anderson et al. 2005). The ataxia (axJ) mice contain a spontaneously occurring mutation in Usp14 that causes loss of 95% of the USP14 protein (Wilson et al. 2002). Loss of USP14 results in synaptic deficits in hippocampus, including reduced paired pulse facilitation (PPF) at Schaffer collateral synapses (Wilson et al. 2002), but no change in long-term potentiation (Wilson et al. 2002; Walters et al. 2008) or long-term depression (Walters et al. 2008). These changes in short-term plasticity without changes in long-term plasticity suggest that the effects of loss of USP14 in hippocampal synapses are specific to presynaptic function.
Because the amount of PPF is normally inversely related to the initial release probability of synapses (Dobrunz & Stevens, 1997; Regehr & Stevens, 2001), changes in PPF are almost always caused by changes in the initial release probability (Zucker & Regehr, 2002). However, the mechanism underlying the PPF deficit in axJ mice is not yet known. While recent work from our laboratory has demonstrated that the ubiquitin recycling activity of USP14 is essential for the development and function of neuromuscular junction synapses (Chen et al. 2009, 2011), the function of USP14 in regulating synaptic transmission at central system synapses is unknown. It is therefore critical to understand whether loss of USP14's ubiquitin recycling activity underlies the changes in presynaptic plasticity seen in axJ mice.
Here we use a comprehensive approach that includes electrophysiology, mathematical modelling, structural analysis, protein biochemistry, pharmacology and genetics to investigate the mechanisms by which USP14 regulates synaptic function and plasticity in hippocampus. Surprisingly, we discovered that the PPF deficit is not caused by a change in basal release probability, but instead occurs through a novel mechanism involving changes to synaptic architecture. Furthermore, transgenic expression of ubiquitin did not rescue the short-term plasticity deficit in hippocampus, unlike at the neuromuscular junction (Chen et al. 2009), indicating that USP14 has additional roles at central synapses independent of ubiquitin recycling. Our results instead reveal a novel role for a ubiquitin hydrolase-independent activity of USP14 to control presynaptic synaptic structure and plasticity at hippocampal synapses. Finally, our results suggest that inappropriate protein degradation underlies the deficit in PPF, as proteasome inhibition is also able to restore PPF at axJ synapses. This study demonstrates an unanticipated new function for proteasomal deubiquitinating enzymes, and represents a major step forward in the burgeoning field of proteasomal regulation of synaptic function, plasticity, learning and memory.
Methods
Ethical approval
Ethical approval was obtained for all experimental protocols from the University of Alabama at Birmingham Institutional Animal Care and Use Committee. All research complied with the United States Animal Welfare Act and other federal statutes and regulations relating to experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, United States National Research Council.
Animals
Wild-type C57BL/6J and Usp14axJ mice (The Jackson Laboratory, Bar Harbor, ME, USA) have been maintained in our breeding colony at the University of Alabama at Birmingham, which is accredited fully by the Association for Assessment and Accreditation of Laboratory Animal Care International. Homozygous USP14axJ mice (which we refer to as axJ mice) were generated by intercrossing axJ/+ mice and could be identified phenotypically by 3 weeks of age. Transgenic animals expressing USP14 (TgUsp14) and ubiquitin (TgUb) under the Thy1.2 promoter have been described previously (Crimmins et al. 2006; Chen et al. 2011). Transgenic mice expressing catalytically inactive USP14 (TgUsp14CA) under the Thy1.2 promoter were generated by pronuclear injection of the Usp14CA transgene (see below) into C57BL/6J fertilized eggs.
Construction of the Usp14CA transgene
The full-length Usp14 cDNA was used as a template for generating Usp14CA cDNA. PCR-mediated site-directed mutagenesis was performed to produce a cDNA with a cysteine to alanine change at amino acid position 114 in the Usp14 cDNA. This amino acid change blocks the ubiquitin hydrolase activity of USP14. The Usp14CA cDNA was then cloned into the XhoI site of the Thy1.2 expression cassette (gift from Dr Pico Caroni at the Friedrich Institute, Basel, Switzerland). The transgene was excised from the vector by using EcoRI and NdeI and prepared for microinjection via standard procedures.
Slice preparation
Male mice aged 4–6 weeks were anaesthetized with isoflurane, decapitated, and their brains removed rapidly. Coronal slices of the brain, 400 μm thick, were cut using a vibrating microtome (VT1000S; Leica, Bannockburn, IL, USA) as previously described (Speed & Dobrunz, 2009). Slices were cut in ice-cold (1–3°C) dissecting solution containing the following (in mm): 120 NaCl, 3.5 KCl, 0.7 CaCl2, 4.0 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose, bubbled with 95% O2–5% CO2, pH 7.35–7.45. The CA3 region of the hippocampus was surgically removed from all slices to prevent recurrent activity. Slices were incubated at room temperature in a holding chamber containing the dissecting solution and bubbled with 95% O2–5% CO2 for >1 h before recording.
Electrophysiology
Slices were held in a submersion recording chamber perfused with external recording solution (ERS) composed of (in mm): 120 NaCl, 3.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. The solution was bubbled with 95% O2–5% CO2, pH 7.35–7.45. All experiments were performed at ∼25°C. Stimulation was provided by a Grass S48 stimulator (Grass Tech, West Warwick, RI, USA) and applied with a BSI–2 biphasic stimulus isolator (BAK Electronics, Mount Airy, MD, USA). Bipolar tungsten electrodes (FHC Bowdoin, ME, USA) were used to stimulate and 2–4 MΩ electrodes pulled from borosilicate glass were used to record.
Whole cell recording
CA1 pyramidal neurons were patched ‘blindly’ in the voltage-clamp configuration and recorded at a holding potential of −60 mV or −40 mV, as indicated, using an Axopatch 200B amplifier (Molecular Devices, Union City, CA, USA). Patch electrodes (2–4 MΩ) were filled with internal recording solution composed of (in mm): 100 caesium gluconate, 0.6 EGTA, 5.0 MgCl2, 10 Hepes, 10 ATP, 0.3 GTP, and 5 N-ethyllidocaine chloride (QX-314), to block voltage dependent Na+ channels; pH was adjusted to 7.2 with CsOH. The access resistance and holding current (<200 pA) were monitored continuously. Recordings were rejected if either access resistance or holding current changed >20% during the experiment.
Measurement of release probability
Initial release probability was assayed using the rate of block of the NMDA EPSCs by the open channel blocker MK-801 (40 μm) during stimulation at 0.1 Hz (Hessler et al. 1993; Sun et al. 2005). NMDA EPSCs were pharmacologically isolated via blocking AMPA/kainate receptors with 10 μm NBQX, blocking GABAA-mediated inhibition with 100 μm picrotoxin, and blocking GABAB-mediated inhibition with 10 μm CGP55845, and recorded at a holding potential of −40 mV in 2.5 mm extracellular calcium and 1.3 mm extracellular magnesium. A stable baseline was obtained at 0.1 Hz prior to the addition of 40 μm MK-801. After a 10 min wash-in period with no stimulus, stimulation was resumed and 60–120 EPSCs were recorded. The release probability of facilitated pulses was assayed in a similar manner, except that 20 μm MK-801 was used to block the NMDA EPSCs and the rate of block was measured in response to 100 Hz 3-pulse trains with a 30 s inter-train interval. The NMDA receptor EPSC amplitude was normalized to the initial response, and a single exponential was fitted to the resulting decay curve. The decay constant (τ) was obtained for each experiment.
Field potential recordings
Dendritic field excitatory postsynaptic potentials (fEPSPs) were recorded from stratum radiatum of CA1 using glass micropipettes filled with ERS in response to stimulation of Schaffer collateral axons or in the distal region of dentate stratum moleculare in response to stimulation of lateral perforant path axons. The initial slope of the fEPSP was used as a measure of synaptic response. Stimulation was applied as pairs of pulses (interval 50–500 ms) at 0.1 Hz. Where stated, the following drugs were added to the ERS during field potential recordings: 50 μm d-2-amino-5-phosphonovaleric acid (APV) to block NMDA receptors, 1 mm cyclothiazide to inhibit AMPA receptor desensitization, 100 μm picrotoxin to block GABAA-mediated inhibition, 10 μm CGP55845 to block GABAB-mediated inhibition.
Mathematical modelling
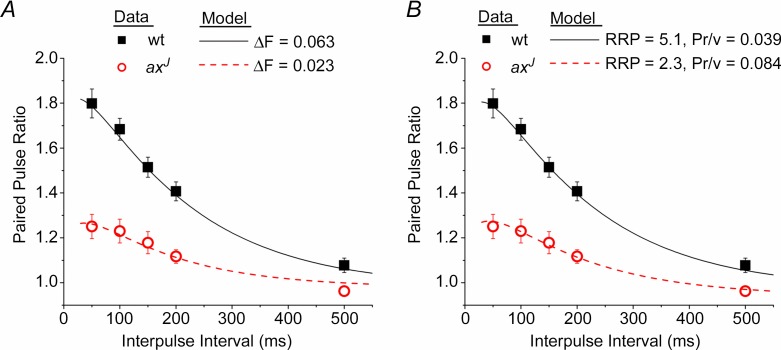
We used a simple mathematical model of vesicle release probability and short-term plasticity that has been previously published (Sun et al. 2005). The model parameter representing the initial (basal) readily releasable pool size, which is referred to here as RRP, was previously called nT (Sun et al. 2005, 2009; Sun & Dobrunz, 2006). The model parameter representing the initial (basal) release probability per vesicle, which here is referred to as Pr/v, was previously called α1 (Sun et al. 2005, 2009; Sun & Dobrunz, 2006). The model was fitted to wild-type (wt) data for PPF vs. interpulse interval (Fig. 2A and B). In Fig. 2A, the model was fitted to the axJ data with only a change in the amplitude of facilitation (ΔF), which caused no change in the initial release probability. In Fig. 2B, the model was fitted to axJ data with a change in RRP, and with the initial release probability held constant, which caused a change in Pr/v. The values of model parameters that changed are given in Fig. 2. Model parameters that are the same for wt and axJ are shown in Table 1.
Figure 2.
A, a decrease in the magnitude of facilitation (ΔF) causes a decrease in paired pulse facilitation with no change in the initial release probability. B, a decrease in the number of readily releasable vesicles (RRP) and increase in the release probability per vesicle (Pr/v) also causes a decrease in paired pulse facilitation with no change in the initial release probability. Model is from Sun et al. (2005); parameter values held constant are given in Table 1. In A, RRP = 5.1, Pr/v = 0.039. In B, ΔF = 0.063.
Table 1.
Model parameters that are held constant in the model simulations of data from wt and axJ mice
| Symbol | Definition | Value | Unit |
|---|---|---|---|
| τin | Time constant for entry into refractory state | 3 | ms |
| k0 | Baseline recovery rate from the refractory state | 2 | s−1 |
| kmax | Maximum recovery rate from the refractory state | 30 | s−1 |
| ΔD | Incremental increase in CaXD after a stimulus | 4.7 | (normalized) |
| KD | Dissociation constant of CaXD | 1 | N/A |
| τD | Decay time constant of CaXD after a stimulus | 48.8 | ms |
| KF | Dissociation constant of CaXF | 1 | N/A |
| τF | Decay constant of CaXF | 181.8 | ms |
| R | Refilling rate of readily releasable vesicle pool | 0.1 | s−1 |
CaXD, calcium-bound molecule governing the recovery from inactivation; CaXF, calcium-bound molecule governing facilitation.
Transmission electron microscopy
Hippocampal sections were prepared for electron microscopy using standard methods (Chapleau et al. 2012). Mice were deeply anaesthetized with ketamine (100 mg kg−1) and xylazine (10 mg kg−1) and transcardially perfused with PBS followed by Karnovsky's fixative (2.5% gluteraldehyde, 2% paraformaldehyde, 50% Sorenson's phosphate buffer, pH 7.4). After 1 h of fixation brains were sliced on a vibrating microtome (VTS 1000, Vibratome, Bannockburn, IL, USA) and hippocampal sections containing Schaffer collateral and/or lateral perforant path synapses were isolated. The slices were washed twice for 10 min each in 0.1 m NaPO4, post-fixed in 1% osmium tetroxide (in 0.2 m sodium cacodylate buffer, pH 7.4) for 1 h in the dark, washed 4 times for 15 min each in maleate buffer (pH 5.2), and stained in 2% uranyl acetate (in maleate buffer, pH 6.0) for 1 h in the dark. Slices were then washed 4 times for 15 min each in maleate buffer (pH 5.2), dehydrated in a graded ethanol series followed by propylene oxide, and finally incubated in propylene oxide:resin in a 1:1 ratio (Embed 812, Electron Microscopy Sciences, Hatfield, PA, USA) overnight. Slices were moved to 100% Embed 812, washed for 4 h with shaking, embedded in fresh resin, and heated to 60°C for 72 h.
Schaffer collateral or lateral perforant path regions were identified in semi-thin (0.5 μm) sections, and ultra-thin sections (70 nm) were photographed at ×21,000 using an FEI Tecnai F20 FEG transmission electron microscope operated at 80 kV (FEI, Hillsboro, OR, USA). Asymmetric synapses onto dendritic spines were identified by electron-dense postsynaptic densities, and total vesicles, docked vesicles, active zone length, and bouton area were quantified using ImageJ software (National Institutes of Health) and standard methods (Dickinson-Nelson & Reese, 1983).
Immunoblotting
Hippocampal–entorhinal cortex extracts were prepared as previously described (Crimmins et al. 2006). Proteins were resolved on either 8% Tris-glycine gels or 4–20% Tris-glycine NUPAGE gels (Invitrogen, Grand Island, NY) and transferred onto polyvinylidene difluoride (PVDF) membranes. The polyclonal USP14 R138 antisera (Anderson et al. 2005), anti-ubiquitin (Mab1510; Millipore, Temecula, CA) and anti-actin antibody (Developmental Studies Hybridoma Bank) were diluted in a mixture of Tris-buffered saline and Tween 20 (TBST) containing 3% bovine serum albumin. Primary antibodies were detected using an anti-mouse or anti-rabbit HRP-conjugated secondary antibody (Southern Biotechnology Associates, Birmingham, AL) and luminol reagents (Pierce, Rockford, IL).
Statistical analysis
Data are presented as mean ± SEM. Statistical comparisons were made using Student's t test, with P < 0.05 considered significant.
Results
axJ mice show a reduction in paired pulse facilitation with no change in the initial release probability
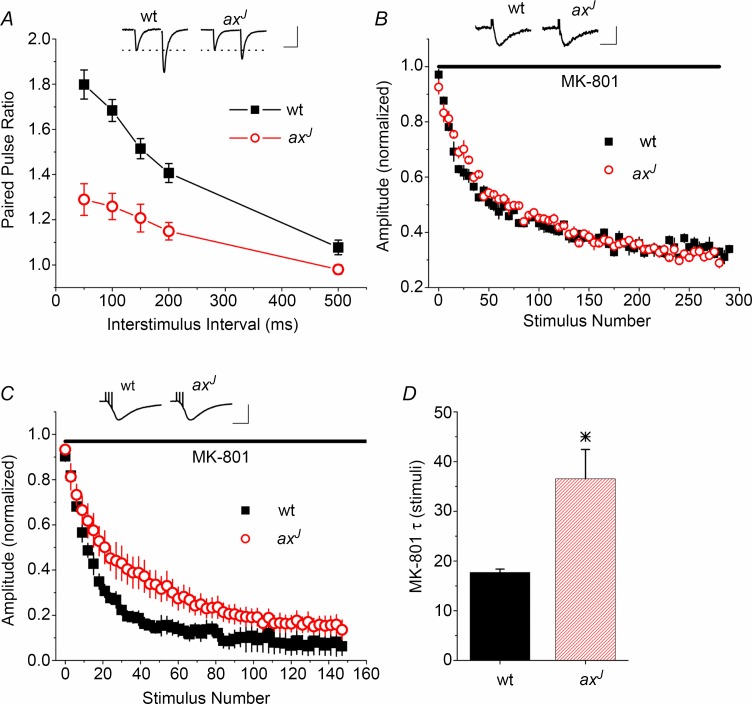
Using dendritic field potential recordings from acute hippocampal slices, we confirmed a significant decrease in paired pulse facilitation (PPF) at Schaffer collateral synapses in axJ mice as compared to wild-type (wt) controls (Fig. 1A), as previously reported (Wilson et al. 2002). Because the amount of PPF is usually inversely related to initial release probability (Dobrunz & Stevens, 1997; Regehr & Stevens, 2001), we hypothesized that the decrease in PPF in axJ mice derives from an increase in initial release probability. We tested this by measuring the rate of the use-dependent block of NMDA receptors by MK-801 (Hessler et al. 1993; Speed & Dobrunz, 2009), an established method for testing for differences in release probability (Castro-Alamancos, 1997; Sun et al. 2005; Sun & Dobrunz, 2006). An increase in release probability would cause an increase in the rate of block of NMDA EPSCs by MK-801; however, we found no difference in the rate of block by 40 μm MK-801 in axJ mice in response to single pulse stimulation at 0.1 Hz (Fig. 1B), indicating that increased initial release probability does not underlie the PPF deficit in axJ mice. Further, the GluN2B-specific antagonist Ro25-6981 (1 μm) blocked NMDA EPSCs to the same extent in wt and axJ (wt 50 ± 6.5% n = 7, axJ 42 ± 9% n = 5, P > 0.5), indicating that an altered ratio of GluN2A to GluN2B in axJ does not confound the interpretation of the MK-801 experiment (see also Speed & Dobrunz, 2009).
Figure 1.
A, Schaffer collateral synapses from axJ mice (n = 5) show a reduction in PPF when compared to wt mice (n = 5). Inset: example traces at the 50 ms interval. Scale bars: 20 ms, 0.15 mV wt, 0.35 mV axJ. B, blocking rate of the NMDA response by MK-801 is not significantly different between axJ (n = 5) and wt (n = 5). Results are displayed as 5 point bins. Inset: example traces of NMDA EPSCs prior to MK-801 block. Scale bars: 20 ms, 35 pA wt, 25.3 pA axJ. C, blocking rate of the NMDA response by MK-801 is slower in slices from axJ mice (n = 5) than wt mice (n = 5) in response to short trains (3 pulses, 10 ms interval, 0.05 Hz). Points are displayed as 3 point bins. Inset: example traces of NMDA EPSCs prior to MK-801 block. Scale bars: 50 ms, 500 pA wt, 350 pA axJ. D, decay constant (τ) for block by MK-801 during stimulation with short trains, in stimuli. n = 5 wt, n = 5 axJ, *P < 0.05.
An increase in release probability may also be reflected by an increase in mEPSC frequency (Zucker & Regehr, 2002). However, there was no difference between wt and axJ mice in the average frequency of mEPSCs measured in CA1 pyramidal cells (Supplemental Fig. S1A, available online), consistent with the finding that initial release probability is unaltered. In addition, there was no difference in the average amplitude of mEPSCs (Supplemental Fig. S1B), and no change in the density of spines (Supplemental Fig. S1C), indicating there is no change in the postsynaptic quantal response or in synapse number. Together with previous results showing no difference in the input–output relationship (Wilson et al. 2002), these results support our finding that the decrease in PPF in axJ mice is not caused by an increase in the initial release probability.
Reduction in paired pulse facilitation in axJ mice is not accounted for by common postsynaptic mechanisms
Although reduced PPF is typically caused by an increase in the initial release probability (Dobrunz & Stevens, 1997; Regehr & Stevens, 2001), the PPF deficit observed in axJ mice could instead stem from a postsynaptic mechanism. AMPA receptor desensitization (Koike-Tani et al. 2008), NMDA receptor activation (Joy & Albertson, 1993), and GABA-mediated inhibition (Sylantyev et al. 2005), for example, have been shown to affect PPF at some synapses. However, we found no difference in PPF in wt or axJ animals in the presence of cyclothiazide (to block AMPA receptor desensitization), APV (to block NMDA receptors), or picrotoxin (to block GABAA receptors) (Supplemental Fig. S1D and E). Together these data suggest that the PPF deficit induced by loss of USP14 does not originate postsynaptically.
Release probability of facilitated pulses is decreased in axJ mice
The MK-801 method can be used to test for differences in release probability of facilitated pulses if the initial release probability is unaltered (Sippy et al. 2003). To test whether the probability of release on facilitated pulses in a short train is reduced in axJ mice as compared to wt, we measured the rate of block by MK-801 (20 μm) in response to 3-pulse, 100 Hz trains, which resulted in two facilitated pulses per train. The rate of block by MK-801 was slower at Schaffer collateral synapses from axJ mice (Fig. 1C) and the decay constant was larger (Fig. 1D, P < 0.05), indicating a lower release probability of facilitated responses. This suggests that the observed decrease in PPF is due to a reduction in the release probability of the second pulse in the axJ mice as compared to wt mice.
Mathematical modelling predicts possible mechanisms
We next used mathematical modelling to elucidate mechanisms that could lead to a decrease in PPF with no change in initial release probability. We used a simple model of release probability and short-term plasticity (Sun et al. 2005) in which release probability is governed by the size of the readily releasable vesicle pool (RRP) and the release probability per vesicle (Pr/v) (Dobrunz, 2002). Facilitation occurs through an activity-dependent increase in Pr/v, the magnitude of which is governed by the parameter ΔF (Sun et al. 2005). The decrease in PPF in the axJ mice can be fitted by a 63% decrease in ΔF, which corresponds to an impairment of the mechanism of facilitation (Fig. 2A). Alternatively, the decreased PPF can be fitted by a decrease in the RRP and increase in Pr/v, such that the initial release probability is unchanged (Fig. 2B). The model predicts that a 55% decrease in the RRP together with about a 2–fold increase in Pr/v would be needed to cause the magnitude of PPF deficit observed in the axJ mice.
Levels of proteins involved in neurotransmitter release are unchanged in axJ animals
We next looked for evidence for the first explanation predicted by our model, that the mechanism of facilitation is altered in axJ animals. Loss of USP14 has been reported, both in yeast and mammalian systems, to increase the rate of turnover of some proteasomal substrates (Hanna et al. 2006; Koulich et al. 2008; Lee et al. 2010). We reasoned, therefore, that the levels of key presynaptic proteins involved in neurotransmitter release might be reduced in axJ mice. For example, overexpression of neuronal calcium sensor 1 (NCS1) in cultured hippocampal neurons has been shown to increase short-term facilitation with no change in initial release probability (Sippy et al. 2003), making it an attractive candidate for altering the ‘mechanism of facilitation’ as predicted by our model. To test this, we examined the steady-state level of several candidate presynaptic proteins in hippocampal extracts from wt and axJ mice. We found no change in the expression of NCS1, MUNC13, MUNC18, RAB3a, synaptophysin 1, synaptojanin, syntaxin 1B, synaptobrevin, synaptotagmin 1 or synapsin 1 (Supplemental Fig. S2, available online). These data indicate that the loss of USP14 does not result in global changes in the expression of presynaptic proteins, and suggest that a loss of NCS1 does not underlie the observed PPF deficit in axJ mice.
axJ mice have a reduction in synaptic vesicles
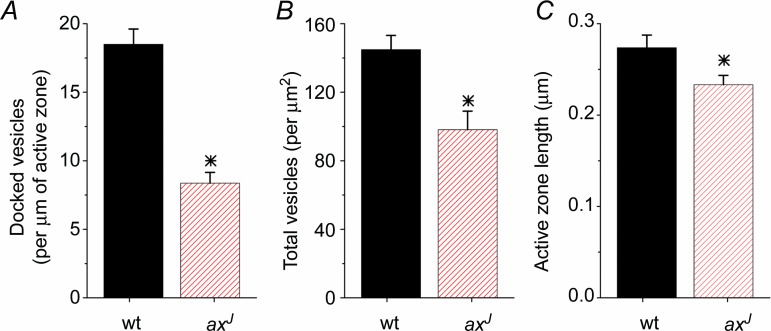
To test whether a reduced RRP contributes to the PPF deficit in axJ mice, we compared the ultrastructure of Schaffer collateral synapses from axJ and wt mice using electron microscopy (Fig. 3). The number of docked vesicles (vesicles located within one vesicle width from the active zone) and the total number of synaptic vesicles were quantified using standard methods (Dickinson-Nelson & Reese, 1983). Strikingly, we find a 55% reduction in docked vesicles at axJ synapses compared to wt (Fig. 3A). Because docked vesicles are the anatomical correlate of the physiologically defined readily releasable pool of vesicles (Schikorski & Stevens, 2001), this supports the model's prediction that the readily releasable pool is reduced at Schaffer collateral synapses in axJ mice. In addition, there was a reduction in the total number of synaptic vesicles (per μm2) at synapses from axJ mice as compared to wt (Fig. 3B). Finally, a small but statistically significant decrease was observed in active zone length at axJ synapses (Fig. 3C). These results suggest that a decrease in the number of docked vesicles, rather than an impairment of the mechanism of facilitation, contributes to the PPF deficit observed in axJ mice. This represents a novel mechanism through which short-term plasticity can be modulated.
Figure 3.
Transmission electron micrographs of Schaffer collateral synapses from wt mice and axJ mice were analysed. There is a decrease in docked vesicles (A), total vesicles (B) and active zone length (C) at Schaffer collateral synapses from axJ mice (n = 57) compared to synapses from wt controls (n = 60). *P < 0.05.
Transgenic expression of USP14 rescues the PPF deficit in the axJ mice
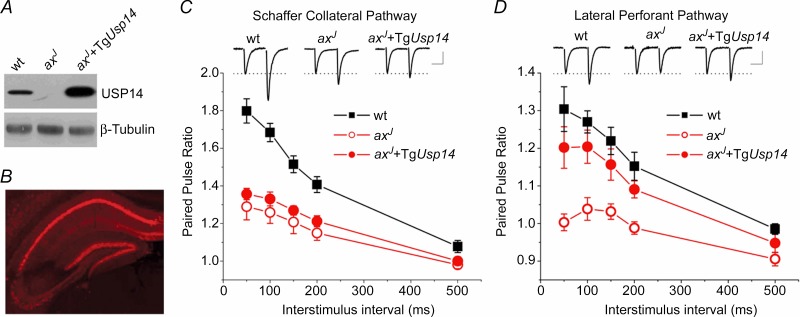
We next sought to determine which activity of USP14 is required for the normal expression of short-term plasticity at hippocampal synapses. Transgenic expression of Usp14 (TgUsp14) under the neuronal Thy 1.2 promoter rescues synaptic transmission deficits at the axJ neuromuscular junction (Chen et al. 2009), and should also restore hippocampal short-term plasticity provided it is expressed in that region. Using wt, axJ and axJ + TgUsp14 mice (Chen et al. 2009), we investigated transgene expression in the hippocampus and entorhinal cortex via immunoblot analysis (Fig. 4A). Robust expression of USP14 was observed in extracts of axJ mice containing the Usp14 transgene. Consistent with the mosaic nature of the Thy1.2 promoter (Caroni, 1997), we observed only faint USP14 expression in CA3 pyramidal cells (Fig. 4B and Chen et al. 2009), and PPF was not restored in the axJ + TgUsp14 mice at Schaffer collateral synapses (Fig. 4C). At lateral perforant path synapses, in which the transgene is expressed both pre- and postsynaptically, expression of TgUsp14 does rescue PPF in axJ mice (Fig. 4D). We therefore assayed PPF in the lateral perforant path for all experiments using Thy1.2 transgenic mice.
Figure 4.
A, immunoblot of USP14 expression in extracts from wt, axJ and axJ + TgUsp14 mice. B, indirect immunofluorescence shows that USP14 expression is strong in CA1 but not in CA3 in axJ + TgUsp14 mice. C, PPF is not restored at Schaffer collateral synapses from axJ + TgUsp14 mice (n = 5 wt, n = 5 axJ, n = 6 axJ + TgUsp14). Inset: example traces at the 50 ms interval. Scale bars: 20 ms, 0.30 mV wt, 0.35 mV axJ, 0.37 mV axJ + TgUsp14. D, lateral perforant path synapses show a deficit in PPF in axJ mice that is restored in axJ + TgUsp14 mice (n = 7 wt, n = 6 axJ, n = 5 axJ + TgUsp14). Inset: example traces at the 50 ms interval. Scale bars: 20 ms, 0.25 mV wt, 0.30 mV axJ, 0.24 mV axJ + TgUsp14.
Ubiquitin over-expression does not restore PPF in the axJ mice
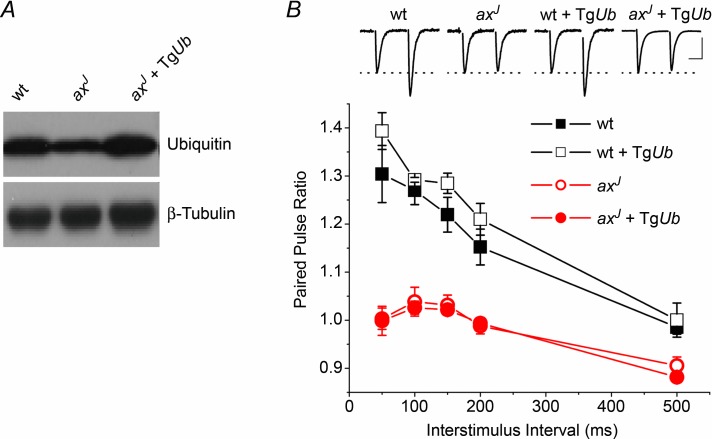
Loss of USP14 results in a significant decrease in free ubiquitin in both the central and peripheral nervous systems of axJ mice (Anderson et al. 2005), and transgenic restoration of ubiquitin in neurons rescues the synaptic deficits at the axJ neuromuscular junction (Chen et al. 2011). This implies that peripheral nervous system synapses require the ubiquitin recycling activity of USP14 for proper structure and function. To determine if the diminished ubiquitin pool causes the hippocampal PPF deficit, we measured PPF at lateral perforant path synapses in transgenic mice that express ubiquitin under the Thy1.2 promoter (TgUb). Immunoblot analysis indicates that the ubiquitin transgene was able to restore hippocampal ubiquitin levels to the TgUb axJ mice (Fig. 5A). However, restoration of ubiquitin did not rescue the PPF deficit observed in axJ mice (axJ + TgUb, Fig. 5B) nor did transgenic expression of ubiquitin have a significant effect on PPF in wt mice (wt + TgUb, Fig. 5B). Depletion of ubiquitin pools, therefore, does not underlie the PPF deficit observed in axJ mice.
Figure 5.
A, immunoblot performed against ubiquitin in extracts from wt, ax and axJ + TgUb mice. B, expression of ubiquitin is unable to restore PPF at lateral perforant path synapses in slices from axJ + TgUb mice (n = 7 wt, n = 6 axJ, n = 4 wt + TgUb, n = 4 axJ + TgUb. Insets: example traces of EPSCs from lateral perforant path synapses. Scale bars: 20 ms, 0.25 mV wt, 0.30 mV axJ, 0.4 mV wt + TgUb, 0.7 mV axJ + TgUb.
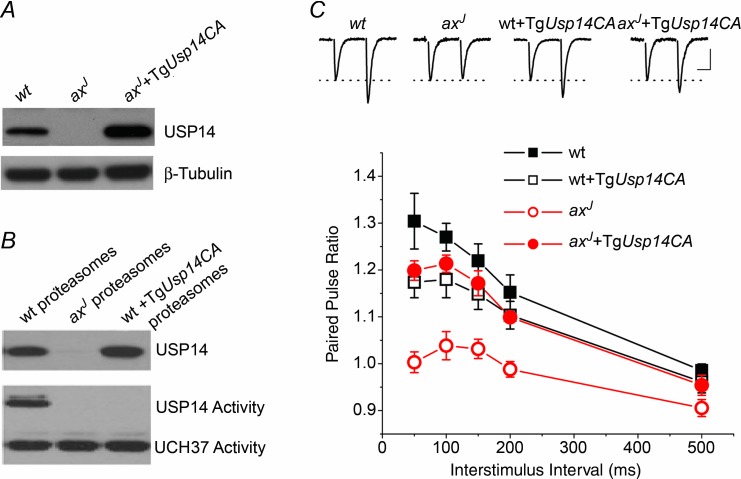
The lack of restoration of PPF observed in axJ + TgUb animals suggests that ubiquitin recycling, and thus the ubiquitin hydrolase activity of USP14, may be dispensable for short-term plasticity in the hippocampus. In support of a ubiquitin hydrolase-independent activity for USP14, several recent studies have demonstrated that catalytically inactive USP14 can modulate proteasome function (Hanna et al. 2006; Lee et al. 2010). To test the hypothesis that loss of USP14's ubiquitin hydrolase-independent activity is responsible for the PPF deficits in axJ mice, we generated transgenic mice expressing a ubiquitin hydrolase-inactive form of USP14 (TgUsp14CA, Fig. 6A). We measured ubiquitin hydrolase activity by performing ubiquitin vinyl-methyl ester labelling assays to confirm that proteasomes from TgUsp14CA mice are devoid of USP14 hydrolase activity. Although USP14 was present on proteasomes from both wt and axJ + TgUsp14CA animals (Fig. 6A), USP14's ubiquitin hydrolase activity could only be detected in proteasomes isolated from wt mice (Fig. 6B). This indicates that USP14CA has no ubiquitin hydrolase activity, and that USP14CA can replace endogenous USP14 on proteasomes of wt + TgUsp14CA mice.
Figure 6.
A, immunoblot against USP14 in extracts from wt, axJ and axJ + TgUsp14CA mice. B, USP14CA displaces all catalytically active USP14 from the proteasome. Purified proteasomes from wt, axJ and wt + TgUsp14CA mice were exposed to hemagglutinin tagged ubiquitin vinlyl-sulfone (HA-Ub-VS), which will label active deubiquitinating enzymes with HA, and immunoblotted for HA. C, expression of TgUsp14CA is able to restore PPF in axJ mice. Acute slice electrophysiology was performed at lateral perforant path synapses from wt (n = 7), axJ (n = 6), wt + TgUsp14CA (n = 6) and axJ + TgUsp14CA (n = 4) mice. Insets are example traces at the 50 ms interval. Scale bars; 20 ms, 0.25 mV wt, 0.30 mV axJ, 0.50 mV wt + TgUsp14CA, 0.40 mV axJ + TgUsp14CA.
Replacing endogenous USP14 with USP14CA had little effect on PPF in wt + TgUsp14CA mice (Fig. 6C), suggesting that the ubiquitin hydrolase activity of USP14 is not required for normal expression of PPF. However, expression of USP14CA restored PPF to normal levels at lateral perforant path synapses in slices from axJ + TgUsp14CA mice (Fig. 6C), indicating that the presence of USP14, even without its ubiquitin hydrolase activity, can restore PPF in axJ mice. The ubiquitin hydrolase-independent activity of USP14 is therefore critical for the normal expression of short-term plasticity at hippocampal lateral perforant path synapses.
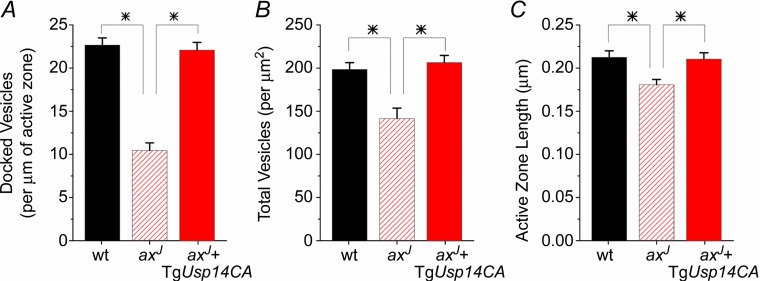
We next tested whether USP14CA also rescues the readily releasable pool of synaptic vesicles. Lateral perforant path synapses from axJ mice have reduced docked vesicles (Fig. 7A), total vesicles (Fig. 7B), and a smaller active zone length (Fig. 7C) compared to wt synapses, comparable to the reductions observed at Schaffer collateral synapses (Fig. 3). Expression of TgUsp14CA on the axJ background restores the number of docked vesicles (Fig. 7A, P < 0.01 vs. axJ, n.s. vs. wt), the total number of vesicles (Fig. 7B, P < 0.01 vs. axJ, n.s. vs. wt), and active zone length (Fig. 7C, P < 0.05 vs. axJ, n.s. vs. wt). Along with the mathematical modelling, these data support the hypothesis that a reduction in the readily releasable pool of vesicles underlies the PPF deficit in axJ mice, and indicate that USP14's ubiquitin hydrolase-independent activity is required for the maintenance of synaptic vesicles and PPF.
Figure 7.
Transmission electron micrographs of lateral perforant path synapses from wt mice, axJ mice and axJ + TgUsp14CA mice were analysed. There is a decrease in docked vesicles (A), total vesicles (B), and active zone length (C) at lateral perforant path synapses from axJ mice (n = 69) compared to synapses from wt controls (n = 65), P < 0.01. All three measures are restored at synapses from axJ + TgUsp14CA mice (n = 65). *P < 0.05.
Proteasome inhibition restores PPF
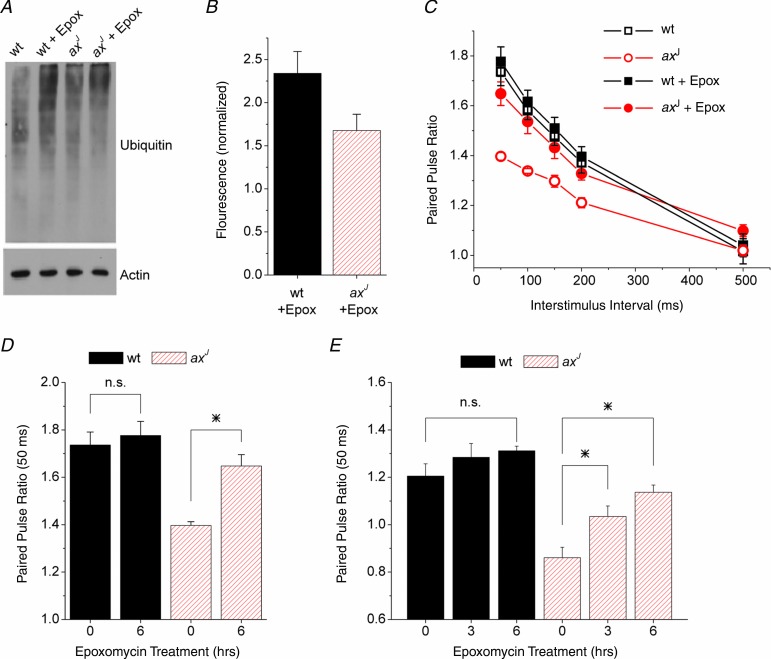
Recent studies have suggested that the loss of USP14 may result in accelerated protein degradation by the proteasome in vitro (Koulich et al. 2008; Lee et al. 2010). We therefore tested whether slowing protein degradation would restore PPF to the axJ hippocampus. Acute wt and axJ hippocampal slices were treated with the proteasome-specific inhibitor epoxomycin for 6 h and the levels of ubiquitin conjugates were measured as an indicator of effective proteasome inhibition (Fig. 8A). Immunoblot analysis demonstrated that a 6 h epoxomycin treatment of hippocampal slices led to 2.2-fold and 1.5-fold increases in wt and axJ ubiquitin conjugates, respectively (Fig. 8B). The smaller increase in ubiquitin conjugates in the axJ hippocampus is probably due to the 40% reduction in free ubiquitin due to loss of USP14 (Anderson et al. 2005). Proteasome inhibition by epoxomycin did not result in a significant change in PPF at Schaffer collateral synapses from wt mice at any interstimulus interval during the 6 h time period (Fig. 8C and D). In contrast, we observed a significant increase in the axJ PPF after the 6 h treatment with epoxomycin (Fig. 8C and D). Similarly, inhibition of the proteasome with epoxomycin increased PPF at lateral perforant path synapses from axJ mice at both 3 h and 6 h of epoxomycin treatment (Fig. 8E). These results demonstrate that proteasome inhibition can rescue the axJ PPF deficit, and suggest that loss of USP14 results in accelerated protein degradation by the proteasome that leads to a deficit in PPF in the axJ mice.
Figure 8.
A, immunoblot for ubiquitinated proteins isolated from hippocampal slices after 6 h of proteasome inhibition with epoxomycin (10 μm). B, quantification of immunoblots as in A, n = 5 wt, n = 5 axJ. Results are normalized to fluorescence from slices with no epoxomycin. C, paired pulse facilitation measured at Schaffer collateral synapses in slices from wt mice (n = 4) and axJ mice (n = 5) after 6 h of exposure to epoxomycin or vehicle. axJ slices showed a robust increase in PPF while wt slices showed no change. D, paired pulse facilitation at Schaffer collateral synapses at the 50 ms interval for 0 and 6 h treatment with epoxomycin (10 μm), n = 4 wt, n = 5 axJ. *P < 0.05. E, paired pulse facilitation at lateral perforant path synapses at the 50 ms interval for 0, 3 h and 6 h treatment with epoxomycin (10 μm), n = 4 wt, n = 4 axJ. *P < 0.05.
Discussion
Our results demonstrate a novel role for the ubiquitin proteasome system in regulating short-term plasticity. Loss of the proteasome-associated deubiquitinating enzyme USP14 results in a reduction of PPF that is not caused by changes in the initial release probability. Since the loss of USP14 results in fewer docked and total presynaptic vesicles, our data suggest that the ubiquitin proteasome system is able to modulate PPF by controlling the number of vesicles available to the presynaptic compartment. This is a novel mechanism by which the ubiquitin proteasome system can regulate presynaptic plasticity without changes in basal synaptic strength. These defects are caused by the loss of a ubiquitin hydrolase-independent function of USP14, and not by ubiquitin depletion, because transgenic expression of USP14CA, but not ubiquitin, is able to rescue the deficits in synaptic vesicles and PPF. We suggest that the ubiquitin hydrolase-independent function of USP14 can spare certain proteins necessary for normal PPF, because proteasome inhibition was also able to restore PPF to axJ synapses. This study therefore provides the first in vivo evidence for a ubiquitin hydrolase-independent function of a deubiquitinating enzyme in mammals and suggests a novel regulatory mechanism for USP14 to control synaptic structure and short-term plasticity.
It is well established that changes in PPF are often caused by changes in the initial release probability of synapses (Zucker & Regehr, 2002). The amount of PPF is inversely correlated with the initial release probability across a population of the same type of synapses (Dobrunz & Stevens, 1997) and also between different types of synapses (Sun et al. 2005; Sun & Dobrunz, 2006). However, there are also examples of changes in PPF that are not caused by changes in the initial release probability (Kokaia et al. 1998; Sippy et al. 2003; Speed & Dobrunz, 2009), as we have shown here, making it important to directly test whether changes in PPF are caused by inverse changes in the initial release probability using the MK-801 method. In cases where there is not a change in the initial release probability, a multiple-pulse MK-801 protocol can be used to demonstrate changes in the release probability of facilitating pulses (Sippy et al. 2003, present study). One potential issue with using the MK-801 method to test for differences in release probability is whether there could be differences in NMDA receptor subunit composition that could affect the MK-801 blocking rate. However, we found no difference between wt and axJ synapses in the percentage of the NMDA current that is blocked by an GluN2B specific antagonist. In addition, a previous study by our lab has directly shown that the GluN2A/GluN2B subunit composition does not affect the MK-801 blocking rate (Speed & Dobrunz, 2009), alleviating this concern. Our results using the multiple-pulse MK-801 protocol demonstrate that the release probability of facilitating pulses is reduced at synapses from axJ mice compared to wt mice.
Mathematical modelling suggests that a reduction in PPF with no change in initial release probability could be caused by a decrease in the number of readily releasable vesicles, together with an increase in the release probability per vesicle. Our electron microscopy data demonstrate a large (55%) reduction in the number of vesicles docked at the active zone. Since the number of docked vesicles has been shown to correspond with the size of the readily releasable vesicle pool (Schikorski & Stevens, 2001), this is consistent with the 55% reduction in readily releasable vesicles proposed by the model. While it is possible that under some conditions the readily releasable pool could instead comprise only a subset of the docked vesicles, it is unlikely that such a large reduction in anatomically docked vesicles would fail to decrease the number of readily releasable vesicles. Therefore, our data indicate that loss of USP14 causes a reduction in readily releasable vesicles at hippocampal synapses, which is observed at both Schaffer collateral synapses and lateral perforant path synapses. Consistent with this, a decrease in the size of the readily releasable vesicle pool in axJ mice is also observed at neuromuscular junction synapses using FM dye imaging (Bhattacharyya et al. 2012). Our results do not rule out the possibility that there is also a change in the magnitude of facilitation itself, in addition to a reduction in the number of synaptic vesicles. A change in PPF with no change in initial release probability has also been shown to occur upon alterations in the levels of the calcium binding protein NCS1 (Sippy et al. 2003). In particular, increased levels of NCS1 caused an increase in facilitation in cultured hippocampal synapses, with no change in the initial release probability (Sippy et al. 2003). However, we saw no change in the level of NCS1 in axJ mice, suggesting that a reduction in NCS1 is not the mechanism for the observed decreased PPF. In addition, the extremely close agreement between the model prediction and the magnitude of the decrease in docked vesicles suggests that a decrease in RRP alone is sufficient to account for the decrease in PPF in the axJ mice. The modulation of synaptic vesicles by USP14 therefore appears to be an important mechanism through which the ubiquitin proteasome system could modulate the function and plasticity of hippocampal synapses.
In addition to the reduction in docked vesicles, hippocampal synapses from axJ mice also have a reduction in active zone length, and total synaptic vesicles, indicating that the synapses are smaller. This could potentially indicate that the synapses have failed to mature or are delayed in their normal developmental maturation, since synapses normally show an increase in size and in synaptic vesicle number during early postnatal development (Dyson & Jones, 1980; Mozhayeva et al. 2002). Consistent with this, the loss of USP14 results in a developmental delay in the maturation of neuromuscular junction synapses in the axJ mice (Chen et al. 2011). However, the reduction in PPF in hippocampal synapses from axJ mice is not consistent with a developmental delay, in that PPF is larger at Schaffer collateral synapses from juvenile rodents compared to young adults (Dekay et al. 2006). Although loss of USP14 may cause a delay in structural maturation of synapses, it appears to also have other effects on synaptic function and plasticity.
Because a large decrease in readily releasable vesicles by itself should decrease the initial release probability (Dobrunz & Stevens, 1997; Dobrunz, 2002), which is not observed in the axJ mice, there must also be an increase in the release probability per vesicle. Consistent with this, an increase in release probability per vesicle is also observed at neuromuscular junction synapses from axJ mice (Bhattacharyya et al. 2012). It is not known whether the release probability per vesicle is directly regulated by USP14, and if so, by what mechanism. Alternatively, the increase in release probability per vesicle could reflect a homeostatic compensation that helps to normalize the initial release probability despite such a large decrease in synaptic vesicles. Other components of the ubiquitin proteasome system have been shown to be important for homeostatic scaling of postsynaptic strength (Jakawich et al. 2010; Djakovic et al. 2012) and presynaptic strength (Lazarevic et al. 2011) in response to changes in neuronal activity; it remains to be shown whether the loss of USP14 alters presynaptic function through homeostatic mechanisms.
USP14 is one of three deubiquitinating enzymes that reside on the regulatory particle of the proteasome and are involved in the turnover of ubiquitinated proteins at the proteasome (Guterman & Glickman, 2004). Our previous work has shown that the loss of USP14 in the axJ mice results in a significant depletion of ubiquitin at synapses, indicating that USP14 functions to stabilize ubiquitin pools (Anderson et al. 2005). Using ubiquitin transgenic mice, we previously demonstrated that restoring ubiquitin levels to the axJ mice was sufficient to prevent perinatal lethality and restore synaptic transmission at the neuromuscular junction (Chen et al. 2011). The results from our present work demonstrate that restoration of ubiquitin levels in the hippocampus of axJ mice was not sufficient to correct the PPF deficit. This indicates that the decreased levels of ubiquitin in axJ mice are not responsible for the PPF deficit. Consistent with this, we have previously shown that mice deficient in UCH-L1, who also have decreased ubiquitin, do not have the PPF deficit at hippocampal synapses (Walters et al. 2008). Together, these results suggest that USP14 may have functions other than recycling ubiquitin that can regulate synaptic activity at central synapses.
Our finding that transgenic expression of a ubiquitin hydrolase-inactive form of USP14, USP14CA, can restore synaptic vesicle pools and hippocampal PPF to the axJ mice indicates that this additional role for USP14 does not require its canonical enzymatic activity. Analysis of the yeast orthologue of USP14, Ubp6, has demonstrated that the ability of Ubp6 to slow substrate degradation by the proteasome is independent of its ubiquitin hydrolase activity (Hanna et al. 2006). Work from several labs has shown that loss of USP14 also results in an increase in the degradation of synthetic proteasome substrates in vitro and in immortalized cell lines (Koulich et al. 2008; Lee et al. 2010), although loss of USP14 does not alter the proteolytic activity of the proteasome (Anderson et al. 2005). This suggests that the 20S proteasome itself is not directly affected by loss of UPS14, but instead that the rate that ubiquitinated substrates are processed by the proteasome is increased. Consistent with this, we find that pharmacological inhibition of the proteasome restores PPF at both Schaffer collateral and lateral perforant path synapses from axJ mice, with no significant effect on PPF from wt mice. Although we currently do not know if proteasome inhibition also restores synaptic vesicle pools to the axJ hippocampus, previous work on primary neurons has indicated that proteasome inhibition increases synaptic vesicle pools (Willeumier et al. 2006). Therefore the rescue of PPF observed at axJ synapses with proteasome inhibitors may in part be due to restoring the expression of a protein(s) required for the maintenance of synaptic vesicle pools.
The most direct model for USP14's function at hippocampal synapses would involve the non-catalytic activity of USP14 resulting in sparing of a synaptic protein (or proteins) important for regulating synaptic vesicle numbers. When this protein is present at a reduced concentration, as may occur in the axJ mice, the result is a reduction in the number of vesicles at the synapse. However, immunoblot studies of several candidate presynaptic proteins did not show any significant decreases, indicating that there is not a widespread decrease in presynaptic protein levels due to increased proteasomal protein degradation. Therefore, it is likely that USP14 controls the stability of a limited set of proteins; however, the mechanism underlying this selectivity is not yet known. A more thorough investigation of the synaptic proteome of axJ mice will be required to identify the specific substrates for USP14 and determine which is (are) important for regulating synaptic vesicles.
The mechanism by which loss of USP14 may cause accelerated protein degradation by the proteasome is not yet understood. Since deubiquitination of substrates by the proteasomal deubiquitinating enzyme Rpn11 is coupled to degradation (Yao & Cohen, 2002), it has been suggested that Ubp6 could slow substrate degradation by reducing the activity of Rpn11 (Hanna et al. 2006). While USP14 and Rpn11/POH1 have not been shown to physically interact, there are several examples where subunits in different regions of the proteasome have been shown to influence each other's activity (Borodovsky et al. 2001; Peth et al. 2009; Djakovic et al. 2012). USP14 has also been shown to possess a stimulatory activity on the proteasome that does not require its catalytic activity (Peth et al. 2009, 2013). However, our study does not directly support a role for this stimulatory activity of USP14 in hippocampal synaptic transmission since the effect of USP14CA on axJ PPF mimics that observed with proteasome inhibition.
Proteasome inhibition not only alters protein turnover but has also been shown to deplete monomeric ubiquitin from cells (Mimnaugh et al. 1997; Patnaik et al. 2000). It is possible that the effect of proteasome inhibitors on PPF at axJ synapses does not occur through changes in protein abundance, but instead is caused by an alteration in non-proteolytic ubiquitin signalling. Several studies have demonstrated that neurons rely on ubiquitin signalling to regulate cellular pathways independently of protein abundance (Chen & Sun, 2009; Clague et al. 2012). Therefore, the further lowering of ubiquitin pools following proteasome inhibition in the axJ hippocampus could result in decreased non-proteolytic ubiquitin signalling and indirectly suppress the effects caused by the loss of USP14. Since our data show that the deubiquitinating activity of USP14 is dispensable for normal PPF, we favour the model whereby proteasome inhibition restores PPF via an alteration in protein abundance and not aberrant ubiquitin signalling.
Many neurodegenerative diseases show protein aggregation, prompting researchers to suggest that promoting proteasome activity would facilitate the clearance of aggregate-prone proteins. Our observations suggest that therapies aimed at increasing proteasomal activity may also cause deleterious changes within synapses that could reduce the therapeutic benefits of this approach. However, drugs to block USP14's ubiquitin hydrolase activity, which are currently being developed, would not be expected to be detrimental to the hippocampal synapses examined in this study.
In conclusion, our study has revealed an unexpected activity for the proteasomal deubiquitinating enzyme USP14 in regulating synaptic function. Although previous studies have demonstrated that the catalytic activity of USP14 is required to maintain ubiquitin levels necessary for synaptic transmission at the neuromuscular junction (Chen et al. 2011), we now show that USP14 also possesses a ubiquitin hydrolase-independent activity that is required to maintain synaptic vesicles and short-term plasticity in the hippocampus. This new activity would allow for the precise control of the abundance of proteins involved in the maintenance of synaptic vesicles. These results establish a new role for proteasomal deubiquitinating enzymes in controlling synaptic function by regulating synaptic vesicles that does not require their canonical enzymatic activity.
Key points
Mice carrying the ataxia (axJ) mutation have a 95% reduction in the deubiquitinating enzyme USP14, which results in a reduction in hippocampal paired pulse facilitation, a form of short-term synaptic plasticity.
Hippocampal synapses in axJ mice have a 50% reduction in synaptic vesicles but no change in the initial release probability, which is a novel mechanism for regulating paired pulse facilitation.
USP14 modulates hippocampal short-term plasticity and structure independent of its deubiquitinating activity, as overexpression of a catalytically inactive form of USP14 restores hippocampal paired pulse facilitation and vesicle number to the ataxia mice.
Pharmacological inhibition of the proteasome also rescues the deficits in hippocampal short-term plasticity in ataxia mice, implying that the loss of USP14 causes increased protein degradation.
These results suggest that USP14 plays a modulatory role in regulating protein turnover by the proteasome that is independent of its canonical role in the disassembly of polyubiquitin conjugates.
Glossary
- ERS
external recording solution
- NCS1
neuronal calcium sensor 1
- PPF
paired pulse facilitation
- RRP
readily releasable pool size
- USP14
ubiquitin-specific protease 14
- wt
wild-type
Additional information
Competing interests
S.M.W. is a paid consultant for Progenra.
Author contributions
L.E.D., B.J.W. and S.M.W. designed the experiments. B.J.W., S.M.W. and L.E.D. wrote the manuscript. B.J.W. performed all electrophysiology experiments and analysed the data. S.M.W. created the transgenic mouse lines and B.J.W. examined transgene expression patterns. L.E.D. did the mathematical modelling. B.J.W. and J.J.H. did the electron microscopy experiments. B.J.W. did the deubiquitinating enzyme activity assay. C.S.T. and H.L.P. contributed the HA-Ub-VS. All authors approved the final version of the manuscript. All experiments were conducted at the University of Alabama at Birmingham School of Medicine.
Funding
This work was supported by grants from the National Institutes of Health (R01 NS047533 and R21 NS074456 to S.M.W., T32GM0811 to B.J.W. and R01 MH065328 to L.E.D.), the Civitan International Research Center and the Evelyn F. McKnight Brain Institute.
References
- Anderson C, Crimmins S, Wilson JA, Korbel GA, Ploegh HL, Wilson SM. Loss of Usp14 results in reduced levels of ubiquitin in ataxia mice. J Neurochem. 2005;95:724–731. doi: 10.1111/j.1471-4159.2005.03409.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Wilson SM, Jung H, Miller RJ. Altered neurotransmitter release machinery in mice deficient for the deubiquitinating enzyme Usp14. Am J Physiol Cell Physiol. 2012;302:C698–C708. doi: 10.1152/ajpcell.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingol B, Sheng M. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron. 2011;69:22–32. doi: 10.1016/j.neuron.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 2001;20:5187–5196. doi: 10.1093/emboj/20.18.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. doi: 10.1016/s0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Short-term plasticity in thalamocortical pathways: cellular mechanisms and functional roles. Rev Neurosci. 1997;8:95–116. doi: 10.1515/revneuro.1997.8.2.95. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Boggio EM, Calfa G, Percy AK, Giustetto M, Pozzo-Miller L. Hippocampal CA1 pyramidal neurons of Mecp2 mutant mice show a dendritic spine phenotype only in the presymptomatic stage. Neural Plast. 2012;2012:976164. doi: 10.1155/2012/976164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Bhattacharyya BJ, Hanna J, Minkel H, Wilson JA, Finley D, Miller RJ, Wilson SM. Ubiquitin homeostasis is critical for synaptic development and function. J Neurosci. 2011;31:17505–17513. doi: 10.1523/JNEUROSCI.2922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Qin LN, Li XM, Walters BJ, Wilson JA, Mei L, Wilson SM. The proteasome-associated deubiquitinating enzyme Usp14 is essential for the maintenance of synaptic ubiquitin levels and the development of neuromuscular junctions. J Neurosci. 2009;29:10909–10919. doi: 10.1523/JNEUROSCI.2635-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Liu H, Urbé S. Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev Cell. 2012;23:457–467. doi: 10.1016/j.devcel.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Crimmins S, Jin Y, Wheeler C, Huffman AK, Chapman C, Dobrunz LE, Levey A, Roth KA, Wilson JA, Wilson SM. Transgenic rescue of ataxia mice with neuronal-specific expression of ubiquitin-specific protease 14. J Neurosci. 2006;26:11423–11431. doi: 10.1523/JNEUROSCI.3600-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekay JG, Chang TC, Mills N, Speed HE, Dobrunz LE. Responses of excitatory hippocampal synapses to natural stimulus patterns reveal a decrease in short-term facilitation and increase in short-term depression during postnatal development. Hippocampus. 2006;16:66–79. doi: 10.1002/hipo.20132. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci. 2004;27:223–246. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- Dickinson-Nelson A, Reese TS. Structural changes during transmitter release at synapses in the frog sympathetic ganglion. J Neurosci. 1983;3:42–52. doi: 10.1523/JNEUROSCI.03-01-00042.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic SN, Marquez-Lona EM, Jakawich SK, Wright R, Chu C, Sutton MA, Patrick GN. Phosphorylation of Rpt6 regulates synaptic strength in hippocampal neurons. J Neurosci. 2012;32:5126–5131. doi: 10.1523/JNEUROSCI.4427-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE. Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Int J Dev Neurosci. 2002;20:225–236. doi: 10.1016/s0736-5748(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dyson SE, Jones DG. Quantitation of terminal parameters and their inter-relationships in maturing central synapses: a perspective for experimental studies. Brain Res. 1980;183:43–59. doi: 10.1016/0006-8993(80)90118-3. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Ubiquitin and synaptic dysfunction: ataxic mice highlight new common themes in neurological disease. Trends Neurosci. 2003;26:4–7. doi: 10.1016/s0166-2236(02)00013-9. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Byrne JH. Protein degradation and memory formation. Brain Res Bull. 2011;85:14–20. doi: 10.1016/j.brainresbull.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Guterman A, Glickman MH. Deubiquitinating enzymes are IN/(trinsic to proteasome function) Curr Protein Pept Sci. 2004;5:201–211. doi: 10.2174/1389203043379756. [DOI] [PubMed] [Google Scholar]
- Hanna J, Hathaway NA, Tone Y, Crosas B, Elsasser S, Kirkpatrick DS, Leggett DS, Gygi SP, King RW, Finley D. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell. 2006;127:99–111. doi: 10.1016/j.cell.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Hu M, Li P, Song L, Jeffrey PD, Chenova TA, Wilkinson KD, Cohen RE, Shi Y. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005;24:3747–3756. doi: 10.1038/sj.emboj.7600832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakawich SK, Neely RM, Djakovic SN, Patrick GN, Sutton MA. An essential postsynaptic role for the ubiquitin proteasome system in slow homeostatic synaptic plasticity in cultured hippocampal neurons. Neuroscience. 2010;171:1016–1031. doi: 10.1016/j.neuroscience.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy RM, Albertson TE. NMDA receptors have a dominant role in population spike-paired pulse facilitation in the dentate gyrus of urethane-anaesthetized rats. Brain Res. 1993;604:273–282. doi: 10.1016/0006-8993(93)90379-2. [DOI] [PubMed] [Google Scholar]
- Kaang BK, Choi JH. Synaptic protein degradation in memory reorganization. Adv Exp Med Biol. 2012;970:221–240. doi: 10.1007/978-3-7091-0932-8_10. [DOI] [PubMed] [Google Scholar]
- Koike-Tani M, Kanda T, Saitoh N, Yamashita T, Takahashi T. Involvement of AMPA receptor desensitization in short-term synaptic depression at the calyx of Held in developing rats. J Physiol. 2008;586:2263–2275. doi: 10.1113/jphysiol.2007.142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia M, Asztely F, Olofsdotter K, Sindreu CB, Kullmann DM, Lindvall O. Endogenous neurotrophin–3 regulates short-term plasticity at lateral perforant path–granule cell synapses. J Neurosci. 1998;18:8730–8739. doi: 10.1523/JNEUROSCI.18-21-08730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulich E, Li X, Demartino GN. Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell. 2008;19:1072–1082. doi: 10.1091/mbc.E07-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Schone C, Heine M, Gundelfinger ED, Fejtova A. Extensive remodeling of the presynaptic cytomatrix upon homeostatic adaptation to network activity silencing. J Neurosci. 2011;31:10189–10200. doi: 10.1523/JNEUROSCI.2088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimnaugh EG, Chen HY, Davie JR, Celis JE, Neckers L. Rapid deubiquitination of nucleosomal histones in human tumor cells caused by proteasome inhibitors and stress response inducers: effects on replication, transcription, translation, and the cellular stress response. Biochemistry. 1997;36:14418–14429. doi: 10.1021/bi970998j. [DOI] [PubMed] [Google Scholar]
- Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A, Chau V, Wills JW. Ubiquitin is part of the retrovirus budding machinery. Proc Natl Acad Sci U S A. 2000;97:13069–13074. doi: 10.1073/pnas.97.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. Dysfunction of the ubiquitin-proteasome system in multiple disease conditions: therapeutic approaches. Bioessays. 2008;30:1172–1184. doi: 10.1002/bies.20852. [DOI] [PubMed] [Google Scholar]
- Peth A, Besche HC, Goldberg AL. Ubiquitinated proteins activate the proteasome by binding to Usp14/Ubp6, which causes 20S gate opening. Mol Cell. 2009;36:794–804. doi: 10.1016/j.molcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Kukushkin N, Bossé M, Goldberg AL. Ubiquitinated proteins activate the proteasomal ATPases by binding to Usp14 or Uch37 homologs. J Biol Chem. 2013;288:7781–7790. doi: 10.1074/jbc.M112.441907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Stevens CF. Physiology of synaptic transmission and short-term plasticity. In: Cowan WM, Sudhof TC, Stevens CF, editors. Synapses. Baltimore, MD, USA: Johns Hopkins University; 2001. pp. 135–176. [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Singhal S, Taylor MC, Baker RT. Deubiquitylating enzymes and disease. BMC Biochem. 2008;9(Suppl. 1):S3. doi: 10.1186/1471-2091-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippy T, Cruz-Martin A, Jeromin A, Schweizer FE. Acute changes in short-term plasticity at synapses with elevated levels of neuronal calcium sensor–1. Nat Neurosci. 2003;6:1031–1038. doi: 10.1038/nn1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed HE, Dobrunz LE. Developmental changes in short-term facilitation are opposite at temporoammonic synapses compared to Schaffer collateral synapses onto CA1 pyramidal cells. Hippocampus. 2009;19:187–204. doi: 10.1002/hipo.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Bartley AF, Dobrunz LE. Calcium-permeable presynaptic kainate receptors involved in excitatory short-term facilitation onto somatostatin interneurons during natural stimulus patterns. J Neurophysiol. 2009;101:1043–1055. doi: 10.1152/jn.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Dobrunz LE. Presynaptic kainate receptor activation is a novel mechanism for target cell-specific short-term facilitation at Schaffer collateral synapses. J Neurosci. 2006;26:10796–10807. doi: 10.1523/JNEUROSCI.2746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Lyons SA, Dobrunz LE. Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats. J Physiol. 2005;568:815–840. doi: 10.1113/jphysiol.2005.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylantyev SO, Lee CM, Shyu BC. A parametric assessment of GABA antagonist effects on paired-pulse facilitation in the rat anterior cingulate cortex. Neurosci Res. 2005;52:362–370. doi: 10.1016/j.neures.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Campbell SL, Chen PC, Taylor AP, Schroeder DG, Dobrunz LE, Artavanis-Tsakonas K, Ploegh HL, Wilson JA, Cox GA, Wilson SM. Differential effects of Usp14 and Uch-L1 on the ubiquitin proteasome system and synaptic activity. Mol Cell Neurosci. 2008;39:539–548. doi: 10.1016/j.mcn.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeumier K, Pulst SM, Schweizer FE. Proteasome inhibition triggers activity-dependent increase in the size of the recycling vesicle pool in cultured hippocampal neurons. J Neurosci. 2006;26:11333–11341. doi: 10.1523/JNEUROSCI.1684-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Bhattacharyya B, Rachel RA, Coppola V, Tessarollo L, Householder DB, Fletcher CF, Miller RJ, Copeland NG, Jenkins NA. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat Genet. 2002;32:420–425. doi: 10.1038/ng1006. [DOI] [PubMed] [Google Scholar]
- Yao I, Takagi H, Ageta H, Kahyo T, Sato S, Hatanaka K, Fukuda Y, Chiba T, Morone N, Yuasa S, Inokuchi K, Ohtsuka T, MacGregor GR, Tanaka K, Setou M. SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release. Cell. 2007;130:943–957. doi: 10.1016/j.cell.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev. 2007;59:14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.